Helminths in Invasive Raccoons (Procyon lotor) from Southwest Germany
Abstract
1. Introduction
2. Materials and Methods
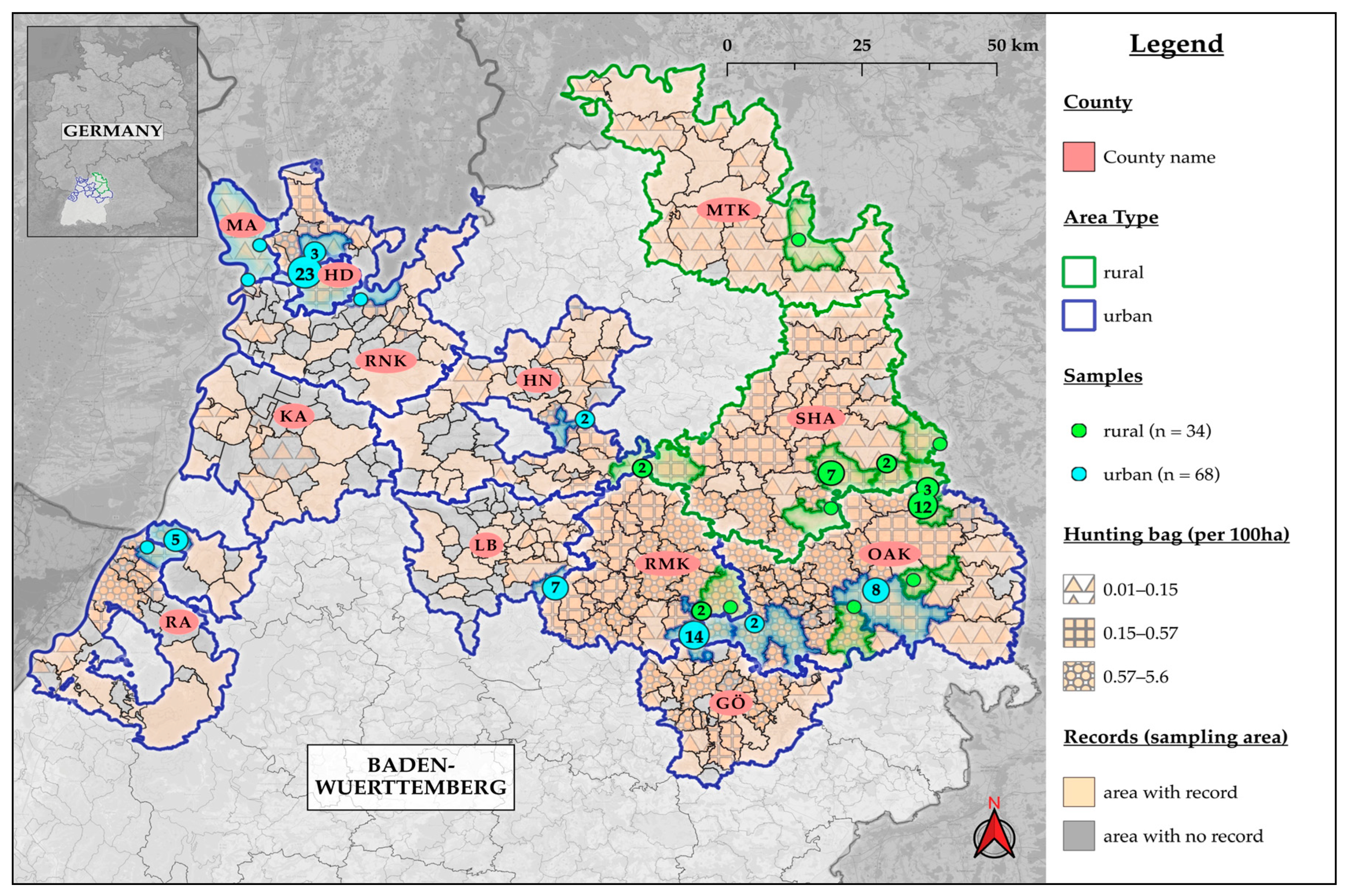
2.1. Sample Collection
2.2. Necropsy
2.3. Parasitic Examinations
2.4. Nematode and Cestode Identification by PCR
2.4.1. Sample Preparation
2.4.2. DNA Amplification and Sequencing
2.5. Statistics and Graphics
3. Results
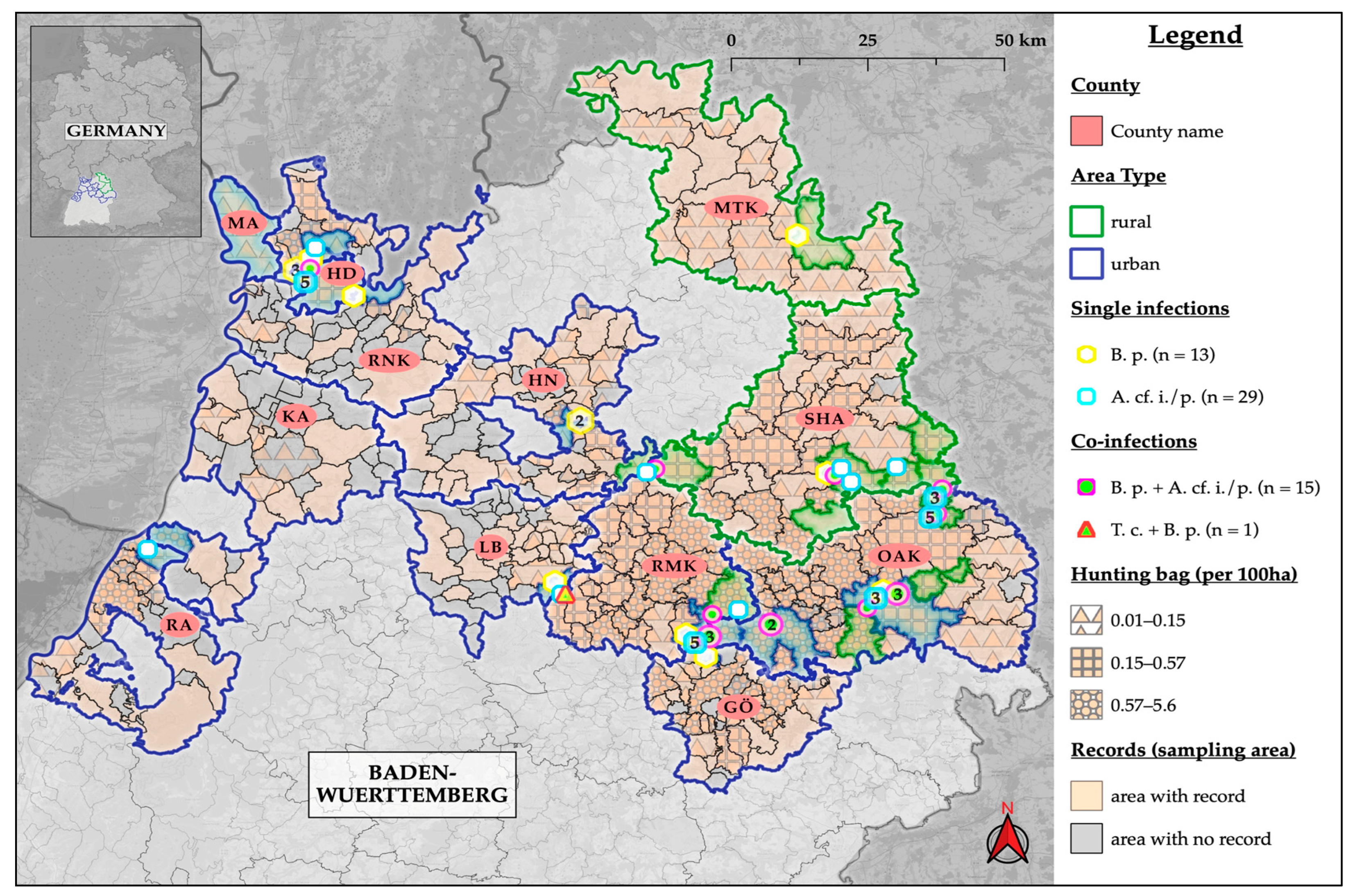
3.1. Nematodes
3.2. Cestodes
3.3. Trematodes
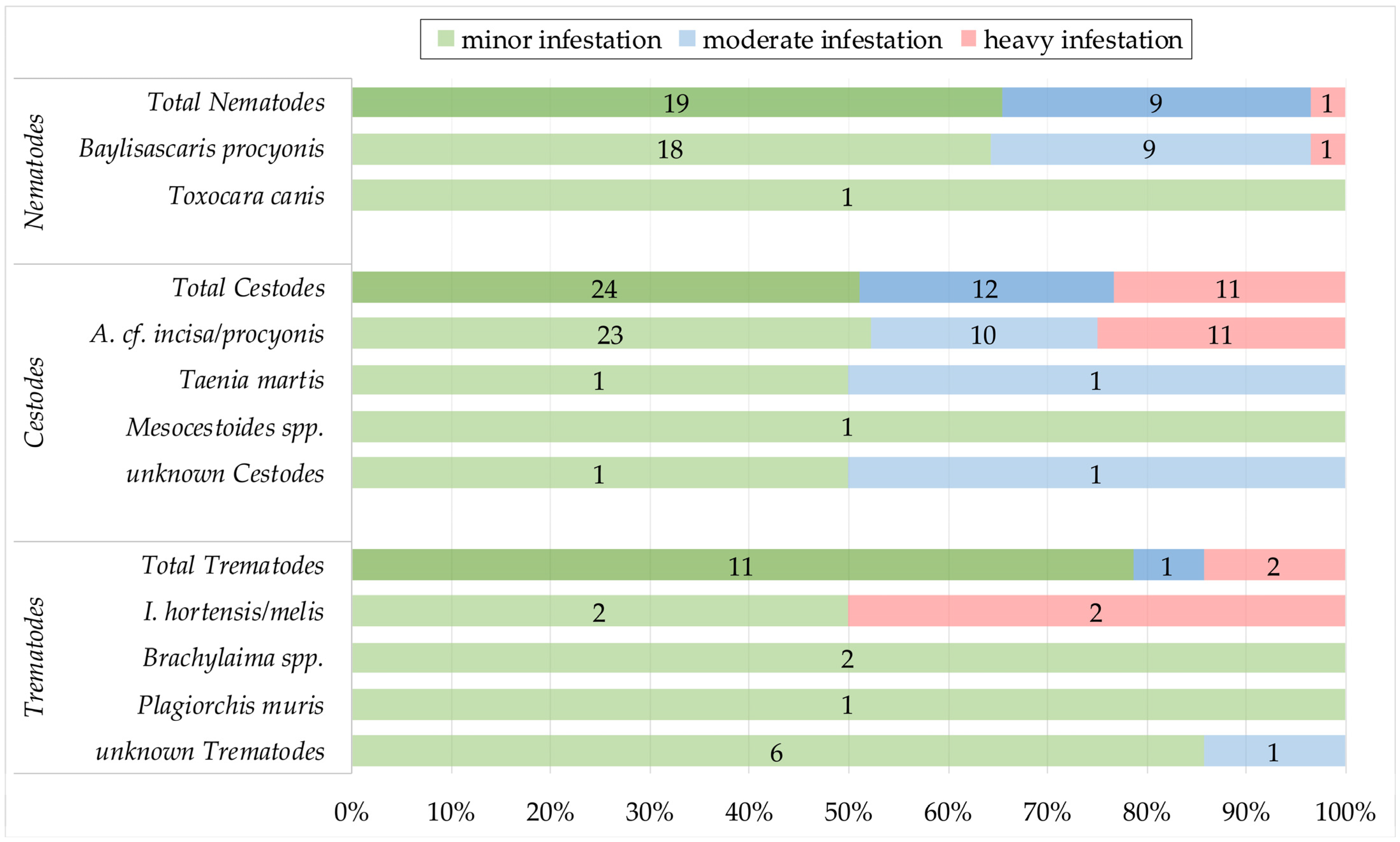
3.4. Intensity of Infestation and Co-Infections
4. Discussion
4.1. Methodological Limitations
4.2. Nematodes
4.2.1. Baylisascaris procyonis
4.2.2. Toxocara canis, Capillaria spp. and Trichuris spp.
4.3. Cestodes
4.4. Trematodes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirzo, R.; Ceballos, G.; Ehrlich, P.R. Circling the drain: The extinction crisis and the future of humanity. Philos. Trans. R. Soc. B. 2022, 377, 20210378. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; McManus, D.P.; Jones, M.K.; Gray, D.J.; Gobert, G.N. The Increase of Exotic Zoonotic Helminth Infections: The Impact of Urbanization, Climate Change and Globalization. Adv. Parasitol. 2016, 91, 311–397. [Google Scholar] [CrossRef]
- Yon, L.; Duff, J.P.; Agren, E.O.; Erdelyi, K.; Ferroglio, E.; Godfroid, J.; Hars, J.; Hestvik, G.; Horton, D.; Kuiken, T.; et al. Recent Changes in Infectious Diseases in European Wildlife. J. Wildl. Dis. 2019, 55, 3–43. [Google Scholar] [CrossRef]
- Louis, M.E. Baylisascaris procyonis, a Zoonotic Threat Requiring an Integrated One Health Approach. Master’s Thesis, North Carolina State University, Releigh, NC, USA, 2020. [Google Scholar]
- Thompson, R.C. Parasite zoonoses and wildlife: One health, spillover and human activity. Int. J. Parasitol. 2013, 43, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife–Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Sapp, S.G.H. Baylisascaris procyonis Infection Dynamics and Transmission among Wildlife, Domestic Animal, and Human Hosts. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2018. [Google Scholar]
- Reinhardt, N.P.; Köster, J.; Thomas, A.; Arnold, J.; Fux, R.; Straubinger, R.K. Bacterial and Viral Pathogens with One Health Relevance in Invasive Raccoons (Procyon lotor, Linne 1758) in Southwest Germany. Pathogens 2023, 12, 389. [Google Scholar] [CrossRef]
- Peter, N.; Dörge, D.D.; Cunze, S.; Schantz, A.V.; Skaljic, A.; Rueckert, S.; Klimpel, S. Raccoons contraband—The metazoan parasite fauna of free-ranging raccoons in central Europe. Int. J. Parasitol. Parasites Wildl. 2023, 20, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Waindok, P.; Raue, K.; Grilo, M.L.; Siebert, U.; Strube, C. Predators in northern Germany are reservoirs for parasites of One Health concern. Parasitol. Res. 2021, 120, 4229–4239. [Google Scholar] [CrossRef]
- Fischer, M.L.; Sullivan, M.J.P.; Greiser, G.; Guerrero-Casado, J.; Heddergott, M.; Hohmann, U.; Keuling, O.; Lang, J.; Martin, I.; Michler, F.-U.; et al. Assessing and predicting the spread of non-native raccoons in Germany using hunting bag data and dispersal weighted models. Biol. Invasions 2016, 18, 57–71. [Google Scholar] [CrossRef]
