Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs
Abstract
1. Introduction
2. Probiotics as a Source of Functional Components
2.1. Antimicrobial Agents
2.2. Vitamins
2.3. Peptides
2.4. Biosurfactants
2.5. EPSs
2.6. Enzymes
3. Source of Postbiotics and Their Potential Benefits
3.1. Lipoteichoic Acid
3.2. Peptidoglycans
3.3. Surface-Layer Proteins
3.4. Cell-Free Supernatants and Soluble Factors
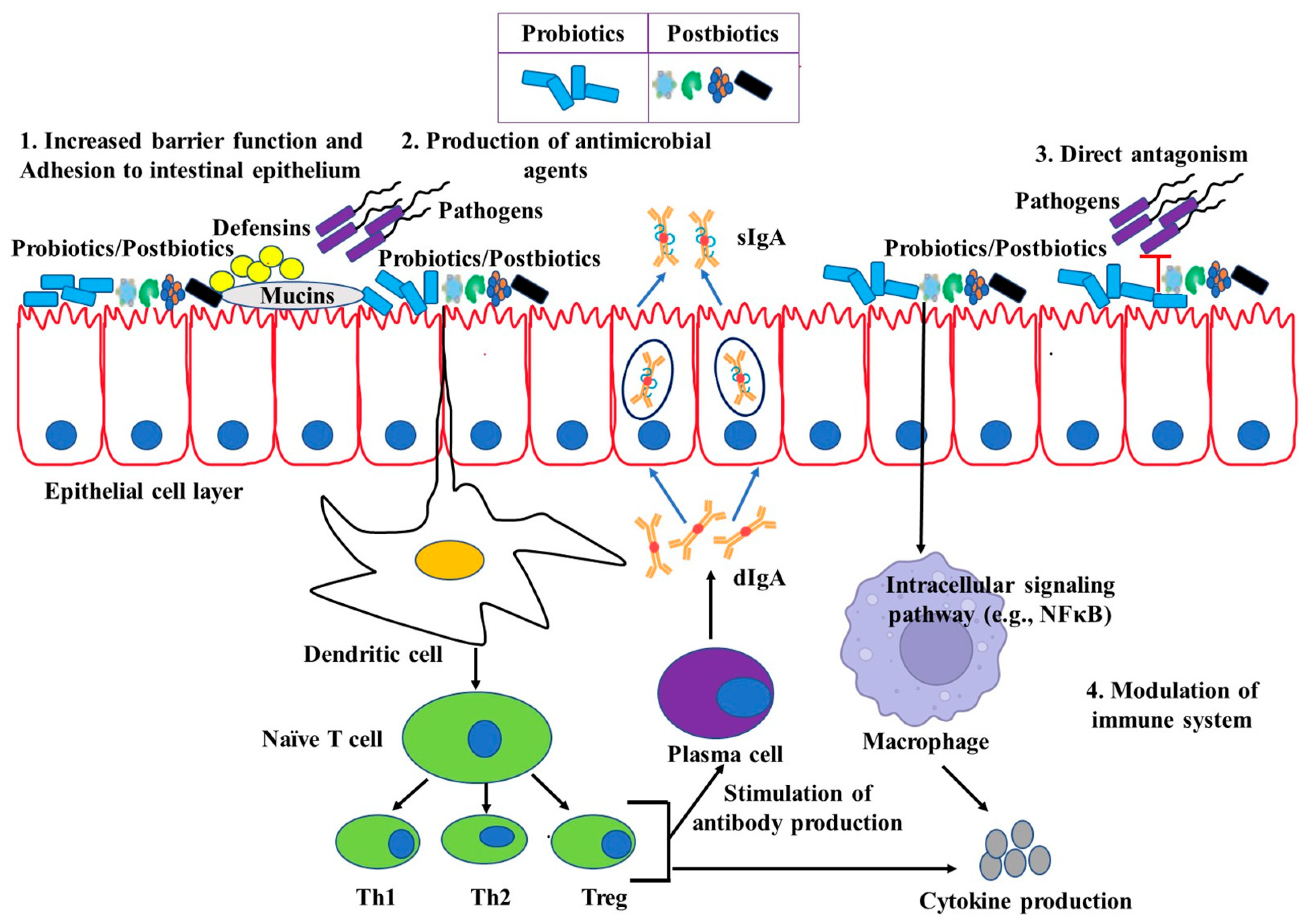
4. Potential Immunomodulation by Live Probiotics
5. Beneficial Effects of Probiotics on Pigs
6. Probiotics as Alternatives to Antibiotics and Zinc Oxide in Weaned Piglets
7. Cost–Benefit Analysis of Using Probiotics in the Swine Industry
8. Safety Concerns Involving the Use of Live Probiotics
9. Postbiotics and Their Effects on Immunomodulation
9.1. Heat Treatment
9.2. UV Irradiation
9.3. Sonication
10. Beneficial Effects of Inactivated Probiotics on Pigs
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LAB | Lactic acid bacteria |
| GIT | Gastrointestinal tract |
| EPS | Exopolysaccharides |
| UV | Ultraviolet |
| TLR | Toll-like receptor |
| SCFA | Short-chain fatty acid |
| BS | Biosurfactants |
| IL | Interleukin |
| INF | Interferon |
| TNF | Tumor necrotic factor |
| S-layer | Surface layer |
| PBMC | Peripheral blood mononuclear cell |
| Treg | Regulatory T cell |
| NK | Natural killer |
| sIgA | Secretory immunoglobulin A |
| dIgA | Dimeric Immunoglobulin A |
| Caco-2 | Colon carcinoma-2 |
| ETEC | Enterotoxigenic E. coli |
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar]
- Zhang, M.; Liu, Y.-S.; Zhao, J.-L.; Liu, W.-R.; Chen, J.; Zhang, Q.-Q.; He, L.-Y.; Ying, G.-G. Variations of antibiotic resistome in swine wastewater during full-scale anaerobic digestion treatment. Environ. Int. 2021, 155, 106694. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Visschers, V.H.M.; Iten, D.M.; Riklin, A.; Hartmann, S.; Sidler, X.; Siegrist, M. Swiss pig farmers’ perception and usage of antibiotics during the fattening period. Livest. Sci. 2014, 162, 223–232. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Delsart, M.; Pol, F.; Dufour, B.; Rose, N.; Fablet, C. Pig farming in alternative systems: Strengths and challenges in terms of animal welfare, biosecurity, animal health and pork safety. Agriculture 2020, 10, 261. [Google Scholar]
- Racewicz, P.; Ludwiczak, A.; Skrzypczak, E.; Składanowska-Baryza, J.; Biesiada, H.; Nowak, T.; Nowaczewski, S.; Zaborowicz, M.; Stanisz, M.; Ślósarz, P. Welfare health and productivity in commercial pig herds. Animals 2021, 11, 1176. [Google Scholar]
- Eurostat, G.D.P.; Components, M. Available online: https://ec.europa.eu/eurostat/databrowser/view.NAMQ_10_GDP/default/Table2020 (accessed on 10 May 2023).
- Han, M.; Yu, W.; Clora, F. Boom and bust in China’s pig sector during 2018–2021: Recent recovery from the ASF shocks and longer-term sustainability considerations. Sustainability 2022, 14, 6784. [Google Scholar] [CrossRef]
- Shin, S.B.; Cho, S.R.; Mok, J.S. Antimicrobial Resistance of Fecal-Indicator Bacteria Isolated from Aquatic Animal Farms along the Korean Coast. Aquac. Res. 2023, 2023, 5014754. [Google Scholar] [CrossRef]
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action; World Health Organization: Geneva, Switzerland, 2012; ISBN 9244503182. [Google Scholar]
- Wegener, H.C. Antibiotic resistance—Linking human and animal health. In Improving Food Safety through a One Health Approach: Workshop Summary; National Academies Press: Washington, DC, USA, 2012; p. 331. [Google Scholar]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics overuse in animal agriculture: A call to action for health care providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Teillant, A.; Laxminarayan, R. Economics of antibiotic use in US swine and poultry production. Choices 2015, 30, 1–11. [Google Scholar]
