A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats
Abstract
1. Introduction
2. Materials and Methods
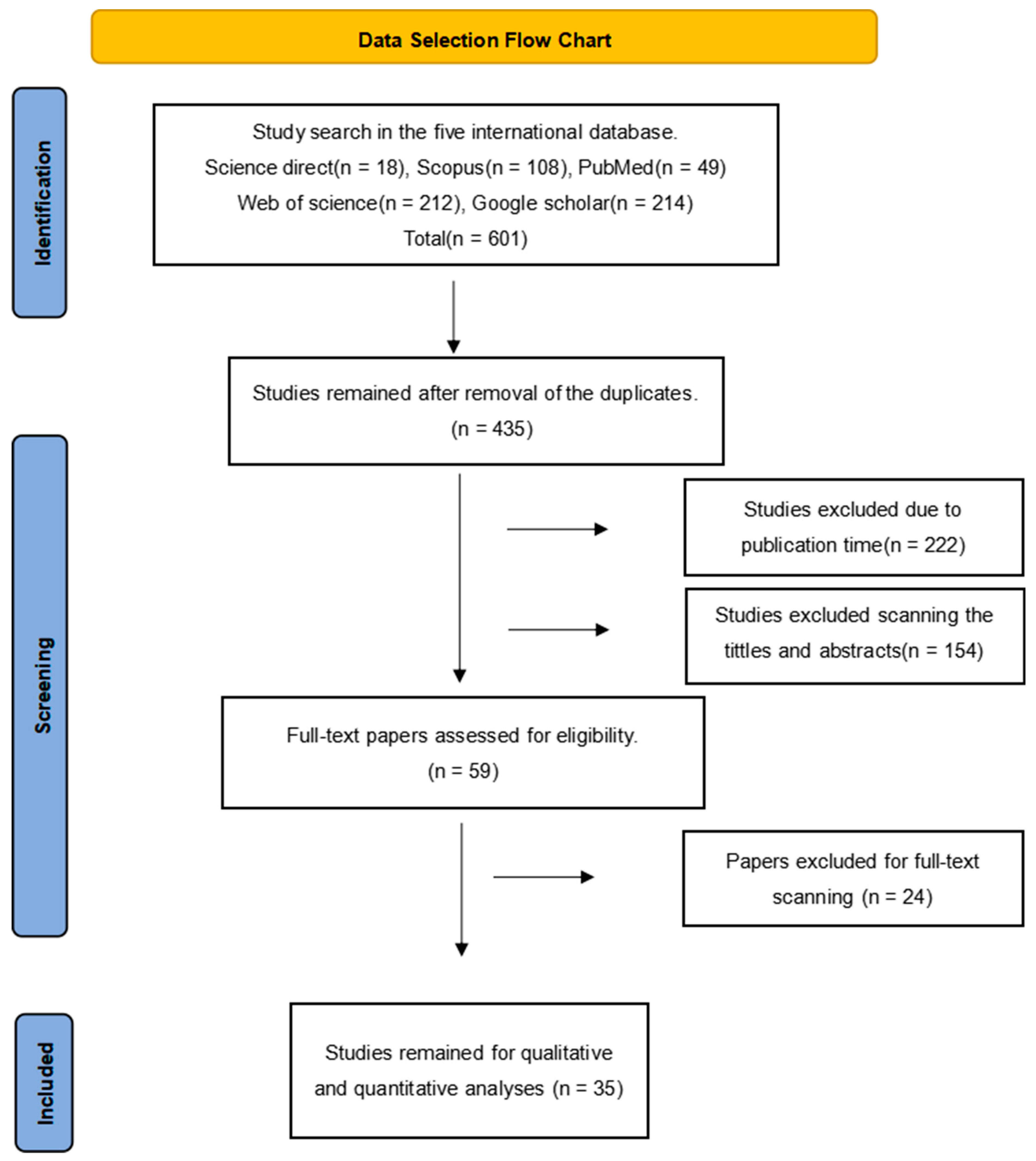
2.1. Article Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Screening
2.4. Data Extraction Formula
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenthal, B.M. Zoonotic Sarcocystis. Res. Vet. Sci. 2021, 136, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zeng, H.; Van Damme, I.; Kabi, T.W.; Oba, B.; Gabril, S. Sarcocystis species in bovine carcasses from a Belgian abattoir: A cross-sectional study. Parasites Vectors 2021, 14, 271. [Google Scholar] [CrossRef]
- Sheikhi, M.; Salahi-Moghaddam, A.; Asl, M.N.; Farahani, A.; Shamseddin, J. A survey on the Frequency of Sarcocystis in Bandar Abbas, Iran in 2019–2020. Gene Cell Tissue 2020, 7, e109990. [Google Scholar] [CrossRef]
- Dahmani, A.; Aissi, M.; Zenia, S.; Harhoura, K.; Saadi, A. Morphometric Study of Microscopic Cysts of Sarcocystis Sp. in Sheep Carcasses. Folia Vet. 2020, 64, 38–46. [Google Scholar] [CrossRef]
- Xue, S.; Xiang, C.R.; Ma, C.F.; Yan, W.C. Serological investigation of Sarcocystis infection in dairy cattle in parts of Henan Province. Adv. Vet. Med. 2022, 43, 142–144. [Google Scholar]
- Xue, R.; Han, L.F.; Qian, W.F.; He, B.; Yan, W.C. Tissue squash and molecular identification of Sarcocystis in pigs in Luoyang City. J. Henan Univ. Sci. Technol. Nat. Sci. Ed. 2018, 39, 6. [Google Scholar]
- Moré, G.; Schares, S.; Maksimov, A.; Conraths, F.J.; Venturini, M.C.; Schares, G. Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet. Parasitol. 2013, 197, 85–94. [Google Scholar] [CrossRef]
- Hu, J.J.; Huang, S.; Wen, T.; Esch, W.G.; Liang, Y.; Li, H.L. Sarcocystis spp. in domestic sheep in Kunming City, China: Prevalence, morphology, and molecular characteristics. Parasite. 2017, 24, 30. [Google Scholar] [CrossRef]
- Prakas, P.; Oksanen, A.; Butkauskas, D.; Sruoga, A.; Liaugaudaitė, S. Identification and intraspecific genetic diversity of Sarcocystis rileyi from ducks, Anas spp., in Lithuania and Finland. J. Parasitol. 2014, 100, 657–661. [Google Scholar] [CrossRef]
- Sudan, V.; Shanker, D.; Paliwal, S.; Sruoga, A.; Liaugaudaitė, S. Phylogenetics of Sarcocystis fusiformis isolates based on 18S rRNA and cox 1 genes. Microb. Pathog. 2021, 159, 105144. [Google Scholar] [CrossRef]
- Ma, C.L.; Ye, Y.L.; Wen, T.; Huang, Z.M.; Song, J.L. Prevalence and morphological and molecular characteristics of Sarcocystis bertrami in horses in China. Parasite 2020, 27, 1. [Google Scholar] [CrossRef]
