Molecular Characterization and Expression Analysis of IL-10 and IL-6 in Channel Catfish (Ictalurus punctatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Cloning of IL-10 and IL-6
2.3. Bioinformatics Analysis of IL-10 and IL-6
2.4. Tissue Expression Analysis of IL-10 and IL-6
2.5. In Vitro CCK Culture and Immune Induction
2.6. Bacterial Challenge
2.7. Viral Infection
2.8. Deltamethrin Challenge of the Channel Catfish
2.9. Statistical Analysis
3. Results
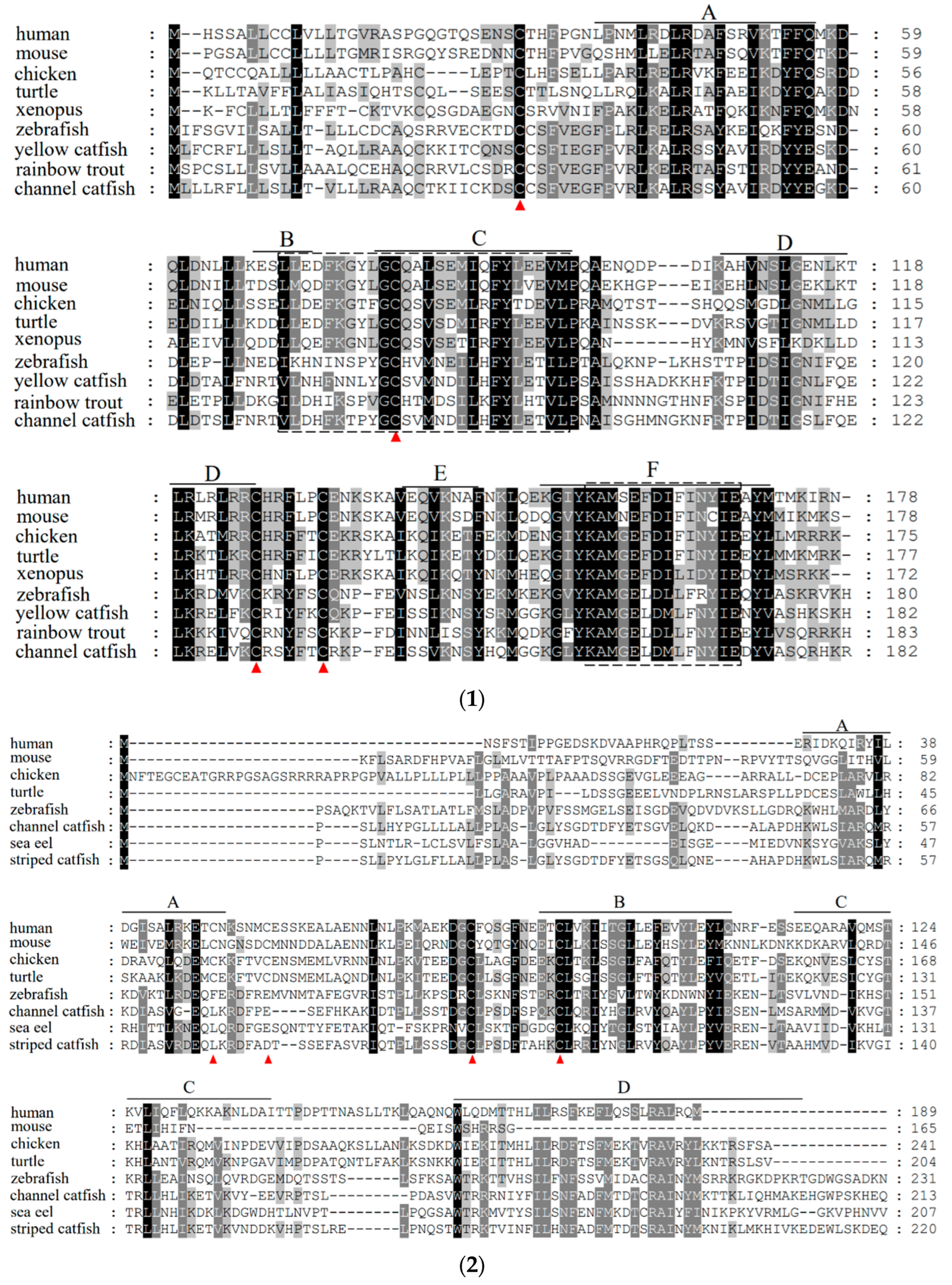
3.1. Sequence Analysis of the IL-10 and IL-6 Genes in Channel Catfish
3.2. Phylogenetic Analysis and Multiple Alignment
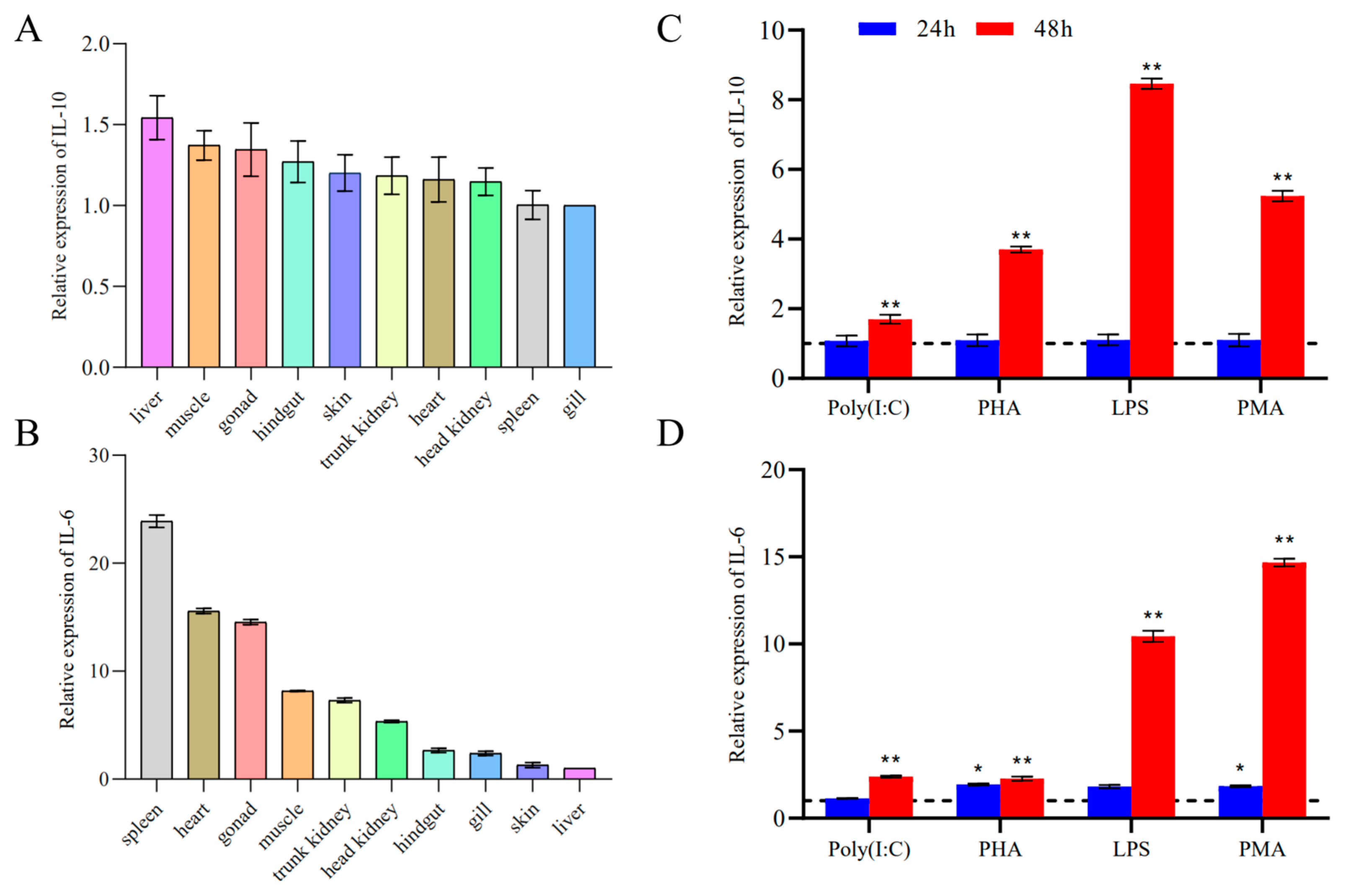
3.3. Tissue Expression Analysis of IL-10 and IL-6
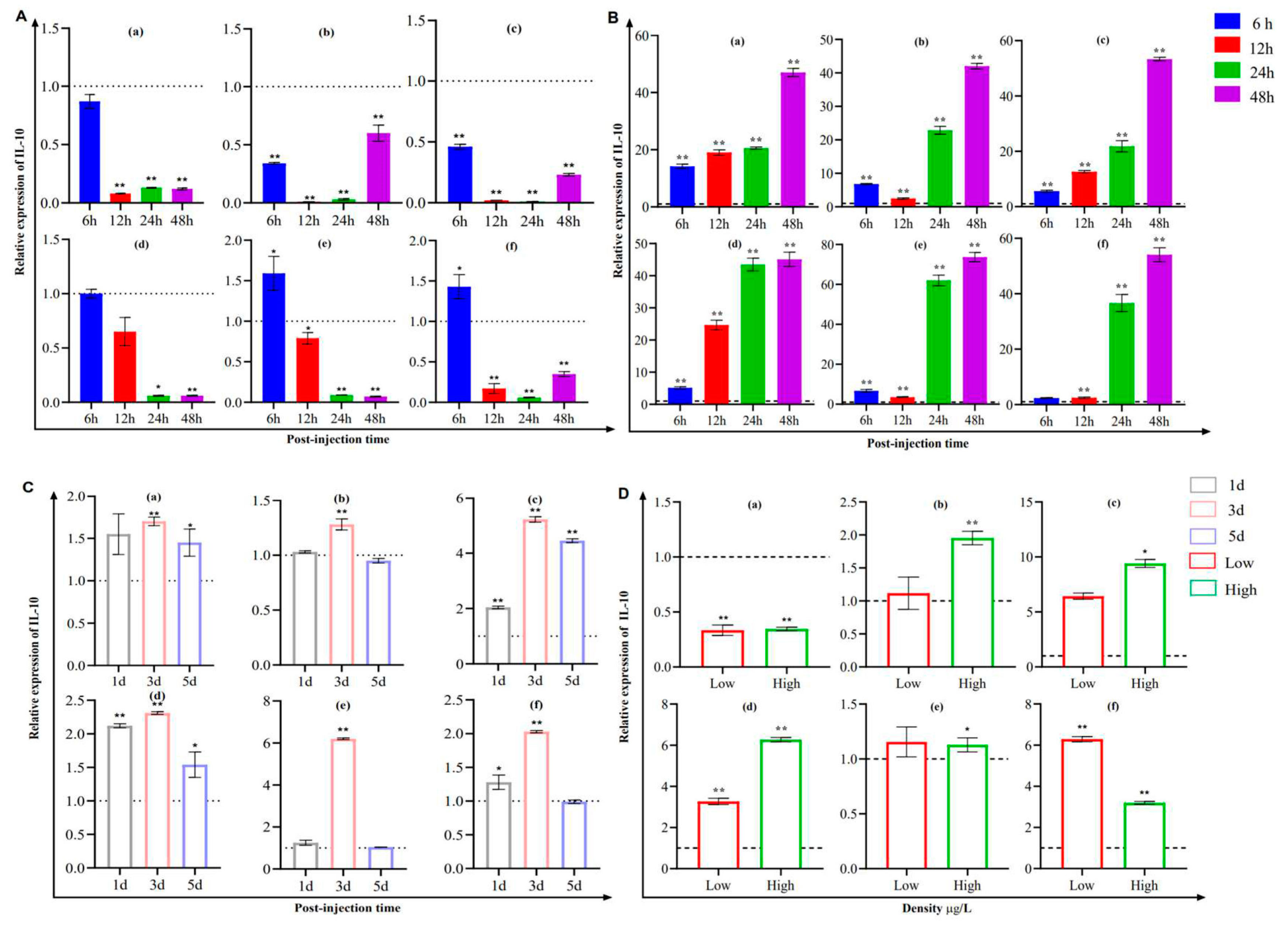
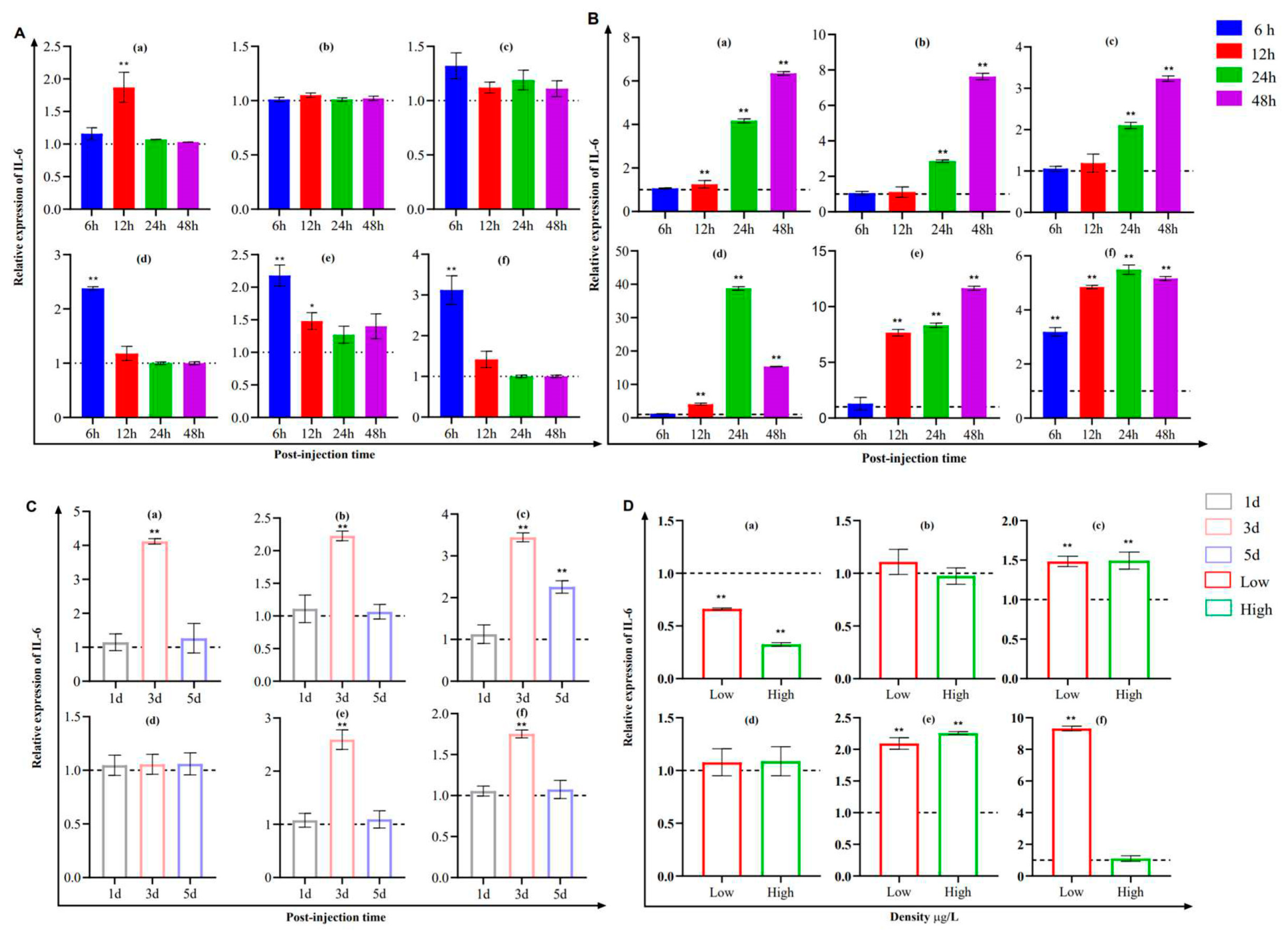
3.4. Modulation of IL-10 and IL-6 Expression in Channel Catfish Kidney (CCK) Cells
3.5. Analysis of IL-10 and IL-6 Expression in Fish
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Wang, J.; Xu, J.; Liu, Q.; Wang, Z.; Zhu, X.; Ai, X.; Gao, Q.; Chen, X.; Zou, J. Characterization of IL-22 Bioactivity and IL-22-Positive Cells in Grass Carp Ctenopharyngodon idella. Front. Immunol. 2020, 11, 586889. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; Vieira, P.; Fiorentino, D.F.; Trounstine, M.L.; Khan, T.A.; Mosmann, T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar] [CrossRef]
- Peng, Y.; Cai, X.; Zhang, G.; Wang, J.; Li, Y.; Wang, Z.; Wang, B.; Xiong, X.; Wu, Z.; Jian, J. Molecular characterization and expression of interleukin-10 and interleukin-22 in golden pompano (Trachinotus ovatus) in response to Streptococcus agalactiae stimulus. Fish. Shellfish Immunol. 2017, 65, 244–255. [Google Scholar] [CrossRef]