- Fischer, M.L.; Hochkirch, A.; Heddergott, M.; Schulze, C.; Anheyer-Behmenburg, H.E.; Lang, J.; Michler, F.U.; Hohmann, U.; Ansorge, H.; Hoffmann, L.; et al. Historical Invasion Records Can Be Misleading: Genetic Evidence for Multiple Introductions of Invasive Raccoons (Procyon lotor) in Germany. PLoS ONE 2015, 10, e0125441. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Beck, B.; García, F.J.; Gortázar, C. Raccoons in Europe: Disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012, 58, 5–15. [Google Scholar] [CrossRef]
- Deutscher Jagdverband, e.V. Infografik_Jahresjagdstrecke_Waschbär_2020/2021. Available online: https://www.jagdverband.de/sites/default/files/2022-02/2022-01_Infografik_Jahresjagdstrecke_Waschbaer_2020_2021.jpg (accessed on 14 June 2023).
- Heddergott, M.; Steinbach, P.; Schwarz, S.; Anheyer-Behmenburg, H.E.; Sutor, A.; Schliephake, A.; Jeschke, D.; Striese, M.; Muller, F.; Meyer-Kayser, E.; et al. Geographic Distribution of Raccoon Roundworm, Baylisascaris procyonis, Germany and Luxembourg. Emerg. Infect. Dis. 2020, 26, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.L.; Salgado, I.; Beninde, J.; Klein, R.; Frantz, A.C.; Heddergott, M.; Cullingham, C.I.; Kyle, C.J.; Hochkirch, A.; Leung, B. Multiple founder effects are followed by range expansion and admixture during the invasion process of the raccoon (Procyon lotor) in Europe. Divers. Distrib. 2017, 23, 409–420. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Popiolek, M.; Zalewski, A. Host-parasite interactions in non-native invasive species are dependent on the levels of standing genetic variation at the immune locus. BMC Evol. Biol. 2020, 20, 43. [Google Scholar] [CrossRef]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 17, 71–79. [Google Scholar] [CrossRef]
- Michler, B.A. Koproskopische Untersuchungen zum Nahrungsspektrum des Waschbären Procyon lotor (Linné, 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern) unter spezieller Berücksichtigung des Artenschutzes und des Endoparasitenbefalls. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2017. [Google Scholar]
- Mazzamuto, M.V.; Panzeri, M.; Bisi, F.; Wauters, L.A.; Preatoni, D.; Martinoli, A. When management meets science: Adaptive analysis for the optimization of the eradication of the Northern raccoon (Procyon lotor). Biol. Invasions 2020, 22, 3119–3130. [Google Scholar] [CrossRef]
- French, S.K.; Pearl, D.L.; Peregrine, A.S.; Jardine, C.M. Baylisascaris procyonis infection in raccoons: A review of demographic and environmental factors influencing parasite carriage. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100275. [Google Scholar] [CrossRef]
- Fiderer, C.; Göttert, T.; Zeller, U. Spatial interrelations between raccoons (Procyon lotor), red foxes (Vulpes vulpes), and ground-nesting birds in a Special Protection Area of Germany. Eur. J. Wildl. Res. 2019, 65, 14. [Google Scholar] [CrossRef]
- Salgado, I. Is the raccoon (Procyon lotor) out of control in Europe? Biodivers Conserv. 2018, 27, 2243–2256. [Google Scholar] [CrossRef]
- Bauer, C. Baylisascariosis–Infections of animals and humans with ‘unusual’ roundworms. Vet. Parasitol. 2013, 193, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, B.T.; Prange, S.; Hauver, S.A.; Gehrt, S.D. Patterns of latrine use by raccoons (Procyon lotor) and implication for Baylisascaris procyonis transmission. J. Wildl. Dis. 2014, 50, 243–249. [Google Scholar] [CrossRef]
- Graeff-Teixeira, C.; Morassutti, A.L.; Kazacos, K.R. Update on Baylisascariasis, a Highly Pathogenic Zoonotic Infection. Clin. Microbiol. Rev. 2016, 29, 375–399. [Google Scholar] [CrossRef]