- Dessi, G.; Tamponi, C.; Pasini, C.; Porcu, F.; Meloni, L.; Cavallo, L.; Sini, M.F.; Knoll, S.; Scala, A.; Varcasia, A. A survey on Apicomplexa protozoa in sheep slaughtered for human consumption. Parasitol. Res. 2022, 121, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Pipia, A.P.; Varcasia, A.; Zidda, A.; Dessi, G.; Panzalis, R.; Tamponi, C.; Marrosu, R.; Tosciri, G.; Sanna, G.; Dore, F.; et al. Cross-sectional investigation on sheep sarcosporidiosis in Sardinia, Italy. Vet. Parasitol. Reg. Stud. Rep. 2016, 3, 13–17. [Google Scholar] [CrossRef]
- Bacci, C.; Vismarra, A.; Passeri, B.; Dessi, G.; Panzalis, R.; Tamponi, C.; Marrosu, R.; Tosciri, G.; Sanna, G.; Dore, F.; et al. Detection of Toxoplasma gondii and Sarcocystis tenella in indigenous Cornigliese sheep in Italy using serological and molecular methods. Small Rumin. Res. 2016, 135, 13–16. [Google Scholar] [CrossRef]
- Latif, B.; Kutty, K.M.; Muslim, A.; Hussaini, J.; Omar, E.; Heo, C.C.; Rossle, N.F.; Abdullah, S.; Kamarudin, M.A.; Zulkarnain, M.A. Light microscopy and molecular identification of Sarcocystis spp. in meat producing animals in Selangor, Malaysia. Trop. Biomed. 2015, 32, 444–452. [Google Scholar] [PubMed]
- Amairia, S.; Amdouni, Y.; Rouatbi, M.; Rjeibi, M.R.; Awadi, S.; Gharbi, M. First detection and molecular identification of Sarcocystis spp. in small ruminants in North-West Tunisia. Transbound. Emerg. Dis. 2018, 65, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.; Nourani, H.; Razmi, G. Histopathological, ultrastructural and molecular examination of Sarcocystis spp. in sheep of Mashhad area, Khorasan Razavi province, Iran. Iran. J. Vet. Sci. Technol. 2020, 12, 1–9. [Google Scholar]
- Pestechian, N.; Yousefi, H.A.; Kalantari, R.; Jafari, R.; Khamesipour, F.; Keshtkar, M.; Esmaeilifallah, M. Molecular and Microscopic Investigation of Sarcocystis Species Isolated from Sheep Muscles in Iran. J. Food Qual. 2021, 2021, 5562517. [Google Scholar] [CrossRef]
- Farhang-Pajuh, F.; Yakhchali, M.; Mardani, K. Molecular determination of abundance of infection with Sarcocystis species in slaughtered sheep of Urmia, Iran. Vet. Res. Forum Int. Q. J. 2014, 5, 181–186. [Google Scholar]
- Rahdar, M.; Kardooni, T. Molecular identification of Sarcocystis spp. in sheep and cattle by PCR-RFLP from southwest of Iran. Jundishapur J. Microbiol. 2017, 10, e12798. [Google Scholar] [CrossRef]
- Shahabi, S.; Dehbashi, N.; Sarkari, B.; Arefkhah, N.; Sedaghat, B.; Savardashtaki, A. Detection and phylogenetic analysis of Sarcocystis moulei and Sarcocystis spp. (Sarcocystidae: Apicomplexa) from slaughtered sheep in southwest Iran. J. Parasit. Dis. 2022, 46, 215–219. [Google Scholar] [CrossRef]
- Modiri, D.; Hosainvosughi. Survey of contamination rate sheep to Sarcocystis in slaughterhouse Boroujerd City and studied by PCR. Adv. Environ. Biol. 2014, 8, 424–428. [Google Scholar]