- Swain, B.; Samanta, M.; Basu, M.; Panda, P.; Sahoo, B.; Maiti, N.; Mishra, B.; Eknath, A. Molecular characterization, inductive expression and mechanism of interleukin-10 gene induction in the Indian major carp, catla (Catla catla). Aquac. Res. 2012, 43, 897–907. [Google Scholar] [CrossRef]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-parallel inhibition of IL-10 and TGF-β1 controls LPS-induced inflammatory response via NF-κB signaling in grass carp monocytes/macrophages. Fish. Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Shao, Y.Q.; Huang, Y.Q.; Jiang, S.G. Cloning, characterization and expression analysis of interleukin-10 from the zebrafish (Danio rerion). J. Biochem. Mol. Biol. 2005, 38, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kamota, S.; Ito, K.; Yoshiura, Y.; Ototake, M.; Moritomo, T.; Nakanishi, T. Molecular cloning and expression analysis of rainbow trout (Oncorhynchus mykiss) interleukin-10 cDNAs. Fish. Shellfish Immunol. 2005, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Hirano, T.; Taga, T.; Nakano, N.; Yasukawa, K.; Kashiwamura, S.; Shimizu, K.; Nakajima, K.; Pyun, K.H.; Kishimoto, T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc. Natl. Acad. Sci. USA 1985, 82, 5490–5494. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Odaka, T.; Suetake, H.; Tahara, D.; Miyadai, T. Teleost IL-6 promotes antibody production through STAT3 signaling via IL-6R and gp130. Dev. Comp. Immunol. 2012, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Andus, T.; Geiger, T.; Hirano, T.; Northoff, H.; Ganter, U.; Bauer, J.; Kishimoto, T.; Heinrich, P.C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987, 221, 18–22. [Google Scholar] [CrossRef]