- Denkova, R.; Goranov, B.; Teneva, D.; Denkova, Z.; Kostov, G. Antimicrobial Activity of Probiotic Microorganisms: Mechanisms of Interaction and Methods of Examination. Antimicrob. Res. Nov. Bioknowl. Educ. Programs 2017, 1, 201–212. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L.K. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous Probiotic Lactobacillus Isolates Presenting Antibiotic like Activity against Human Pathogenic Bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Sarowska, J.; Choroszy-Król, I.; Regulska-Ilow, B.; Frej-Mądrzak, M.; Jama-Kmiecik, A. The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv. Clin. Exp. Med. Off. Organ. Wroclaw Med. Univ. 2013, 22, 759–766. [Google Scholar]
- Nair, M.S.; Amalaradjou, M.A.; Venkitanarayanan, K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv. Appl. Microbiol. 2017, 98, 1–29. [Google Scholar]
- Olaya Galán, N.N.; Ulloa Rubiano, J.C.; Velez Reyes, F.A.; Fernandez Duarte, K.P.; Salas Cárdenas, S.P.; Gutierrez Fernandez, M.F. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J. Appl. Microbiol. 2016, 120, 1041–1051. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Cerutti, A.; Rescigno, M. The Biology of Intestinal Immunoglobulin A Responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Li, N.; Wynn, J.L.; Neu, J. Probiotic microbes: Do they need to be alive to be beneficial? Nutr. Rev. 2009, 67, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Mater, D.D.G.; Langella, P.; Corthier, G.; Flores, M.-J. A probiotic Lactobacillus strain can acquire vancomycin resistance during digestive transit in mice. J. Mol. Microbiol. Biotechnol. 2008, 14, 123–127. [Google Scholar] [CrossRef]
- Neu, J. Perinatal and Neonatal Manipulation of the Intestinal Microbiome: A Note of Caution. Nutr. Rev. 2007, 65, 282–285. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for preterm neonates—The next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid.-Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Li, S.-C.; Hsu, W.-F.; Chang, J.-S.; Shih, C.-K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef]
- Oerlemans, M.M.P.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef]
- Chandler, C.E.; Ernst, R.K. Bacterial lipids: Powerful modifiers of the innate immune response. F1000Research 2017, 6, 1334. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Malashree, L.; Angadi, V.; Yadav, S.; Prabha, R. “Postbiotics”—One Step Ahead Probiotics. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2049–2053. [Google Scholar] [CrossRef]
- Ou, C.; Lin, S.; Tsai, J.; Lin, M. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260–M267. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, H.; Yao, R.; Odamaki, T.; Xiao, J.Z. Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment. Benef. Microbes 2017, 8, 463–472. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zawistowska-Rojek, A.; Kośmider, A.; Stępień, K.; Tyski, S. Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch. Microbiol. 2022, 204, 285. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Kim, K.W.; Kang, S.-S.; Woo, S.-J.; Park, O.-J.; Ahn, K.B.; Song, K.-D.; Lee, H.-K.; Yun, C.-H.; Han, S.H. Lipoteichoic Acid of Probiotic Lactobacillus plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Gilmore, W.J.; Johnston, E.L.; Zavan, L.; Bitto, N.J.; Kaparakis-Liaskos, M. Immunomodulatory roles and novel applications of bacterial membrane vesicles. Mol. Immunol. 2021, 134, 72–85. [Google Scholar] [CrossRef]
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily Intake of Heat-Killed Lactobacillus plantarum L-137 Augments Acquired Immunity in Healthy Adults. J. Nutr. 2006, 136, 3069–3073. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496. [Google Scholar] [CrossRef]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Piazentin, A.C.M.; Mendonça, C.M.N.; Vallejo, M.; Mussatto, S.I.; de Souza Oliveira, R.P. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuteri. Braz. J. Microbiol. 2022, 53, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Surwase, S.S.; Adsul, G.G.; Jadhav, D.S. Anti-microbial Activity Associated With Bacteriocin From Lactobacillus acidophilus. J. Res. Antimicrob 2011, 1, 5–8. [Google Scholar]