- Kalantari, N.; Khaksar, M.; Ghaffari, S.; Hamidekish, S.M. Molecular Analysis of Sarcocystis Spp. Isolated from Sheep (Ovis aries) in Babol Area, Mazandaran Province, Northern Iran. Iran. J. Parasitol. 2016, 11, 73–80. [Google Scholar]
- Ghazaei, C. Evaluation of Sarcocyst Parasite Strains in Carcasses Obtained from Ardabil Meat Industrial Group. Int. J. Mol. Clin. Microbiol. 2018, 8, 950–956. [Google Scholar]
- Mirzaei, M.; Rezaei, H. The role of sheep in the epidemiology of Sarcocystis spp. in Tabriz area northwest of Iran. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2016, 40, 285–288. [Google Scholar] [CrossRef]
- Dong, H.; Su, R.; Wang, Y.; Tong, Z.; Zhang, L.; Yang, Y.; Hu, J. Sarcocystis species in wild and domestic sheep (Ovis ammon and Ovis aries) from China. BMC Vet. Res. 2018, 14, 377. [Google Scholar] [CrossRef]
- Sun, Y.; Ju, J.; Su, X.; Xie, C.; Li, Y.; Kang, M. Infection survey and morphological characteristics of Sarcocystis spp. in naturally infected Tibetan sheep from Qinghai in northwestern China. Parasitol. Int. 2021, 80, 102219. [Google Scholar] [CrossRef]
- Portella, L.P.; Cadore, G.C.; Sangioni, L.A.; Alves, M.E.M.; Chemeris, R.; Brum, L.P.; Voge, F.S.F. Molecular detection of protozoa of the Sarcocystidae family in sheep from the State of Rio Grande do Sul, Brazil. Ciência Rural 2016, 46, 1613–1617. [Google Scholar] [CrossRef]
- Gerab, R.A.; Edris, A.B.M.; Lamada, H.M.; Elrais, A.M. Prevalence and Distribution of Sarcocystis in Buffaloes and Sheep in Egypt. J. Adv. Vet. Res. 2022, 12, 302–307. [Google Scholar]
- Hussein, N.M. Morphological and Molecular characterization of Sarcocystis arieticanis from the Heart Muscles of Domestic sheep, Ovis aries, in Qena, Upper Egypt. J. Adv. Vet. Res. 2020, 10, 73–80. [Google Scholar]
- El-Morsey, A.; Abdo, W.; Sultan, K.; Elhawary, N.M.; AbouZaid, A.A. Ultrastructural and Molecular Identification of the sarcocysts of Sarcocystis tenella and Sarcocystis arieticanis Infecting Domestic Sheep (Ovis aries) from Egypt. Acta Parasitol. 2019, 64, 501–513. [Google Scholar] [CrossRef]
- El-Morsey, A.; Abdo, W.; Zaid, A.A.A.; Sorour, S.S.G. Morphologic and molecular identification of three macroscopic Sarcocystis species infecting domestic sheep (Ovis aries) and cattle (Bos taurus) in Egypt. Parasitol. Res. 2021, 120, 637–654. [Google Scholar] [CrossRef]
- Nageib, B.; Kuraa, H. Microscopical and serological studies with ultrastructure description of Sarcocystis species in sheep in Assiut. Assiut Vet. Med. J. 2018, 64, 46–55. [Google Scholar]
- Abdullah, S.H. Investigation of Sarcocystis spp. in slaughtered cattle and sheep by peptic digestion and histological examination in Sulaimani Province, Iraq. Vet. World 2021, 14, 468–474. [Google Scholar] [CrossRef]