- Gauldie, J.; Richards, C.; Harnish, D.; Lansdorp, P.; Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7251–7255. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef]
- Bird, S.; Zou, J.; Savan, R.; Kono, T.; Sakai, M.; Woo, J.; Secombes, C. Characterisation and expression analysis of an interleukin 6 homologue in the Japanese pufferfish, Fugu rubripes. Dev. Comp. Immunol. 2005, 29, 775–789. [Google Scholar] [CrossRef]
- Iliev, D.B.; Castellana, B.; Mackenzie, S.; Planas, J.V.; Goetz, F.W. Cloning and expression analysis of an IL-6 homolog in rainbow trout (Oncorhynchus mykiss). Mol. Immunol. 2007, 44, 1803–1807. [Google Scholar] [CrossRef]
- Varela, M.; Dios, S.; Novoa, B.; Figueras, A. Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio). Dev. Comp. Immunol. 2012, 37, 97–106. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zhang, R.; Liu, L.; Ma, G.; Zhu, H. Interleukin-6 in Siberian sturgeon (Acipenser baeri): Molecular characterization and immune functional activity. Fish. Shellfish Immunol. 2020, 102, 296–306. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Zhang, J.; Wang, H.; Liu, H. Classical Signaling and Trans-Signaling Pathways Stimulated by Megalobrama amblycephala IL-6 and IL-6R. Int. J. Mol. Sci. 2022, 23, 2019. [Google Scholar] [CrossRef]
- Wen, C.; Gan, N.; Zeng, T.; Lv, M.; Zhang, N.; Zhou, H.; Zhang, A.; Wang, X. Regulation of Il-10 gene expression by Il-6 via Stat3 in grass carp head kidney leucocytes. Gene 2020, 741, 144579. [Google Scholar] [CrossRef]
- Castellana, B.; Iliev, D.B.; Sepulcre, M.P.; MacKenzie, S.; Goetz, F.W.; Mulero, V.; Planas, J.V. Molecular characterization of interleukin-6 in the gilthead seabream (Sparus aurata). Mol. Immunol. 2008, 45, 3363–3370. [Google Scholar] [CrossRef]