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—Aviable alternative to antibiotics? Nat. Rev. Microbiol. 2012, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Devi, A.T.; Prasad, A. Bacteriocins and their applications in food preservation. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J. Investigation of Antibacterial Activity and Probiotic Properties of Strains Belonging to Lactobacillus and Bifidobacterium Genera for Their Potential Application in Functional Food and Feed Products. Probiotics Antimicrob. Proteins 2021, 13, 1387–1403. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Linares, D.M.; Fitzgerald, G.; Hill, C.; Stanton, C.; Ross, P. Production of vitamins, exopolysaccharides and bacteriocins by probiotic bacteria. Probiotic Dairy Prod. 2018, 2, 359–388. [Google Scholar]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 produces cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef]
- Burgess, C.M.; Smid, E.J.; van Sinderen, D. Bacterial vitamin B2, B11 and B12 overproduction: An overview. Int. J. Food Microbiol. 2009, 133, 1–7. [Google Scholar] [CrossRef]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Mercier, A.; Gauthier, S.F.; Fliss, I. Immunomodulating effects of whey proteins and their enzymatic digests. Int. Dairy J. 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Prioult, G.; Pecquet, S.; Fliss, I. Allergenicity of acidic peptides from bovine β-lactoglobulin is reduced by hydrolysis with Bifidobacterium lactis NCC362 enzymes. Int. Dairy J. 2005, 15, 439–448. [Google Scholar] [CrossRef]
- Tellez, A.; Corredig, M.; Turner, P.V.; Morales, R.; Griffiths, M. A peptidic fraction from milk fermented with Lactobacillus helveticus protects mice against Salmonella infection. Int. Dairy J. 2011, 21, 607–614. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Velraeds, M.M.C.; van de Belt-Gritter, B.; Busscher, H.J.; Reid, G.; van der Mei, H.C. Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli—A teleologic approach. World J. Urol. 2000, 18, 422–426. [Google Scholar] [CrossRef]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Banat, I.M. Potential therapeutic applications of microbial surface-activecompounds. AIMS Bioeng. 2015, 2, 144–162. [Google Scholar] [CrossRef]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Yassin, A.M.; Al-Madboly, L.A.; El-Hawiet, A. A novel purified Lactobacillus acidophilus 20,079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb. Cell Fact. 2018, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chabot, S.; Yu, H.-L.; De Léséleuc, L.; Cloutier, D.; Van Calsteren, M.-R.; Lessard, M.; Roy, D.; Lacroix, M.; Oth, D. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF, IL-6 and IL-12 in human and mouse cultured immunocompetent cells, and IFN-γ in mouse splenocytes. Lait 2001, 81, 683–697. [Google Scholar] [CrossRef]
- Liu, C.; Tseng, K.; Chiang, S.; Lee, B.; Hsu, W.; Pan, T. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Del Carmen, S.; de Moreno de LeBlanc, A.; Martin, R.; Chain, F.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl. Environ. Microbiol. 2014, 80, 869–877. [Google Scholar] [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J.B. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera-A promising approach. Croat. J. food Sci. Technol. 2013, 5, 85–91. [Google Scholar]
- Fardet, A.; Rock, E. In Vitro and in Vivo Antioxidant Potential of Milks, Yoghurts, Fermented Milks and Cheeses: A Narrative Review of Evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.-C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Hirose, Y.; Murosaki, S.; Fujiki, T.; Yamamoto, Y.; Yoshikai, Y.; Yamashita, M. Lipoteichoic acids on Lactobacillus plantarum cell surfaces correlate with induction of interleukin-12p40 production. Microbiol. Immunol. 2010, 54, 143–151. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.-D.; Guo, Y.; Zeng, X. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, N.; Herrera, M.; Salva, S.; Villena, J.; Alvarez, S. Lactobacillus rhamnosus CRL1505 nasal administration improves recovery of T-cell mediated immunity against pneumococcal infection in malnourished mice. Benef. Microbes 2017, 8, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Salva, S.; Kolling, Y.; Ivir, M.; Gutiérrez, F.; Alvarez, S. The Role of Immunobiotics and Postbiotics in the Recovery of Immune Cell Populations from Respiratory Mucosa of Malnourished Hosts: Effect on the Resistance against Respiratory Infections. Front. Nutr. 2021, 8, 704868. [Google Scholar] [CrossRef]