- Barbet, A.F.; Al-Khedery, B.; Stuen, S.; Granquist, E.G.; Felsheim, R.F.; Munderloh, U.G. An emerging tick-borne disease of humans is caused by a subset of strains with conserved genome structure. Pathogens 2013, 2, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.B.; van Wert, J.C.; Kinsella, M.; Tkach, V.V.; Lafferty, K.D. Infection at an ecotone: Cross-system foraging increases satellite parasites but decreases core parasites in raccoons. Ecology 2019, 100, e02808. [Google Scholar] [CrossRef] [PubMed]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The Raccoon Dog (Nyctereutes procyonoides) and the Raccoon (Procyon lotor)–their Role and Impact of Maintaining and Transmitting Zoonotic Diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Popiolek, M.; Szczesna-Staskiewicz, J.; Bartoszewicz, M.; Okarma, H.; Smalec, B.; Zalewski, A. Helminth Parasites of an Introduced Invasive Carnivore Species, the Raccoon (Procyon lotor L.), From the Warta Mouth National Park (Poland). J. Parasitol. 2011, 97, 357–360. [Google Scholar] [CrossRef]
- Bundesinstitut für Bau-Stadt-und Raumforschung (BBSR). Raumabgrenzungen und Raumtypen des BBSR. Available online: https://www.bbsr.bund.de/BBSR/DE/forschung/raumbeobachtung/Raumabgrenzungen/deutschland/gemeinden/Raumtypen2010_vbg/Raumtypen2010_LageSied.html#doc2826692bodyText2 (accessed on 14 June 2023).
- Bundesinstitut für Bau-Stadt-und Raumforschung (BBSR). Raumtypen 2010: Besiedelung und Lage. Available online: https://www.bbsr.bund.de/BBSR/DE/forschung/raumbeobachtung/Raumabgrenzungen/deutschland/gemeinden/Raumtypen2010_vbg/raumTypenBesiedlLage_2020.csv?__blob=publicationFile&v=2 (accessed on 14 June 2023).
- Umhang, G.; Woronoff-Rhen, N.; Combes, B.; Boué, F. Segmental Sedimentation and Counting Technique (SSCT): An adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp. Parasitol. 2011, 128, 57–60. [Google Scholar] [CrossRef]
- Malzacher, P. Eine neue Färbung für zoologische Totalpräparate: Astrablau-Boraxkarmin. Mikrokosmos 1972, 61, 181–182. [Google Scholar]
- Schell, S.C. How to Know the Trematodes; William, C., Ed.; Brown Company Publishers: Dubuque, IA, USA, 1970. [Google Scholar]
- Seo, S.B.; Rim, H.J.; Lee, C.W. Studies on the Parasitic Helminths of Korea: I. Trematodes of Rodents. Korean J. Parasitol. 1964, 2, 20–26. [Google Scholar] [CrossRef]
- Elsemore, D.A.; Geng, J.; Cote, J.; Hanna, R.; Lucio-Forster, A.; Bowman, D.D. Enzyme-linked immunosorbent assays for coproantigen detection of Ancylostoma caninum and Toxocara canis in dogs and Toxocara cati in cats. J. Vet. Diagn. Investig. 2017, 29, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Lavikainen, A.; Yanagida, T.; Ito, A. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). Int. J. Parasitol. 2013, 43, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Manske, M. GENtle, a Free Multi-Purpose Molecular Biology Tool. Ph.D. Thesis, University of Cologne, Cologne, Germany, 2006. [Google Scholar]
- Xie, Y.; Zhang, Z.; Niu, L.; Wang, Q.; Wang, C.; Lan, J.; Deng, J.; Fu, Y.; Nie, H.; Yan, N.; et al. The Mitochondrial Genome of Baylisascaris procyonis. PLoS ONE 2011, 6, e27066. [Google Scholar] [CrossRef] [PubMed]
- Inegbenosun, C.U.; Isaac, C.; Anika, F.U.; Aihebholoria, O.P. Prevalence of intestinal parasites in animal hosts and potential implications to animal and human health in Edo, Nigeria. J. Vet. Sci. 2023, 24, e8. [Google Scholar] [CrossRef] [PubMed]