- Salam, S.M.; Salih Mustafa, B.H. Identification of Sarcocystis Species “Macrocystis” by Visual and Molecular Technique in Sheep and Goats—Sulaymaniyah Slaughterhouse. J. Anim. Poult. Prod. 2021, 12, 331–337. [Google Scholar]
- Metwally, D.M.; Al-Damigh, M.A.; Al-Turaiki, I.M.; Khadragy, M.F. Molecular characterization of Sarcocystis species isolated from sheep and goats in Riyadh, Saudi Arabia. Animals 2019, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Mekibib, B.; Abdisa, D.; Denbarga, Y.; Abebe, R. Muscular Sarcocystis infection in ruminants slaughtered at Municipality abattoir and selected Hotels in Hawassa city, southern Ethiopia: Prevalence and associated risk factors. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100333. [Google Scholar] [CrossRef]
- Januskevicius, V.; Januskeviciene, G.; Prakas, P.; Butkauskas, D.; Petkevicius, S. Prevalence and intensity of Sarcocystis spp. infection in animals slaughtered for food in Lithuania. Vet. Med. 2019, 64, 149–157. [Google Scholar] [CrossRef]
- Gokpinar, S.; Yildiz, K.; Gurcan, I.S. Prevalence and Concentration of Sarcocystis spp. Microscopic Cysts in Sheep Muscles Using Percoll Gradient Centrifugation. Isr. J. Vet. Med. 2014, 69, 16–19. [Google Scholar]
- Kutty, M.K.; Latif, B.; Muslim, A.; Hussaini, J.; Daher, A.M.; Heo, C.C.; Abdullah, S. Detection of sarcocystosis in goats in Malaysia by light microscopy, histology, and PCR. Trop. Anim. Health Prod. 2015, 47, 751–756. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, T.T.; Liu, Q.; Esch, G.W.; Chen, J.Q.; Huang, S.; Wen, T. Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming, China. Parasitol. Res. 2016, 115, 3973–3981. [Google Scholar] [CrossRef]
- Bittencourt, M.V.; Meneses, I.D.S.; Ribeiro-Andrade, M.; de Jesus, R.F.; de Araújo, F.R.; Gondim, L.F.P. Sarcocystis spp. in sheep and goats: Frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol. Res. 2016, 115, 1683–1689. [Google Scholar] [CrossRef]
- Al-Waely, T.N.; Abd AL-Amery, A.M. Prevalence of Sarcocystosis in Goats (Capra hircus) at Wasit Province, Iraq. Plant Arch. 2020, 20, 8939–8944. [Google Scholar]
- Hong, E.J.; Sim, C.; Chae, J.S.; Kim, H.C.; Park, J.; Choi, K.S.; Yu, D.H.; Park, C.H.; Yoo, J.G.; Park, B.K. Ultrastructural and molecular identification of Sarcocystis tenella (Protozoa, Apicomplexa) in naturally infected Korean native goats. Vet. Med. 2016, 61, 374–381. [Google Scholar] [CrossRef]
- Martínez-Navalón, B.; Anastasio-Giner, B.; Cano-Fructuoso, M.; Sanchez-Martínez, P.; Llopis-Morant, A.; Perez-Castarlenas, B.; Goyena, E.; Berriatua, E. Sarcocystis infection: A major cause of carcass condemnation in adult sheep in Spain. Span. J. Agric. Res. 2012, 10, 388–392. [Google Scholar] [CrossRef]