- Sun, Z.; Kong, J.; Hu, S.; Kong, W.; Lu, W.; Liu, W. Characterization of a S-layer protein from Lactobacillus crispatus K313 and the domains responsible for binding to cell wall and adherence to collagen. Appl. Microbiol. Biotechnol. 2013, 97, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.M.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef]
- Ashraf, R.; Vasiljevic, T.; Smith, S.C.; Donkor, O.N. Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J. Dairy Sci. 2014, 97, 2542–2558. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ji, H.; Zhang, D.; Liu, H.; Wang, S.; Wang, J.; Wang, Y. Complete Genome Sequencing of Lactobacillus plantarum ZLP001, a Potential Probiotic That Enhances Intestinal Epithelial Barrier Function and Defense against Pathogens in Pigs. Front. Physiol. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Donato, K.A.; Gareau, M.G.; Wang, Y.J.J.; Sherman, P.M. Lactobacillus rhamnosus GG attenuates interferon-γ and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 2010, 156, 3288–3297. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.-D.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J. Dynamic distribution of gut microbiota in pigs at different growth stages: Composition and contribution. Microbiol. Spectr. 2022, 10, e00688-21. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef]
- Lo Verso, L.; Lessard, M.; Talbot, G.; Fernandez, B.; Fliss, I. Isolation and selection of potential probiotic bacteria from the pig gastrointestinal tract. Probiotics Antimicrob. Proteins 2018, 10, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Lee, E.-B.; Lim, S.-K.; Suk, K.; Park, S.-C. Isolation and Identification of Limosilactobacillus reuteri PSC102 and Evaluation of Its Potential Probiotic, Antioxidant, and Antibacterial Properties. Antioxidants 2023, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, H.; Yu, H.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Screening of Lactobacillus plantarum subsp. plantarum with potential probiotic activities for inhibiting ETEC K88 in weaned piglets. Molecules 2020, 25, 4481. [Google Scholar]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Sobrino, O.J.; Alba, C.; Arroyo, R.; Pérez, I.; Sariego, L.; Delgado, S.; Fernández, L.; de María, J.; Fumanal, P.; Fumanal, A. Replacement of Metaphylactic Antimicrobial Therapy by Oral Administration of Ligilactobacillus salivarius MP100 in a Pig Farm. Front. Vet. Sci. 2021, 8, 666887. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 2018, 24, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with a complex of lactic acid bacteria alone or in combination with Bacillus subtilis and Saccharomyces boulardii. Livest. Sci. 2012, 143, 132–141. [Google Scholar] [CrossRef]
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Moturi, J.; Kim, K.Y.; Hosseindoust, A.; Lee, J.H.; Xuan, B.; Park, J.; Kim, E.B.; Kim, J.S.; Chae, B.J. Effects of Lactobacillus salivarius isolated from feces of fast-growing pigs on intestinal microbiota and morphology of suckling piglets. Sci. Rep. 2021, 11, 6757. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Bach, L.G.; Nguyen, D.C.; Le, T.H.; Pham, K.H.; Nguyen, D.H.; Hoang Thi, T.T. Evaluation of Factors Affecting Antimicrobial Activity of Bacteriocin from Lactobacillus plantarum Microencapsulated in Alginate-Gelatin Capsules and Its Application on Pork Meat as a Bio-Preservative. Int. J. Environ. Res. Public Health 2019, 16, 1017. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Maison, T.; Koontanatechanon, A.; Prapasarakul, N. Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci. Rep. 2021, 11, 12028. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.F.; Hou, C.L.; Wang, S.X.; Zhang, D.Y.; Liu, H.; Shan, D.C.; Wang, Y.M. Effects of Lactobacillus johnsonii XS4 supplementation on reproductive performance, gut environment, and blood biochemical and immunological index in lactating sows. Livest. Sci. 2014, 164, 96–101. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 modulates intestinal host defense peptide expression in the model of IPEC-J2 cells and neonatal piglets. Nutrients 2017, 9, 559. [Google Scholar] [CrossRef]