- Chen, H.H.; Lin, H.T.; Foung, Y.F.; Han-You Lin, J. The bioactivity of teleost IL-6: IL-6 protein in orange-spotted grouper (Epinephelus coioides) induces Th2 cell differentiation pathway and antibody production. Dev. Comp. Immunol. 2012, 38, 285–294. [Google Scholar] [CrossRef]
- Costa, M.M.; Maehr, T.; Diaz-Rosales, P.; Secombes, C.J.; Wang, T. Bioactivity studies of rainbow trout (Oncorhynchus mykiss) interleukin-6: Effects on macrophage growth and antimicrobial peptide gene expression. Mol. Immunol. 2011, 48, 1903–1916. [Google Scholar] [CrossRef]
- Qin, G.; Xu, J.; Ai, X.; Yang, Y. Isolation, Identification, and Pathogenicity of Aeromonas veronii, the Causal Agent of Hemorrhagic Septicemia in Channel Catfish (Ictalurus punctatus) in China. Fishes 2022, 7, 394. [Google Scholar] [CrossRef]
- Cao, H.P.; An, J.; Ou, R.J.; Lu, L.Q.; Ai, X.H.; Yang, Y.B. Enterobacter aerogenes: An Emerging Pathogen for Enteritis in Farmed Channel Catfish Ictalurus punctatus. Isr. J. Aquac.-Bamidgeh 2017, 69. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Zhang, H.; Chen, Y.; Song, Y.; Ai, X. Dual RNA-Seq of Trunk Kidneys Extracted From Channel Catfish Infected With Yersinia ruckeri Reveals Novel Insights Into Host-Pathogen Interactions. Front. Immunol. 2021, 12, 775708. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Peng, Y.H.; Wang, Z.C.; Huang, T.; Xiong, X.Y.; Huang, Y.C.; Wang, B.; Xu, L.W.; Wu, Z.H. Characterization and identification of streptococci from golden pompano in China. Dis. Aquat. Organ. 2016, 119, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, Q.; Wang, Z.; Zhao, W.; Gao, Q. Cloning of interleukin-10 from African clawed frog (Xenopus tropicalis), with the Finding of IL-19/20 homologue in the IL-10 locus. J. Immunol. Res. 2015, 2015, 462138. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Li, M.; Ziwei, Z.; Song, Y.; Xu, W.; Wang, L.; Chen, S. Genome-wide identification, immune response profile and functional characterization of IL-10 from spotted knifejaw (Oplegnathus punctatus) during host defense against bacterial and viral infection. Fish. Shellfish Immunol. 2022, 124, 513–524. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, L.; Gui, G.; Gong, Y.; Wen, X.; Xia, W.; Yang, H.; Zhang, L. Molecular Cloning and Expression Analysis of Interleukin-8 and -10 in Yellow Catfish and in Response to Bacterial Pathogen Infection. Biomed. Res. Int. 2019, 2019, 9617659. [Google Scholar] [CrossRef]
- Zou, J.; Clark, M.S.; Secombes, C.J. Characterisation, expression and promoter analysis of an interleukin 10 homologue in the puffer fish, Fugu rubripes. Immunogenetics 2003, 55, 325–335. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Liu, W.B.; Wu, Q.J.; Gao, X.C.; Ren, H.T. Cloning, characterization and mRNA expression of interleukin-6 in blunt snout bream (Megalobrama amblycephala). Fish. Shellfish Immunol. 2016, 54, 639–647. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Liu, Y.; Che, G.; Di, Z.; Sun, W.; Tian, J.; Ren, M. Calycosin-7-O-β-D-glucoside attenuates myocardial ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway via the regulation of IL-10 secretion in mice. Mol. Cell. Biochem. 2020, 463, 175–187. [Google Scholar] [CrossRef]
- Tancredi, V.; D’Antuono, M.; Cafè, C.; Giovedì, S.; Buè, M.C.; D’Arcangelo, G.; Onofri, F.; Benfenati, F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J. Neurochem. 2000, 75, 634–643. [Google Scholar] [CrossRef]