- Priemer, J.; Lux, E. Atriotaenia incisa (Cestoda), a parasite of the badger, Meles meles, and the raccoon, Procyon lotor, in Brandenburg, Germany. Can. J. Zool. 1994, 72, 1848–1853. [Google Scholar] [CrossRef]
- Giacinti, J.A.; Pearl, D.L.; Ojkic, D.; Jardine, C.M. Comparison of Two Surveillance Components for Investigating the Epidemiology of Canine Distemper Virus in Raccoons (Procyon lotor). J. Wildl. Dis. 2021, 57, 104–115. [Google Scholar] [CrossRef]
- Munscher, E.C. Physical and Health Assessment of a Population of Raccoon (Procyon lotor) in Northeastern Florida. Master Thesis, University of North Florida, Jacksonville, FL, USA, 2006. [Google Scholar]
- Pozio, E. Factors affecting the flow among domestic, synanthropic and sylvatic cycles of Trichinella. Vet. Parasitol. 2000, 93, 241–262. [Google Scholar] [CrossRef]
- Karamon, J.; Kochanowski, M.; Cencek, T.; Bartoszewicz, M.; Kusyk, P. Gastrointestinal helminths of raccoons (Procyon lotor) in western Poland (Lubuskie province)—with particular regard to Baylisascaris procyonis. Bull. Vet. Inst. Pulawy 2014, 58, 547–552. [Google Scholar] [CrossRef]
- Page, L.K.; Gehrt, S.D.; Titcombe, K.K.; Robinson, N.P. Measuring prevalence of raccoon roundworm (Baylisascaris procyonis): A comparison of common techniques. Wildl. Soc. Bull. 2005, 33, 1406–1412. [Google Scholar] [CrossRef]
- Rentería-Solís, Z.; Birka, S.; Schmäschke, R.; Król, N.; Obiegala, A. First detection of Baylisascaris procyonis in wild raccoons (Procyon lotor) from Leipzig, Saxony, Eastern Germany. Parasitol. Res. 2018, 117, 3289–3292. [Google Scholar] [CrossRef]
- Schwarz, S.; Sutor, A.; Mattis, R.; Conraths, F.J. The raccoon roundworm (Baylisascaris procyonis)—No zoonotic risk for Brandenburg? Berl. Und Münchener Tierärztliche Wochenschr. 2015, 128, 34–38. [Google Scholar]
- Duscher, G.G.; Frantz, A.C.; Kuebber-Heiss, A.; Fuehrer, H.P.; Heddergott, M. A potential zoonotic threat: First detection of Baylisascaris procyonis in a wild raccoon from Austria. Transbound. Emerg. Dis. 2020, 00, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabi, M.N.S.; Chriél, M.; Hansen, M.S.; Enemark, H.L. Baylisascaris procyonis in wild raccoons (Procyon lotor) in Denmark. Vet. Parasitol. Reg. Stud. Rep. 2015, 1–2, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Osten-Sacken, N.; Heddergott, M.; Schleimer, A.; Anheyer-Behmenburg, H.E.; Runge, M.; Horsburgh, G.J.; Camp, L.; Nadler, S.A.; Frantz, A.C. Similar yet different: Co-analysis of the genetic diversity and structure of an invasive nematode parasite and its invasive mammalian host. Int. J. Parasitol. 2018, 48, 233–243. [Google Scholar] [CrossRef] [PubMed]
- French, S.K.; Pearl, D.L.; Shirose, L.; Peregrine, A.S.; Jardine, C.M. Demographic and Environmental Factors Associated with Baylisascaris procyonis Infection of Raccoons (Procyon lotor) in Ontario, Canada. J. Wildl. Dis. 2020, 56, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.B. Baylisascaris procyonis Demography and Egg Production in a California Raccoon Population. J. Parasitol. 2016, 102, 622–628. [Google Scholar] [CrossRef]
- Jardine, C.M.; Pearl, D.L.; Puskas, K.; Campbell, D.G.; Shirose, L.; Peregrine, A.S. The impact of land use, season, age, and sex on the prevalence and intensity of Baylisascaris procyonis infections in raccoons (Procyon lotor) from Ontario, Canada. J. Wildl. Dis. 2014, 50, 784–791. [Google Scholar] [CrossRef]