- Liu, H.; Ji, H.F.; Zhang, D.Y.; Wang, S.X.; Wang, J.; Shan, D.C.; Wang, Y.M. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest. Sci. 2015, 178, 251–254. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; Akkermans, A.D.L.; Casini, L.; Trevisi, P.; Mazzoni, M.; De Filippi, S.; Bosi, P.; de Vos, W.M. Feeding of Lactobacillus sobrius reduces Escherichia coli F4 levels in the gut and promotes growth of infected piglets. FEMS Microbiol. Ecol. 2008, 66, 599–607. [Google Scholar] [CrossRef]
- Canibe, N.; Miettinen, H.; Jensen, B.B. Effect of adding Lactobacillus plantarum or a formic acid containing-product to fermented liquid feed on gastrointestinal ecology and growth performance of piglets. Livest. Sci. 2008, 114, 251–262. [Google Scholar] [CrossRef]
- Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Castillo, R.; Ding, X. Effect of Oral Administration with Lactobacillus plantarum CAM6 Strain on Sows during Gestation-Lactation and the Derived Impact on Their Progeny Performance. Mediat. Inflamm. 2021, 2021, 6615960. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.T.; Hoon, J.; Mun, H.-S.; Yang, C.-J. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Afr. J. Microbiol. Res 2014, 8, 96–104. [Google Scholar]
- Wang, C.; Wei, S.; Xu, B.; Hao, L.; Su, W.; Jin, M.; Wang, Y. Bacillus subtilis and Enterococcus faecium co-fermented feed regulates lactating sow’s performance, immune status and gut microbiota. Microb. Biotechnol. 2021, 14, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, Y.; Zhang, J.; Yang, Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res. Vet. Sci. 2013, 94, 62–68. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial impact and molecular mechanism of Bacillus coagulans on piglets’ intestine. Int. J. Mol. Sci. 2018, 19, 2084. [Google Scholar] [CrossRef]
- Suda, Y.; Sasaki, N.; Kagawa, K.; Elean, M.; Zhou, B.; Tomokiyo, M.; Islam, M.A.; Rajoka, M.S.; Kober, A.K.M.H.; Shimazu, T.; et al. Immunobiotic Feed Developed with Lactobacillus delbrueckii subsp. delbrueckii TUA4408L and the Soymilk By-Product Okara Improves Health and Growth Performance in Pigs. Microorganisms 2021, 9, 921. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Zheng, W.; Yang, T.; Wang, X.; Xie, C.; Yan, X. Lactobacillus frumenti mediates energy production via fatty acid β-oxidation in the liver of early-weaned piglets. J. Anim. Sci. Biotechnol. 2019, 10, 95. [Google Scholar] [CrossRef]
- Afonso, E.R.; Parazzi, L.J.; Marino, C.T.; Martins, S.M.M.K.; Araújo, L.F.; Araújo, C.S.D.S.; Vilela, F.G.; Moretti, A.D.S.A. Probiotics association in the suckling and nursery in piglets challenged with Salmonella typhimurium. Braz. Arch. Biol. Technol. 2013, 56, 249–258. [Google Scholar] [CrossRef]
- Kumar, A.; Vlasova, A.N.; Liu, Z.; Chattha, K.S.; Kandasamy, S.; Esseili, M.; Zhang, X.; Rajashekara, G.; Saif, L.J. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut Microbes 2014, 5, 152–164. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.F.; Wang, S.X.; Zhang, D.Y.; Liu, H.; Shan, D.C.; Wang, Y.M. Lactobacillus plantarum ZLP001: In vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1153. [Google Scholar] [CrossRef]
- Tsukida, K.; Takahashi, T.; Iida, H.; Kanmani, P.; Suda, Y.; Nochi, T.; Ohwada, S.; Aso, H.; Ohkawara, S.; Makino, S.; et al. Immunoregulatory effects triggered by immunobiotic Lactobacillus jensenii TL2937 strain involve efficient phagocytosis in porcine antigen presenting cells. BMC Immunol. 2016, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Q.; Chang, J.; Yin, Q.; Song, A.; Li, Z.; Wang, E.; Lu, F. Effects of Lactobacillus casei and Enterococcus faecalis on growth performance, immune function and gut microbiota of suckling piglets. Arch. Anim. Nutr. 2017, 71, 120–133. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Cardarelli, H.R.; Borst, W.; Albrecht, S.; Schols, H.; Gutiérrez, O.P.; Maathuis, A.J.H.; de Melo Franco, B.D.G.; De Martinis, E.C.P.; Zoetendal, E.G.; et al. Effect of galactooligosaccharides and Bifidobacterium animalis Bb-12 on growth of Lactobacillus amylovorus DSM 16698, microbial community structure, and metabolite production in an in vitro colonic model set up with human or pig microbiota. FEMS Microbiol. Ecol. 2013, 84, 110–123. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- Vahjen, W.; Pietruszyńska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef] [PubMed]