- Pinto, R.D.; Nascimento, D.S.; Reis, M.I.; do Vale, A.; Dos Santos, N.M. Molecular characterization, 3D modelling and expression analysis of sea bass (Dicentrarchus labrax L.) interleukin-10. Mol. Immunol. 2007, 44, 2056–2065. [Google Scholar] [CrossRef]
- Huo, H.J.; Chen, S.N.; Li, L.; Nie, P. Functional characterization of IL-10 and its receptor subunits in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2019, 97, 64–75. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, A.R.; Park, J.Y.; Ahn, J.H.; Lee, T.S.; Kim, D.Y.; Lee, M.S.; Hwang, S.; Jeong, Y.J.; Park, J.H. IL-10 Protects Mice From the Lung Infection of Acinetobacter baumannii and Contributes to Bacterial Clearance by Regulating STAT3-Mediated MARCO Expression in Macrophages. Front. Immunol. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Kaushik, H.; Saini, N.; Sahoo, P.K.; Dixit, A.; Garg, L.C. Genomic cloning and sequence analysis of Interleukin-10 from Labeo rohita. Bioinformation 2014, 10, 623–629. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Champsi, J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect. Immun. 1993, 61, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, M.J.; Strieter, R.M.; Kunkel, S.L.; Danforth, J.M.; Goodman, R.E.; Standiford, T.J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 1995, 155, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Harjula, S.E.; Ojanen, M.J.T.; Taavitsainen, S.; Nykter, M.; Rämet, M. Interleukin 10 mutant zebrafish have an enhanced interferon gamma response and improved survival against a Mycobacterium marinum infection. Sci. Rep. 2018, 8, 10360. [Google Scholar] [CrossRef]