- Kazacos, K.R. Baylisascaris procyonis and Related Species. In Parasitic Diseases of Wild Mammals; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University Press: Ames, IA, USA, 2001; Volume 2, pp. 301–341. [Google Scholar]
- Kazacos, K.R.; Jelicks, L.A.; Tanowitz, H.B. Baylisascaris larva migrans. Handb. Clin. Neurol. 2013, 114, 251–262. [Google Scholar] [CrossRef]
- Sorvillo, F.; Ash, L.R.; Berlin, O.G.W.; Morse, S.A. Baylisascaris procyonis: An Emerging Helminthic Zoonosis. Emerg. Infect. Dis. 2002, 8, 355–359. [Google Scholar] [CrossRef]
- Zimmerman, D.M.; Dangoudoubiyam, S.; Kazacos, K.R. Serological Diagnosis of Baylisascaris procyonis in Primates Using a Human Elisa Test. J. Zoo. Wildl. Med. 2019, 50, 414–420. [Google Scholar] [CrossRef]
- Louis, M.M.; Minter, L.J.; Flowers, J.R.; Stoskopf, M.K.; Kennedy-Stoskopf, S. Raccoon roundworm prevalence (Baylisascaris procyonis) at the North Carolina Zoo, USA. PeerJ 2020, 8, e9426. [Google Scholar] [CrossRef]
- Weinstein, S.B.; Lake, C.M.; Chastain, H.M.; Fisk, D.; Handali, S.; Kahn, P.L.; Montgomery, S.P.; Wilkins, P.P.; Kuris, A.M.; Lafferty, K.D. Seroprevalence of Baylisascaris procyonis Infection among Humans, Santa Barbara County, California, USA, 2014–2016. Emerg. Infect. Dis. 2017, 23, 1397–1399. [Google Scholar] [CrossRef]
- Davidson, R.K.; Oines, O.; Hamnes, I.S.; Schulze, J.E. Illegal Wildlife Imports More than Just Animals—Baylisascaris procyonis in Raccoons (Procyon lotor) in Norway. J. Wildl. Dis. 2013, 49, 986–990. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Liu, G.H.; Zheng, W.B.; Hong, S.J.; Sugiyama, H.; Zhu, X.Q.; Elsheikha, H.M. Toxocariasis: A silent threat with a progressive public health impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Results of Parasitological Examinations of Faecal Samples from Cats and Dogs in Germany between 2003 and 2010. Parasitol. Res. 2011, 109, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Strube, C.; Raulf, M.K.; Springer, A.; Waindok, P.; Auer, H. Seroprevalence of human toxocarosis in Europe: A review and meta-analysis. Adv. Parasitol. 2020, 109, 375–418. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, A.; Kornacka, A.; Popiołek, M.; Bień-Kalinowska, J.; Moskwa, B. Use of meat juice from raccoons (Procyon lotor) collected from Central Europe for immunological detection of Trichinella spp. Vet. Parasitol. 2020, 297, 109066. [Google Scholar] [CrossRef] [PubMed]
- Stope, M. Wild raccoons in Germany as a reservoir for zoonotic Agents. Eur. J. Wildl. Res. 2019, 65, 94. [Google Scholar] [CrossRef]
- Mayer-Scholl, A.; Reckinger, S.; Schulze, C.; Nöckler, K. Study on the occurrence of Trichinella spp. in raccoon dogs in Brandenburg, Germany. Vet. Parasitol. 2016, 231, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerta, L.A.; Ticona, D.S.; Lopez-Urbina, M.T.; Gonzalez, A.E. A new species of Atriotaenia (Cestoda: Anoplocephalidae) from the hog-nosed skunk Conepatus chinga (Carnivora: Mephitidae) in Peru. J. Parasitol. 2012, 98, 806–809. [Google Scholar] [CrossRef]
- Kelley, S.W.; Horner, N.V. The Prevalence of Cestodes in Raccoons (Procyon lotor) from North-Central Texas. Comp. Parasitol. 2008, 75, 292–298. [Google Scholar] [CrossRef]