- Satessa, G.D.; Kjeldsen, N.J.; Mansouryar, M.; Hansen, H.H.; Bache, J.K.; Nielsen, M.O. Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets. Animal 2020, 14, 1638–1646. [Google Scholar] [CrossRef]
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Atwill, E.R.; Maga, E.A.; Jørgensen, J.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 52. [Google Scholar] [CrossRef]
- Lin, K.-H.; Yu, Y.-H. Evaluation of Bacillus licheniformis-Fermented Feed Additive as an Antibiotic Substitute: Effect on the Growth Performance, Diarrhea Incidence, and Cecal Microbiota in Weaning Piglets. Animals 2020, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Colbère-Garapin, F.; Martin-Latil, S.; Blondel, B.; Mousson, L.; Pelletier, I.; Autret, A.; François, A.; Niborski, V.; Grompone, G.; Catonnet, G.; et al. Prevention and treatment of enteric viral infections: Possible benefits of probiotic bacteria. Microbes Infect. 2007, 9, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Barba Vidal, E.R. Evaluation of Probiotic Strategies in the Prevention of Piglet Post-Weaning Gastrointestinal Disorders; Universitat Autònoma de Barcelona: Barcelona, Spain, 2017; ISBN 8449069920. [Google Scholar]
- Lodemann, U.; Hübener, K.; Jansen, N.; Martens, H. Effects of Enterococcus faecium NCIMB 10415 as probiotic supplement on intestinal transport and barrier function of piglets. Arch. Anim. Nutr. 2006, 60, 35–48. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Callegari, M.L.; Reggi, S.; Caprarulo, V.; Giromini, C.; Spalletta, A.; Coranelli, S.; Sgoifo Rossi, C.A.; Rossi, L. Lactobacillus plantarum and Lactobacillus reuteri as Functional Feed Additives to Prevent Diarrhoea in Weaned Piglets. Animals 2021, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Evaluation of the Synbiotic Strategy as Prevention and Treatment of Swine Digestive Pathologies; Universitat Autònoma de Barcelona: Barcelona, Spain, 2019. [Google Scholar]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Bouzaiene, T.; Ziadi, M.; Enneifer, M.; Sellami, A.; Aydi, A.; Cherif, A.; Hamdi, M. Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential. Sustainability 2023, 15, 8394. [Google Scholar] [CrossRef]
- Pereira, W.A.; Franco, S.M.; Reis, I.L.; Mendonça, C.M.N.; Piazentin, A.C.M.; Azevedo, P.O.S.; Tse, M.L.P.; De Martinis, E.C.P.; Gierus, M.; Oliveira, R.P.S. Beneficial effects of probiotics on the pig production cycle: An overview of clinical impacts and performance. Vet. Microbiol. 2022, 269, 109431. [Google Scholar] [CrossRef]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Bogusławska-Wąs, E.; Łupkowska, A. Effect of EM® probiotic on gut microbiota, growth performance, carcass and meat quality of pigs. Livest. Sci. 2020, 241, 104206. [Google Scholar] [CrossRef]
- Kenny, M.; Smidt, H.; Mengheri, E.; Miller, B. Probiotics—Do they have a role in the pig industry? Animal 2011, 5, 462–470. [Google Scholar] [CrossRef]
- Li, X.-Q.; Zhu, Y.-H.; Zhang, H.-F.; Yue, Y.; Cai, Z.-X.; Lu, Q.-P.; Zhang, L.; Weng, X.-G.; Zhang, F.-J.; Zhou, D. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: Intestinal microbiota and immune imbalances. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Liong, M.-T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Klein, G. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J. Food Prot. 2008, 71, 1734–1741. [Google Scholar] [CrossRef]