- Zhu, Z.; Ying, Z.; Feng, Z.; Liu, Q.; Liu, J. The Occurrence and Meta-Analysis of Investigations on Sarcocystis Infection among Ruminants (Ruminantia) in Mainland China. Animals 2022, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Anvari, D.; Narouei, E.; Hosseini, M.; Narouei, M.R.; Siyadatpanah, A.; Shariatzadeh, S.A.; Pagheh, A.S.; Gholami, S.; Sarvi, S.; Sargazi, D.; et al. Sarcocystosis in ruminants of Iran, as neglected food-borne disease: A systematic review and meta-analysis. Acta Parasitol. 2022, 65, 555–568. [Google Scholar] [CrossRef]
- Shams, M.; Shamsi, L.; Asghari, A.; Motazedian, M.H.; Mohammadi-Ghalehbin, B.; Omidian, M.; Nazari, N.; Sadrebazzaz, A. Molecular epidemiology, species distribution, and zoonotic importance of the neglected meat-borne pathogen Sarcocystis spp. in cattle (Bos taurus): A global systematic review and meta-analysis. Acta Parasitol. 2022, 67, 1055–1072. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Bahari, P.; Vatanchian, M. First Molecular Identification of Sarcocystis ovicanis (Protozoa, Apicomplexa) in the Brain of Sheep in Iran. Iran. J. Parasitol. 2014, 9, 286–291. [Google Scholar] [PubMed]
- Okita, F.O.; Obadiah, H.I.; Gyegweh, K.T.; Okonkwo, A.A.; Azatyom, J.A. Survey of Sarcocystis species infection in slaughtered goats in Makurdi metropolis. Int. J. Infect. Dis. Ther. 2017, 2, 4–8. [Google Scholar]
- Kaur, M.; Singh, B.B.; Sharma, R.; Gill, J.P.S. Pervasive environmental contamination with human feces results in high prevalence of zoonotic Sarcocystis infection in pigs in the Punjab, India. J. Parasitol. 2016, 102, 229–232. [Google Scholar] [CrossRef] [PubMed]
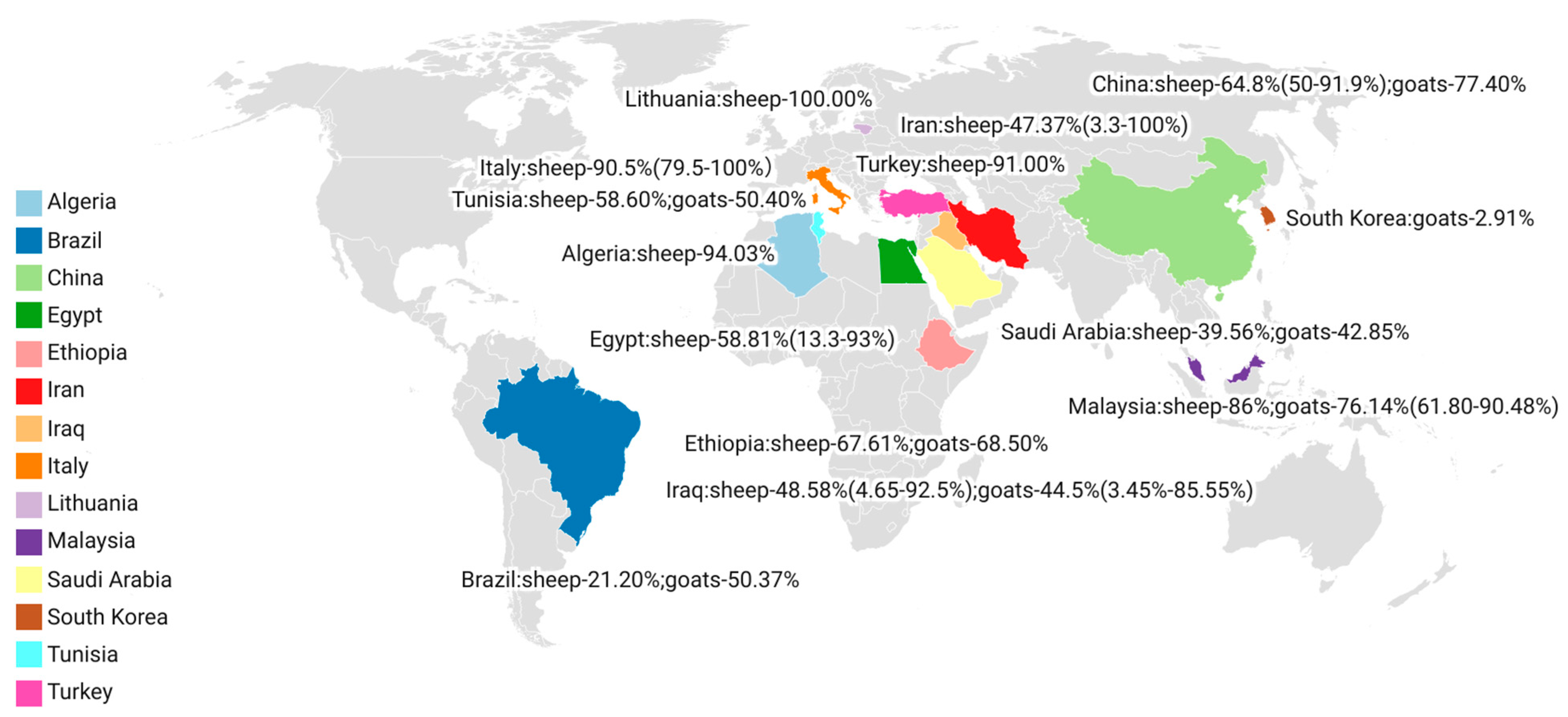
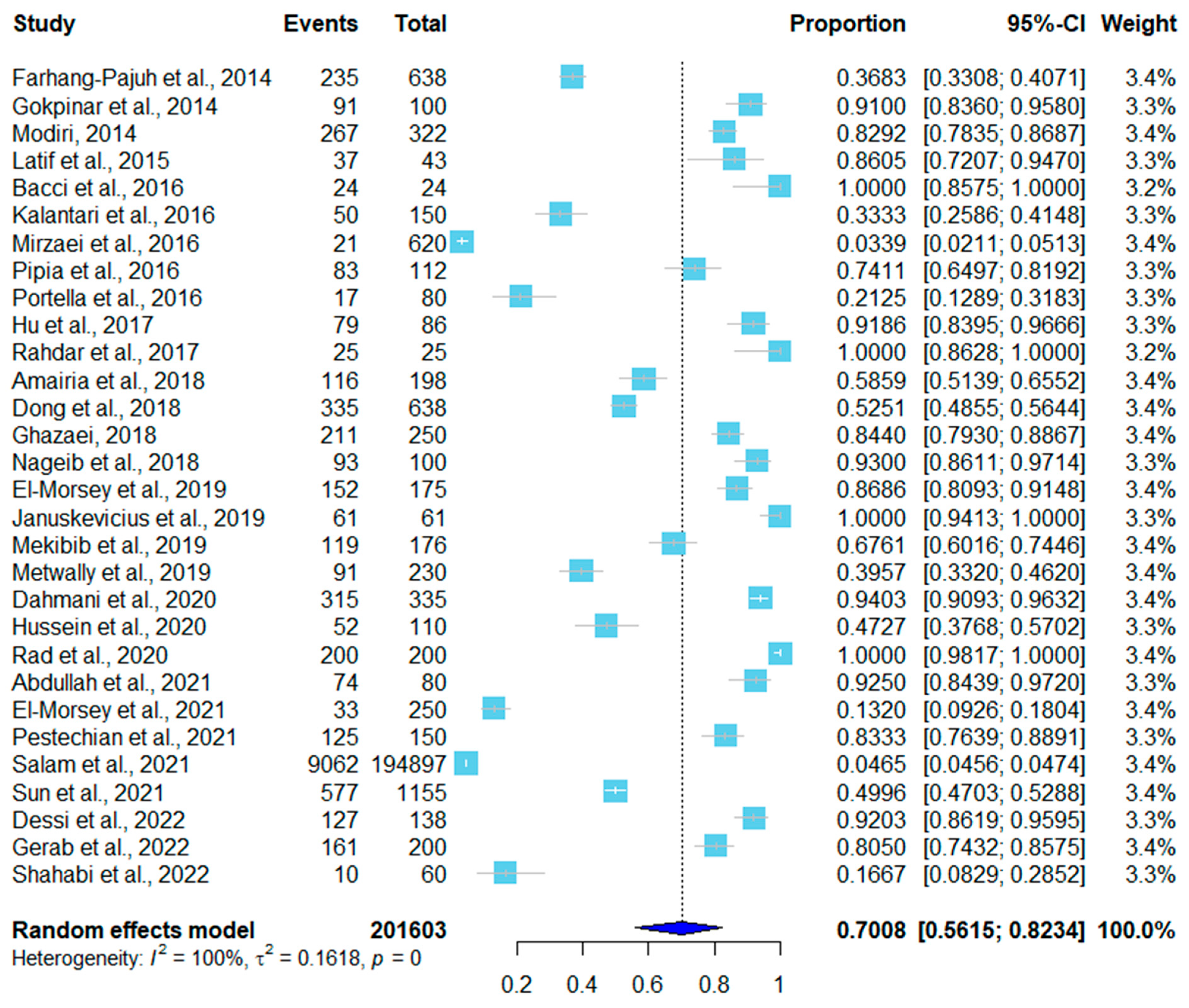
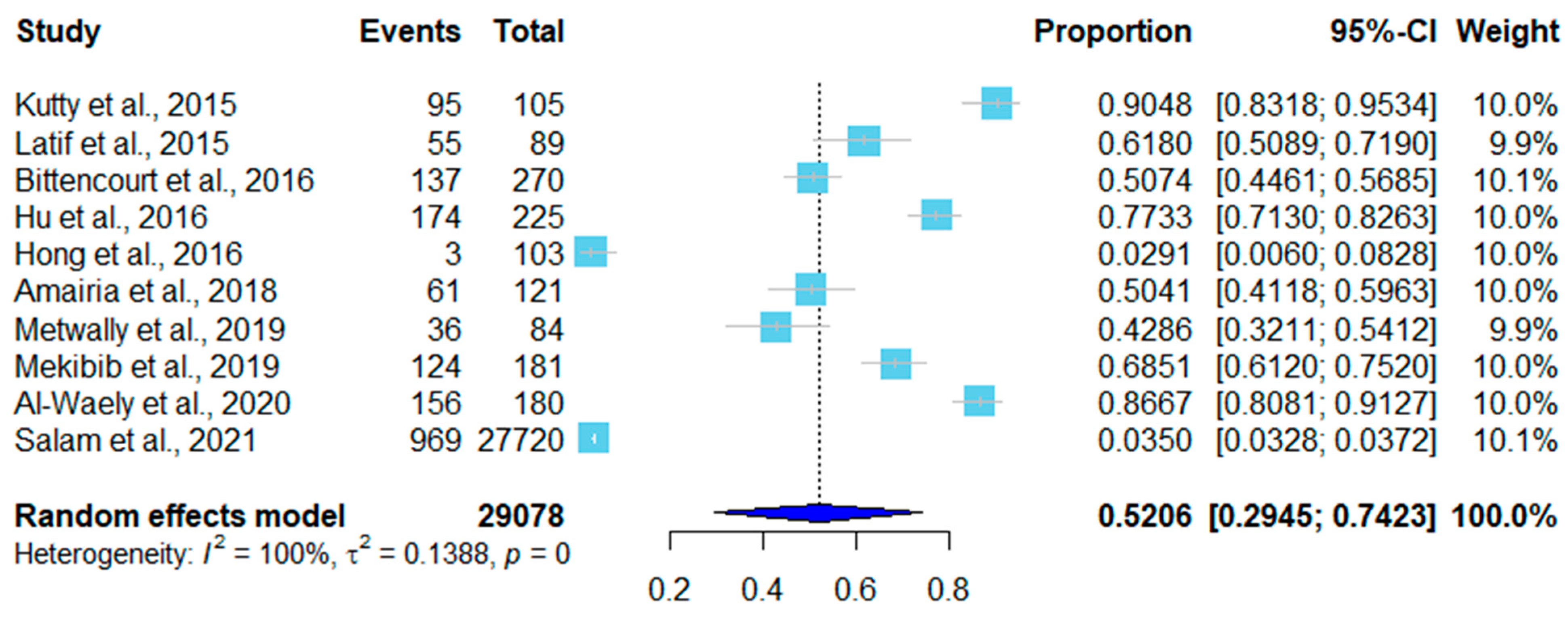



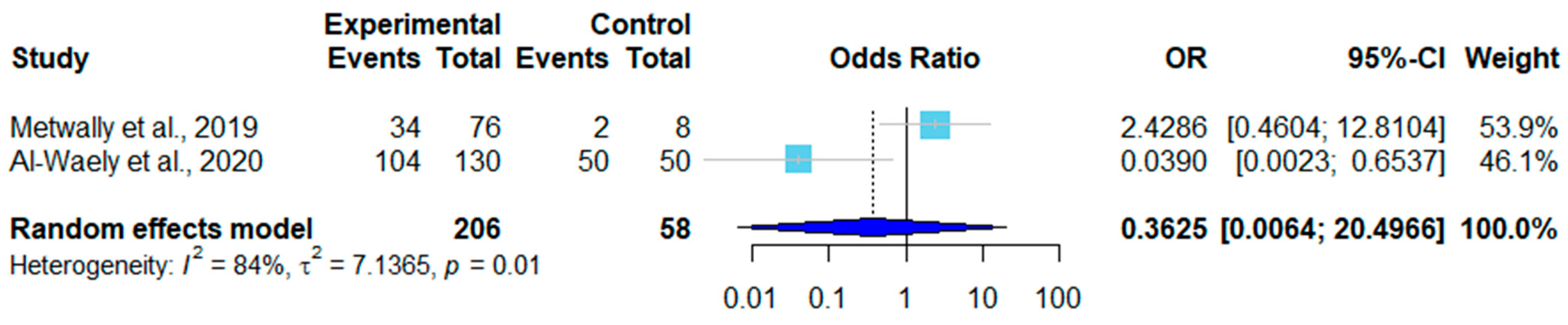
| No. | Country | Sampling Time | Positive/Total | Infection Rate | Detection Method * | Tissue | Species | Reference (year) |
|---|---|---|---|---|---|---|---|---|
| 1 | Iran | 2011.2–2012.1 | 235/638 | 36.83% | PCR-RFLP | Esophagus Diaphragm Skeletal muscle | S. gigantea S. medusiformis | [20] (2014) |
| 2 | Iran | unknown | 267/322 | 82.91% | Digestion method | Esophagus Diaphragm | unknown | [23] (2014) |
| 3 | Turkey | 2011.2–2012.1 | 91/100 | 91.00% | Percoll gradient centrifugation | Skeletal muscle | S. tenella S. arieticanis | [40] (2014) |
| 4 | Malaysia | 2013.2–2013.10 | 37/43 | 86.00% | Digestion method | Tongue Heart Diaphragm Esophagus Skeletal muscle | S. ovicanis | [16] (2015) |
| 5 | Italy | 2013 | 83/112 | 79.50% | Nested PCR | Heart | S. tenella S. arieticanis | [14] (2016) |
| 6 | Italy | 2014.3–2014.4 | 24/24 | 100% | Conventional PCR | Heart | S. tenella | [15] (2016) |
| 7 | Brazil | unknown | 17/80 | 21.20% | Conventional PCR | Heart | S. tenella S. arieticanis | [29] (2016) |