- Gallati, W.W. Life History, Morphology and Taxonomy of Atriotaenia (Ershovia) procyonis (Cestoda: Linstowiidae), a Parasite of the Raccoon. J. Parasitol. 1959, 45, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Miquel, J.; Motjé, M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: A biogeographic approach. Parasitol. Res. 2001, 87, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Monello, R.; Gompper, M. Effects of resource availability and social aggregation on the species richness of raccoon endoparasite infracommunities. Oikos 2011, 120, 1427–1433. [Google Scholar] [CrossRef]
- Brunet, J.; Benoilid, A.; Kremer, S.; Dalvit, C.; Lefebvre, N.; Hansmann, Y.; Chenard, M.P.; Mathieu, B.; Grimm, F.; Deplazes, P.; et al. First case of human cerebral Taenia martis cysticercosis. J. Clin. Microbiol. 2015, 53, 2756–2759. [Google Scholar] [CrossRef]
- Brunet, J.; Pesson, B.; Chermette, R.; Regnard, P.; Grimm, F.; Deplazes, P.; Ferreira, X.; Sabou, M.; Pfaff, A.W.; Abou-Bacar, A.; et al. First case of peritoneal cysticercosis in a non-human primate host (Macaca tonkeana) due to Taenia martis. Parasites Vectors 2014, 7, 422. [Google Scholar] [CrossRef]
- Frank, B.; Zeyhle, E. Larval cestodes in muskrats (Ondatra zibethicus). Nachr. Dtsch. Pflanzenschutzd. 1981, 33, 166–170. [Google Scholar]
- Matoba, Y.; Yamada, D.; Asano, M.; Oku, Y.; Kitaura, K.; Yagi, K.; Tenora, F.; Asakawa, M. Parasitic helminths from feral raccoons (Procyon lotor) in Japan. Helminthologia 2006, 43, 139–146. [Google Scholar] [CrossRef]
- Deplazes, P.; Eichenberger, R.M.; Grimm, F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int. J. Parasitol. Parasites Wildl. 2019, 9, 342–358. [Google Scholar] [CrossRef]
- Varcasia, A.; Sanna, D.; Casu, M.; Lahmar, S.; Dessì, G.; Pipia, A.P.; Tamponi, C.; Gaglio, G.; Hrckova, G.; Otranto, D.; et al. Species delimitation based on mtDNA genes suggests the occurrence of new species of Mesocestoides in the Mediterranean region. Parasit. Vectors 2018, 11, 619. [Google Scholar] [CrossRef]
- Schmidberger, J.; Kratzer, W.; Stark, K.; Gruner, B.; Echinococcosis Working, G. Alveolar echinococcosis in Germany, 1992–2016. An update based on the newly established national AE database. Infection 2018, 46, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.L.; Leighton, B.J.; Stephen, C. Intestinal parasites of raccoons (Procyon lotor) from southwest British Columbia. Can. J. Vet. Res. 2000, 64, 107–111. [Google Scholar] [PubMed]
- Kostadinova, A.; Gibson, D.I. Isthmiophora Lühe, 1909 and Euparyphium Dietz, 1909 (Digenea: Echinostomatidae) re-defined, with comments on their nominal species. Syst. Parasitol. 2002, 52, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.; Adamczyk, M.; Laskowski, Z.; Zalesny, G. Host-dependent morphology of Isthmiophora melis (Schrank, 1788) Luhe, 1909 (Digenea, Echinostomatinae)—Morphological variation vs. molecular stability. Parasit. Vectors 2015, 8, 481. [Google Scholar] [CrossRef]
- Choe, S.; Na, K.J.; Kim, Y.; Jeong, D.H.; Yang, J.J.; Eom, K.S. Infections of Two Isthmiophora Species (Digenea: Echinostomatidae) in Wild Mammals from Republic of Korea with Their Morphological Descriptions. Korean J. Parasitol. 2019, 57, 647–656. [Google Scholar] [CrossRef]
- Romeo, C.; Cafiso, A.; Fesce, E.; Martinez-Rondan, F.J.; Panzeri, M.; Martinoli, A.; Cappai, N.; Defilippis, G.; Ferrari, N. Lost and found: Helminths infecting invasive raccoons introduced to Italy. Parasitol. Int. 2021, 83, 102354. [Google Scholar] [CrossRef]