- van den Nieuwboer, M.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating What They Are and What They Are Not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Honeycutt, T.C.B.; El Khashab, M.; Wardrop, R.M.; McNeal-Trice, K.; Honeycutt, A.L.B.; Christy, C.G.; Mistry, K.; Harris, B.D.; Meliones, J.N.; Kocis, K.C. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: A randomized placebo-controlled trial. Pediatr. Crit. Care Med. 2007, 8, 452–458. [Google Scholar] [CrossRef]
- Shi, H.N.; Walker, A. Bacterial colonization and the development of intestinal defences. Can. J. Gastroenterol. 2004, 18, 493–500. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Meddings, J.; Field, C.; McCargar, L.; Kutsogiannis, D.; Fedorak, R.; Madsen, K. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2007, 85, 816–823. [Google Scholar] [CrossRef]
- Gray, K.D.; Messina, J.A.; Cortina, C.; Owens, T.; Fowler, M.; Foster, M.; Gbadegesin, S.; Clark, R.H.; Benjamin, D.K.J.; Zimmerman, K.O.; et al. Probiotic Use and Safety in the Neonatal Intensive Care Unit: A Matched Cohort Study. J. Pediatr. 2020, 222, 59–64.e1. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis. mSphere 2018, 3, e00104-18. [Google Scholar] [CrossRef]
- Miyazawa, K.; Kawase, M.; Kubota, A.; Yoda, K.; Harata, G.; Hosoda, M.; He, F. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef. Microbes 2015, 6, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Kaur, G.; Kapila, S.; Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486. [Google Scholar] [CrossRef]
- Jang, H.J.; Song, M.W.; Lee, N.-K.; Paik, H.-D. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J. Food Sci. Technol. 2018, 55, 3174–3180. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Nomoto, R.; Mizuno, M.; Osawa, R. An in vitro investigation of immunomodulatory properties of Lactobacillus plantarum and L. delbrueckii cells and their extracellular polysaccharides. Biosci. Microbiota Food Heal 2017, 36, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; De Wolfe, T.J.; Safdar, N.; Darien, B.J.; Steele, J.L. Lactobacillus casei Cell-Surface Components and Their Impact on Immunomodulation and Cecal Microbiata Composition. FASEB J. 2017, 31, 892.2. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic lactobacilli improves the adaptive immune response against influenza virus. Int. Immunopharmacol. 2020, 78, 106115. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Lomax, A.R.; Calder, P.C. Probiotics, immune function, infection and inflammation: A review of the evidence from studies conducted in humans. Curr. Pharm. Des. 2009, 15, 1428–1518. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.J.; Cho, J.H.; Choe, J.; Kyoung, H.; Kim, S.H.; Kim, H.B.; Song, M. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs. J. Anim. Sci. Technol. 2021, 63, 520. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Albarracin, L.; Kitazawa, H.; Villena, J. Screening and characterization of immunobiotic lactic acid bacteria with porcine immunoassay systems. Lact. Acid Bact. Methods Protoc. 2019, 1887, 131–144. [Google Scholar]
- Good, M.; Sodhi, C.P.; Ozolek, J.A.; Buck, R.H.; Goehring, K.C.; Thomas, D.L.; Vikram, A.; Bibby, K.; Morowitz, M.J.; Firek, B.; et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: Evidence in mice for a role of TLR9. Am. J. Physiol. Liver Physiol. 2014, 306, G1021–G1032. [Google Scholar] [CrossRef] [PubMed]
- Tartrakoon, W.; Charoensook, R.; Incharoen, T.; Numthuam, S.; Pechrkong, T.; Onoda, S.; Shoji, G.; Brenig, B. Effects of Heat-Killed Lactobacillus plantarum L-137 Supplementation on Growth Performance, Blood Profiles, Intestinal Morphology, and Immune Gene Expression in Pigs. Vet. Sci. 2023, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kyoung, H.; Park, K.I.; Oh, S.; Song, M.; Kim, Y. Postbiotic heat-killed lactobacilli modulates on body weight associated with gut microbiota in a pig model. AMB Express 2022, 12, 83. [Google Scholar] [CrossRef]
- Bernardeau, M.; Gueguen, M.; Smith, D.G.E.; Corona-Barrera, E.; Vernoux, J.P. In vitro antagonistic activities of Lactobacillus spp. against Brachyspira hyodysenteriae and Brachyspira pilosicoli. Vet. Microbiol. 2009, 138, 184–190. [Google Scholar] [CrossRef]