| 8 | Iran | 2013.9–2013.10 | 50/150 | 33.30% | Tissue squash method | Intra-abdominal Muscle Diaphragm | S. gigantea S. moulei Sarcocystis spp. | [24] (2016) |
| 9 | Iran | 2013.4–2013.10 | 21/620 | 3.30% | Tissue squash method | Esophagus Diaphragm | unknown | [26] (2016) |
| 10 | Iran | unknown | 25/25 | 100% | PCR-RFLP | Heart Tongue Diaphragm Skeletal muscle | S. tenella S. capracanis | [21] (2017) |
| 11 | China | 2015.3–2015.11 | 79/86 | 91.90% | Tissue squash method | Esophagus Diaphragm Skeletal muscle Tongue Heart | S. tenella S. arieticanis | [9] (2017) |
| 12 | Iran | unknown | 211/250 | 84.40% | Tissue squash method | Intervertebral muscle Esophagus Heart Tongue | S. arieticamis S. tenella | [25] (2018) |
| 13 | Tunisia | 2016.5–2016.6 | 116/198 | 58.6% | Conventional PCR | Neck | S. tenella | [17] (2018) |
| 14 | China | 2014.3–2017.10 | 335/638 | 52.51% | Tissue squash method | Heart | S. tenella S. arieticanis | [27] (2018) |
| 15 | Egypt | unknown | 93/100 | 93.00% | Tissue squash method | Esophagus Diaphragm Tongue Skeletal muscle Heart | S. arieticanis S. tenella | [34] (2018) |
| 16 | Saudi Arabia | 2016.3–2017.1 | 91/230 | 39.56% | Digestion method | Tongue Heart Skeletal muscle Diaphragm Esophagus | S. tenella | [37] (2019) |
| 17 | Ethiopia | 2016.11–2017.5 | 119/176 | 67.61% | Tissue squash method | Heart Esophagus | unknown | [38] (2019) |
| 18 | Lithuania | 2012–2014 | 61/61 | 100.00% | Tissue squash method | Esophagus Diaphragm Heart Neck Jaw Back | unknown | [39] (2019) |
| 19 | Egypt | 2017.1–2018.2 | 152/175 | 86.80% | Tissue squash method | Esophagus Diaphragm Skeletal muscle Tongue Heart | S. tenella S. arieticanis | [32] (2019) |
| 20 | Algeria | unknown | 315/335 | 94.03% | Histopathological method | Esophagus Diaphragm | S. arieticanis S. tenella | [5] (2020) |
| 21 | Iran | 2018.10–2019.5 | 200/200 | 100% | Conventional PCR | Esophagus | S. gigantea S. tenella S. arieticanis | [18] (2020) |
| 22 | Egypt | 2018.4–2019.3 | 52/110 | 47.27% | Tissue squash method | Heart | S. arieticanis | [31] (2020) |
| 23 | Iran | unknown | 125/150 | 83.33% | Conventional PCR | Heart | S. gigantea S. tenella | [19] (2021) |
| 24 | China | 2017.2–2018.11 | 577/1155 | 50.00% | Tissue squash method | Heart Diaphragm Esophageal | S. gigantea | [28] (2021) |
| 25 | Egypt | 2018.1–2019.6 | 33/250 | 13.20% | Histopathologic examination | Esophagus Tongues Diaphragm Abdominal muscle Skeletal muscle Heart | S. gigantea S. medusiformis | [33] (2021) |
| 26 | Iraq | 2020.5–2020.7 | 74/80 | 92.50% | Tissue squash method | Diaphragm Esophagus | S. tenella S. arieticanis | [35] (2021) |
| 27 | Iraq | 2020.8–2021.1 | 9062/194,897 | 4.65% | Tissue squash method | Esophagus Diaphragm Heart | S. gigantea S. medisiformis | [36] (2021) |