- Heinecke, R.D.; Buchmann, K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish. Shellfish Immunol. 2013, 34, 521–528. [Google Scholar] [CrossRef]
- Rebl, A.; Korytář, T.; Köbis, J.M.; Verleih, M.; Krasnov, A.; Jaros, J.; Kühn, C.; Köllner, B.; Goldammer, T. Transcriptome profiling reveals insight into distinct immune responses to Aeromonas salmonicida in gill of two rainbow trout strains. Mar. Biotechnol. 2014, 16, 333–348. [Google Scholar] [CrossRef]
- Clasen, B.; Loro, V.L.; Murussi, C.R.; Tiecher, T.L.; Moraes, B.; Zanella, R. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci. Total Environ. 2018, 626, 737–743. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Ren, Z.; Cui, Z. The toxic effects of deltamethrin on Danio rerio: The correlation among behavior response, physiological damage and AChE. RSC Adv. 2016, 6, 109826–109833. [Google Scholar] [CrossRef]
- Sun, X.; Tu, K.; Li, L.; Wu, B.; Wu, L.; Liu, Z.; Zhou, L.; Tian, J.; Yang, A. Integrated transcriptome and metabolome analysis reveals molecular responses of the clams to acute hypoxia. Mar. Environ. Res. 2021, 168, 105317. [Google Scholar] [CrossRef]
- Cengiz, E.I.; Bayar, A.S.; Kizmaz, V. The protective effect of vitamin E against changes in fatty acid composition of phospholipid subclasses in gill tissue of Oreochromis niloticus exposed to deltamethrin. Chemosphere 2016, 147, 138–143. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Xie, M.; Xiang, J.; Zuo, Z.; Tian, X.; Song, R.; Yuan, X.; Wu, Y.; Ou, D. Histopathology and transcriptome analysis reveals the gills injury and immunotoxicity in gibel carp following acute deltamethrin exposure. Ecotoxicol. Environ. Saf. 2022, 234, 113421. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Huang, Y.; Zhang, H.; Liu, Y.; Xu, N.; Fu, G.; Ai, X. RNA-Seq and 16S rRNA Analysis Revealed the Effect of Deltamethrin on Channel Catfish in the Early Stage of Acute Exposure. Front. Immunol. 2022, 13, 916100. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′-3′) | Application |
|---|---|---|
| IL-10-F | ATGGGAAGGAATTTTTGGGC | PCR |
| IL-10-R | TCAGCGTTTATGTCTCTGAG | PCR |
| IL-6-F | ATGCCCTCTCTCCTGCACTATCCTG | PCR |
| IL-6-R | TCATTGCTCGTGTTTGGAGGGCCAC | PCR |
| EF-1α-F | GTTGAAATGGTTCCTGGCAA | qPCR |
| EF-1α-R | TCAACACTCTTGATGACACCAAC | qPCR |
| IL-10-F | GCTTAGGGCCGCTCAGTGCA | qPCR |
| IL-10-R | GCCTTCATAGTAGTCTCGG | qPCR |
| IL-6-F | CAGCCCGCAAAAATGTCTGC | qPCR |
| IL-6-R | TCAGGTAAGGAGGTCGGGCG | qPCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Liu, Y.; Xu, N.; Ai, X.; Yang, Y. Molecular Characterization and Expression Analysis of IL-10 and IL-6 in Channel Catfish (Ictalurus punctatus). Pathogens 2023, 12, 886. https://doi.org/10.3390/pathogens12070886
Zhu X, Liu Y, Xu N, Ai X, Yang Y. Molecular Characterization and Expression Analysis of IL-10 and IL-6 in Channel Catfish (Ictalurus punctatus). Pathogens. 2023; 12(7):886. https://doi.org/10.3390/pathogens12070886
Chicago/Turabian StyleZhu, Xia, Yongtao Liu, Ning Xu, Xiaohui Ai, and Yibin Yang. 2023. "Molecular Characterization and Expression Analysis of IL-10 and IL-6 in Channel Catfish (Ictalurus punctatus)" Pathogens 12, no. 7: 886. https://doi.org/10.3390/pathogens12070886
APA StyleZhu, X., Liu, Y., Xu, N., Ai, X., & Yang, Y. (2023). Molecular Characterization and Expression Analysis of IL-10 and IL-6 in Channel Catfish (Ictalurus punctatus). Pathogens, 12(7), 886. https://doi.org/10.3390/pathogens12070886