- Hong, S.J.; Woo, H.-C.; Chai, J.-Y. A Human Case of Plagiorchis muris (Tanabe, 1922: Digenea) Infection in the Republic of Korea: Freshwater Fish as a Possible Source of Infection. J. Parasitol. 1996, 82, 647–649. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Schurer, J.M.; Gesy, K.M. Old problems on a new playing field: Helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011, 182, 54–69. [Google Scholar] [CrossRef]
| Target Gene | Primer | Primer-Sequence | PCR Product Length | |
|---|---|---|---|---|
| Cestodes 1 | ||||
| nad1 | first PCR | nad1 Forward | 5′-TGATGATTTGTCTAGTC-3′ | ∼990 bp |
| nad1 Reverse | 5′-TTCTTGAAGTTAACAGC-3′ | |||
| nested PCR | nad1 Fornest | 5′-GATTTGTCTAGTCATAGATG-3′ | ∼980 bp | |
| nad1 Revnest | 5′-CTTGAAGTTAACAGCATCACG-3′ | |||
| cox1 | first PCR | cox1H Forward | 5′-WATAAAGGTTTRTTATTTGCTATG-3′ | ∼930 bp |
| cox1H Reverse | 5′-ATCHAWTAAGCATGATGCAAAAGG-3′ | |||
| nested PCR | cox1H Fornest | 5′-TATGTTTTCAATAGTBTGTTTAGG-3′ | ∼890 bp | |
| cox1H Revnest | 5′-CATGATGCAAAAGGCAAAWAAACC-3′ | |||
| Nematodes 2 | ||||
| nad1 | first PCR | Toxo Forward | 5′-ATTGCTTTTATTACTTTGTATGAGC-3′ | ∼480 bp |
| Toxo Reverse | 5′-GCAAATAAATTACCAACAAACTC-3′ | |||
| nested PCR | Toxo Fornest | 5′-CCTAATAAGGTTAGTTTTAT-3′ | ∼380 bp | |
| Toxo Revnest | 5′-AAAAACAAAATATGTTAACATG-3′ |
| % (n, CI 95%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Raccoons (n) | Helminth Positives | Nematodes | B. procyonis | Cestodes | A. cf. incisa/procyonis | Trematodes | I. hortensis/melis |
| Total | 101 | 72.3 (73; 62.5–80.7) | 31.7 (32; 22.8–41.7) | 28.7 (29; 20.1–38.6) | 46.5 (47; 36.5–56.7) | 43.6 (44; 33.7–53.8) | 15.8 (16; 9.3–24.4) | 4 (4; 1.1–9.8) |
| Years | ||||||||
| 2019 | 32.7 (33; 23.7–42.7) | 69.7 (23; 51.3–84.4) | 24.2 (8; 11.1–42.3) | 21.2 (7; 9.0–38.9) | 48.5 (16; 30.8–66.5) | 45.5 (15; 28.1–63.6) | 18.2 (6; 7.0–35.5) | 6.1 (2; 0.7–20.2) |
| 2020 | 67.3 (68; 57.3–76.3) | 73.5 (50; 61.4–83.5) | 35.3 (24; 23.7–47.2) | 32.4 (22; 21.5–44.8) | 45.6 (31; 33.5–58.1) | 42.6 (29; 30.7–55.2) | 14.7 (10; 7.3–25.4) | 2.9 (2; 0.4–10.2) |
| Area | ||||||||
| rural | 32.7 (33; 23.7–42.7) | 75.8 (25; 55.6–87.1) | 27.3 (9; 13.3–45.5) | 24.2 (8; 11.1–42.3) | 63.6 (21; 45.1–79.6) * | 57.6 (19; 39.2–74.5) * | 12.1 (4; 3.4–28.2) | 3 (1; 0.1–15.8) |
| urban | 67.3 (68; 57.3–76.3) | 70.6 (48; 58.3–81.0) | 33.8 (23; 22.8–46.3) | 30.9 (21; 20.2–43.3) | 38.2 (26; 26.7–50.8) * | 36.8 (25; 25.4–49.3) * | 17.6 (12; 9.5–28.8) | 4.4 (3; 0.9–12.4) |
| Sex | ||||||||
| ♀ | 38.6 (39; 29.1–48.8) | 69.2 (27; 52.4–83.0) | 25.6 (10; 13.0–42.1) | 23.1 (9; 11.1–39.9) | 51.3 (20; 34.8–67.6) | 51.3 (20; 34.8–67.6) | 15.4 (6; 5.9–30.5) | 0 (0; 0–9.0) |
| ♂ | 61.4 (62; 51.2–70.9) | 74.2 (46; 61.5–84.5) | 35.5 (22; 23.7–48.7) | 32.3 (20; 20.9–45.3) | 43.5 (27; 31.0–56.7) | 38.7 (24; 26.6–51.9) | 16.1 (10; 8.0–27.7) | 6.5 (4; 1.8–15.7) |
| Age | ||||||||
| >1 a | 54.5 (55; 44.2–64.4) | 80.0 (44; 67.0–89.6) | 34.5 (19; 22.2–48.6) | 32.7 (18; 20.7–46.7) | 58.2 (32; 44.1–71.3) | 52.7 (29; 38.8–66.3) | 14.5 (8; 6.5–26.7) | 3.6 (2; 0.4–12.5) |
| <1 a | 45.5 (46; 35.6–55.8) | 63.0 (29; 47.5–76.8) | 28.3 (13; 16.0–43.5) | 23.9 (11; 12.6–38.8) | 32.6 (15; 19.5–48.0) | 32.6 (15; 19.5–48.0) | 17.4 (8; 7.8–31.4) | 4.4 (2; 0.5–14.8) |
| Age–Sex Class | ||||||||
| >1 a + ♀ | 15.8 (16; 9.3–24.4) | 81.2 (13; 54.4–96.0) | 31.2 (5; 11.0–58.7) | 31.2 (5; 11.0–58.7) | 68.8 (11; 41.3–89.0) | 68.8 (11; 41.3–89.0) | 12.5 (2; 1.6–38.3) | 0 (0; 0–20.6) |