- Maidana, L.G.; Gerez, J.; Pinho, F.; Garcia, S.; Bracarense, A.P. Achados histopatológicos e ultraestruturais induzidos por Lactobacillus plantarum inativado pelo calor na mucosa intestinal de leitões: Estudo ex-vivo. Arq. Bras. Med. Veterinária Zootec. 2019, 71, 11–20. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P.; Gueguen, M. Safety and efficacy of probiotic lactobacilli in promoting growth in post-weaning Swiss mice. Int. J. Food Microbiol. 2002, 77, 19–27. [Google Scholar] [CrossRef]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18 + Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef]
| Probiotics | Effects | References |
|---|---|---|
| Lactobacillus delbrucei TUAA4408L | Boost immunity and modulate intestinal microbiota; improve growth performance and meat quality | [130] |
| L. acidophilus | Improve feed efficiency | [131] |
| Lactobacillus frumenti | Decrease incidence of diarrhea | [132] |
| L. reuteri | Improve feed intake and weight gain | [133] |
| L reuteri and Bacillus licheniformis | Increase nutrient digestibility | [126] |
| L. rhamnosus GG | Improve gut integrity | [134] |
| L. fermentum ZLP001 | Enhance catalase and superoxide dismutase | [135] |
| L. salivarius 160 | Elevate the levels of beneficial bacteria and decrease harmful bacteria | [117] |
| L. plantarum VTT E-78076 | Improve growth performance and gastrointestinal ecology | [124] |
| L. brevis ZLB004 | Improve intestinal microbiota balance, immunity and growth performance | [122] |
| L. jensenii TL2937 | Modulate porcine mononuclear phagocytic activity | [136] |
| L. plantarum SC01 | Inhibit the growth of Salmonella, E. coli, and S. aureus | [118] |
| L. casei | Improve immune functions and gut microbiota balance | [137] |
| L. reuteri I5007 | Improve intestinal infection defense | [121] |
| L. johnsenii XS4 | Improve reproductive performance and immunological index | [120] |
| L. plantarum CAM6 | Develop milk quality | [125] |
| L. amylovorus DSM16698 | Enhance microbial community structure and metabolite production | [138] |
| L. salivarius B1 | Develop intestinal mucosal immunity | [128] |
| L. plantarum | Promote intestinal barrier functions | [139] |
| L. sobrius | Improve ETEC infections | [123] |
| Probiotics | Mode of Inactivation | Effects | References |
|---|---|---|---|
| L. planturum L-137 | Heat | Improve growth performance, intestinal morphology, and immune gene expression | [180] |
| Ligitolactobacillus salivarius 189 | Heat | Modulate body weight and gut microbiota composition | [181] |
| L. rhamnosus | Heat | Develop growth performance and immune response | [177] |
| L. farciminis 3699 | Heat | Decrease the incidence of dysentery | [182] |
| L. plnatarum | Heat | Elongate villi | [183] |
| L. rhamnosus MA27/6B and L. acidophilus MA27/6R | Heat | Promote growth performance | [184] |
| L. rhamnosus CRL1505 | Heat | Boost immunity | [178] |
| L. reuteri I5007 | Heat | Improve intestinal infection defense | [121] |
| Limosilactobacillus fermentum | Heat | Prevent post-weaning diarrhea and improve growth efficiency | [185] |
| L. rhamnosus HN001 | UV | Attenuate necrotizing enterocolitis | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.S.; Lee, E.-B.; Hsu, W.H.; Suk, K.; Sayem, S.A.J.; Ullah, H.M.A.; Lee, S.-J.; Park, S.-C. Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens 2023, 12, 874. https://doi.org/10.3390/pathogens12070874
Ali MS, Lee E-B, Hsu WH, Suk K, Sayem SAJ, Ullah HMA, Lee S-J, Park S-C. Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens. 2023; 12(7):874. https://doi.org/10.3390/pathogens12070874
Chicago/Turabian StyleAli, Md. Sekendar, Eon-Bee Lee, Walter H. Hsu, Kyoungho Suk, Syed Al Jawad Sayem, H. M. Arif Ullah, Seung-Jin Lee, and Seung-Chun Park. 2023. "Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs" Pathogens 12, no. 7: 874. https://doi.org/10.3390/pathogens12070874
APA StyleAli, M. S., Lee, E.-B., Hsu, W. H., Suk, K., Sayem, S. A. J., Ullah, H. M. A., Lee, S.-J., & Park, S.-C. (2023). Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens, 12(7), 874. https://doi.org/10.3390/pathogens12070874