| 28 | Italy | 2019.4–2019.7 | 127/138 | 92.00% | Conventional PCR | Heart | S. tenella | [13] (2022) |
| 29 | Egypt | 2020.7–2021.6 | 161/200 | 80.50% | Tissue squash method | Esophagus Tongue Heart Masseter Skeletal muscle | unknown | [30] (2022) |
| 30 | Iran | 2019.3–2019.5 | 10/60 | 16.66% | Tissue squash method | Muscles # | S. moulei | [22] (2022) |
| No. | Country | Sampling Time | Positive/Total | Infection Rate | Detection Method * | Tissue | Species | Reference (year) |
|---|---|---|---|---|---|---|---|---|
| 1 | Malaysia | 2014.1–2014.2 | 95/105 | 90.48% | Conventional PCR | Skeletal muscle | S. capracanis | [41] (2015) |
| 2 | Malaysia | 2013.2–2013.10 | 55/89 | 61.80% | Tissue squash method | Tongue Heart Diaphragm Esophagus Skeletal muscle | S. capracanis | [16] (2015) |
| 3 | South Korea | 2014.1–2014.8 | 3/103 | 2.91% | Conventional PCR | Heart | S. tenella | [45] (2016) |
| 4 | China | 2014.7–2015.9 | 174/225 | 77.40% | Tissue squash method | Esophagus Diaphragm Skeletal muscle Tongue Heart | S. capracanis S. hircicanis | [42] (2016) |
| 5 | Brazil | 2012.1–2013.6 | 137/270 | 50.37% | Conventional PCR | Tongue Esophagus Heart | S. capracanis | [43] (2016) |
| 6 | Tunisia | 2016.5–2016.6 | 61/121 | 50.4% | Conventional PCR | Neck | S. capracanis | [17] (2018) |
| 7 | Saudi Arabia | 2016.3–2017.1 | 36/84 | 42.85% | Tissue squash method | Tongue Heart Skeletal muscle Diaphragm Esophagus | S. capracanis | [37] (2019) |
| 8 | Ethiopia | 2016.11–2017.5 | 124/181 | 68.50% | Tissue squash method | Heart Esophagus | unknown | [38] (2019) |
| 9 | Iraq | 2019.10–2020.3 | 156/180 | 85.55% | Digestion method | Esophagus Diaphragm | unknown | [44] (2020) |
| 10 | Iraq | 2020.8–2021.1 | 969/27,720 | 3.45% | Tissue squash method | Esophagus Diaphragm Heart | S. moulei | [36] (2021) |
| Reference (year) | Country | Region | Total | Male | Infected | % | Female | Infected | % |
|---|---|---|---|---|---|---|---|---|---|
| [20] (2014) | Iran | Urmia | 638 | 498 | 38 | 7.63 | 140 | 49 | 35 |
| [23] (2014) | Iran | Boroujerd | 322 | 81 | 30 | 37.03 | 241 | 237 | 98.34 |
| [40] (2014) | Turkey | Kirikkale | 100 | 77 | 68 | 88.3 | 23 | 23 | 100 |
| [26] (2016) | Iran | Tabriz | 620 | 395 | 26 | 6.58 | 225 | 9 | 4.0 |
| [25] (2018) | Iran | Ardabil | 250 | 94 | 24 | 26.96 | 156 | 65 | 73.03 |
| [34] (2018) | Egypt | Assiut | 100 | 42 | 40 | 95.2 | 58 | 53 | 91.4 |
| [37] (2019) | Saudi Arabia | Riyadh | 230 | 200 | 83 | 41.5 | 30 | 12 | 40 |
| [31] (2020) | Egypt | Qena | 110 | 93 | 41 | 44.08 | 17 | 11 | 64.70 |
| [36] (2021) | Iraq | Sulaymaniyah | 194,897 | 129,635 | 6128 | 67.6 | 65,262 | 2934 | 32.4 |
| [30] (2022) | Egypt | Tanta | 200 | 100 | 68 | 68 | 100 | 93 | 93 |
| Reference (year) | Country | Region | Total | Male | Infected | % | Female | Infected | % |