| >1 a + ♂ | 38.6 (39; 29.1–48.8) | 79.5 (31; 61.5–89.2) | 35.9 (14; 21.2–52.8) | 33.3 (13; 19.1–50.2) | 53.8 (21; 37.2–69.9) | 46.2 (18; 30.1–62.8) | 15.4 (6; 5.9–30.5) | 5.1 (2; 0.6–17.3) |
| <1 a + ♀ | 22.8 (23; 15.0–32.2) | 60.9 (14; 38.5–80.3) | 21.7 (5; 7.5–43.7) | 17.4 (4; 5.0–38.8) | 39.1 (9; 19.7–61.5) | 39.1 (9; 19.7–61.5) | 17.4 (4; 5.0–38.8) | 0 (0; 0–14.8) |
| <1 a + ♂ | 22.8 (23; 15.0–32.2) | 65.2 (15; 41.7–83.6) | 34.8 (8; 16.4–57.3) | 30.4 (7; 13.2–52.9) | 26.1 (6; 10.2–48.8) | 26.1 (6; 10.2–48.8) | 17.4 (4; 5.0–38.8) | 8.7 (2; 1.1–28.0) |
| % (n Positives, CI 95%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | n Positives | Coinfections | Nem/Cest | Nem/Trem | Cest/Trem | Nem/Cest/Trem |
| Total | 73 | 32.9 (24; 22.3–44.9) | 21.9 (16; 13.1–33.1) | 2.7 (2; 0.3–9.5) | 2.7 (2; 0.3–9.5) | 1.4 (1; 0–7.4) |
| Years | ||||||
| 2019 | 23 | 34.8 (8; 16.4–57.3) | 17.4 (4; 5.0–38.8) | 0 (0; 0–14.8) | 4.3 (1; 0.1–21.9) | 4.3 (1; 0.1–21.9) |
| 2020 | 50 | 32 (16; 19.5–46.7) | 24 (12; 13.1–38.2) | 4 (2; 0.5–13.7) | 2 (1; 0.1–10.6) | 0 (0; 0–7.1) |
| Area | ||||||
| rural | 25 | 40 (10; 21.1–61.3) | 28 (7; 12.1–49.4) | 0 (0; 0–13.7) | 5.9 (2; 0.7–19.7) | 0 (0; 0–13.7) |
| urban | 48 | 29.2 (14; 17.0–44.1) | 18.8 (9; 9.0–32.6) | 4.2 (2; 0.5–14.3) | 0 (0; 0–5.3) | 2.1 (1; 0.1–11.1) |
| Sex | ||||||
| ♀ | 27 | 33.3 (9; 16.5–54.0) | 25.9 (7; 11.1–46.3) | 3.7 (1; 0.1–19.0) | 3.7 (1; 0.1–19.0) | 0 (0; 0–12.8) |
| ♂ | 46 | 32.6 (15; 19.5–48.0) | 19.6 (9; 9.4–33.9) | 2.2 (1; 0.1–11.5) | 2.2 (1; 0.1–11.5) | 2.2 (1; 0.1–11.5) |
| Age | ||||||
| >1 a | 44 | 36.4 (16; 22.4–52.2) | 27.3 (12; 15.0–42.8) | 4.5 (2; 0.6–15.5) | 2.3 (1; 0.1–12.0) | 0 (0; 0–8.0) |
| <1 a | 29 | 27.6 (8; 12.7–47.2) | 13.8 (4; 3.9–31.7) | 0 (0; 0–11.9) | 3.4 (1; 0.1–17.8) | 3.4 (1; 0.1–17.8) |
| Age–Sex Class | ||||||
| >1 a + ♀ | 13 | 38.5 (5; 13.9–68.4) | 30.8 (4; 9.1–61.4) | 7.7 (1;0.2–36.0) | 0 (0; 0–24.7) | 0 (0; 0–24.7) |
| >1 a + ♂ | 31 | 35.5 (11; 19.2–54.6) | 25.8 (8; 11.9–44.6) | 3.2 (1; 0.1–16.7) | 3.2 (1; 0.1–16.7) | 0 (0; 0–11.2) |
| <1 a + ♀ | 14 | 28.6 (4; 8.4–58.1) | 21.4 (3; 4.7–50.8) | 0 (0; 0–23.2) | 7.1 (1; 0.2–33.9) | 0 (0; 0–23.2) |
| <1 a + ♂ | 15 | 26.7 (4; 7.8–55.1) | 6.7 (1; 0.2–31.9) | 0 (0; 0–21.8) | 0 (0; 0–21.8) | 6.7 (1; 0.2–31.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, N.P.; Wassermann, M.; Härle, J.; Romig, T.; Kurzrock, L.; Arnold, J.; Großmann, E.; Mackenstedt, U.; Straubinger, R.K. Helminths in Invasive Raccoons (Procyon lotor) from Southwest Germany. Pathogens 2023, 12, 919. https://doi.org/10.3390/pathogens12070919
Reinhardt NP, Wassermann M, Härle J, Romig T, Kurzrock L, Arnold J, Großmann E, Mackenstedt U, Straubinger RK. Helminths in Invasive Raccoons (Procyon lotor) from Southwest Germany. Pathogens. 2023; 12(7):919. https://doi.org/10.3390/pathogens12070919
Chicago/Turabian StyleReinhardt, Nico P., Marion Wassermann, Jessica Härle, Thomas Romig, Lina Kurzrock, Janosch Arnold, Ernst Großmann, Ute Mackenstedt, and Reinhard K. Straubinger. 2023. "Helminths in Invasive Raccoons (Procyon lotor) from Southwest Germany" Pathogens 12, no. 7: 919. https://doi.org/10.3390/pathogens12070919
APA StyleReinhardt, N. P., Wassermann, M., Härle, J., Romig, T., Kurzrock, L., Arnold, J., Großmann, E., Mackenstedt, U., & Straubinger, R. K. (2023). Helminths in Invasive Raccoons (Procyon lotor) from Southwest Germany. Pathogens, 12(7), 919. https://doi.org/10.3390/pathogens12070919