|---|---|---|---|---|---|---|---|---|---|
| [37] (2019) | Saudi Arabia | Riyadh | 84 | 64 | 30 | 46.87 | 20 | 6 | 30 |
| [44] (2020) | Iraq | Wasit | 180 | 104 | 83 | 79.80 | 76 | 71 | 93.42 |
| [36] (2021) | Iraq | Sulaymaniyah | 27,720 | 17,809 | 491 | 50.7 | 9911 | 478 | 49.3 |
| Reference (year) | Country, Region | No. of Positive Samples | Positivity (%) | Age Groups (year) | No. of Samples | Age-Adjusted No. of Positive Samples | Age-Adjusted Positivity (%) |
|---|---|---|---|---|---|---|---|
| [40] (2014) | Turkey, Kirikkale | 91 | 91 | 1 2 3 4 | 63 21 12 4 | 53 21 12 4 | 84.12 100 100 100 |
| [26] (2016) | Iran, Tabriz | 35 | 5.64 | <1 1–3 >3 | 96 423 101 | 6 18 11 | 6.25 4.25 10.89 |
| [25] (2018) | Iran, Ardabil | 89 | 35.6 | <1 >1 | 97 153 | 24 65 | 24.74 42.48 |
| [34] (2018) | Egypt, Assiut | 93 | 93 | 0.5–2 2–4 ≥4 | 40 41 19 | 38 38 17 | 95 92.7 89.5 |
| [38] (2019) | Ethiopia, Hawassa | 176 | 67.61 | 1.5–2.5 | 176 | 119 | 67.61 |
| [37] (2019) | Saudi Arabia, Riyadh | 95 | 41.30 | <1 >1 | 168 62 | 73 22 | 43.45 35.48 |
| [32] (2019) | Egypt, Gharbia | 152 | 86.8 | >2 | 175 | 152 | 86.8 |
| [31] (2020) | Egypt, Qena | 52 | 47.27 | <1 >1 | 41 69 | 11 41 | 26.82 59.42 |
| [28] (2021) | China, Qinghai | 577 | 50 | >1 | 1155 | 577 | 50 |
| [30] (2022) | Egypt, Tanta | 161 | 80.5 | 1–2 3–5 | 100 100 | 68 93 | 68 93 |
| Reference (year) | Country, Region | No. of Positive Samples | Positivity (%) | Age Groups (year) | No. of Samples | Age-Adjusted No. of Positive Samples | Age-Adjusted Positivity (%) |
|---|---|---|---|---|---|---|---|
| [37] (2019) | Saudi Arabia, Riyadh | 36 | 42.85 | <1 >1 | 76 8 | 34 2 | 44.73 25 |
| [38] (2019) | Ethiopia, Hawassa | 124 | 68.50 | 1.5–2.5 | 181 | 124 | 68.50 |
| [44] (2020) | Iraq, Wasit | 154 | 85.55 | <1 1–2 >7 | 130 41 9 | 104 41 9 | 80 100 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Guo, R.; Sang, X.; Zhang, X.; Li, M.; Li, X.; Yang, N.; Jiang, T. A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats. Pathogens 2023, 12, 902. https://doi.org/10.3390/pathogens12070902
Feng Y, Guo R, Sang X, Zhang X, Li M, Li X, Yang N, Jiang T. A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats. Pathogens. 2023; 12(7):902. https://doi.org/10.3390/pathogens12070902
Chicago/Turabian StyleFeng, Ying, Ruiying Guo, Xiaoyu Sang, Xiaohan Zhang, Meiqi Li, Xiang Li, Na Yang, and Tiantian Jiang. 2023. "A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats" Pathogens 12, no. 7: 902. https://doi.org/10.3390/pathogens12070902
APA StyleFeng, Y., Guo, R., Sang, X., Zhang, X., Li, M., Li, X., Yang, N., & Jiang, T. (2023). A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats. Pathogens, 12(7), 902. https://doi.org/10.3390/pathogens12070902








