Growth of V. parahaemolyticus in Tropical Blacklip Rock Oysters
Abstract
1. Introduction
2. Methods
2.1. Isolation of V. parahaemolyticus Strains from Oysters and Preparation of Inoculum
2.2. Oyster Inoculation, Incubation and Processing
2.3. Analyses
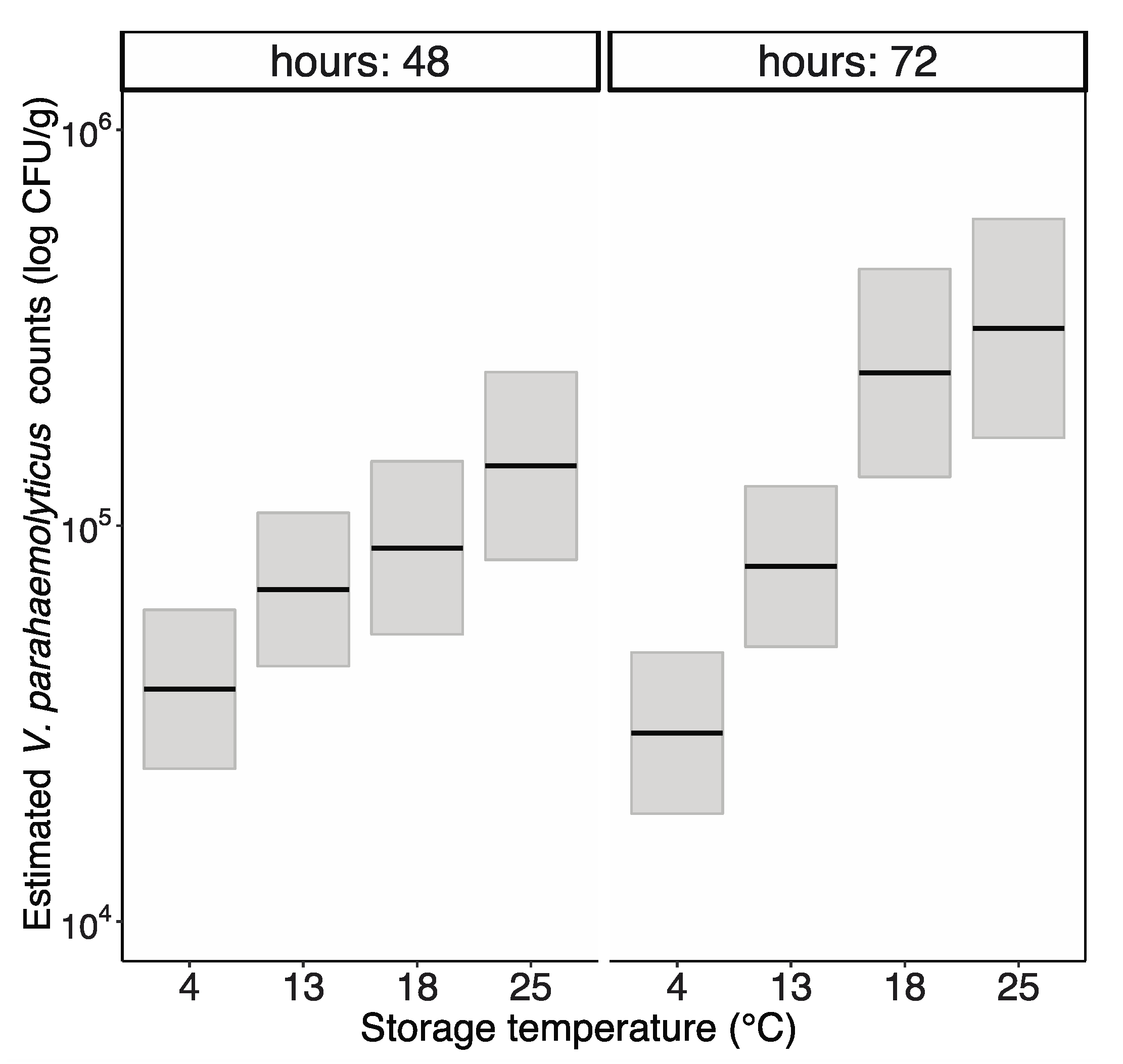
3. Results
3.1. V. parahaemolyticus Growth Rates in Injected Oysters
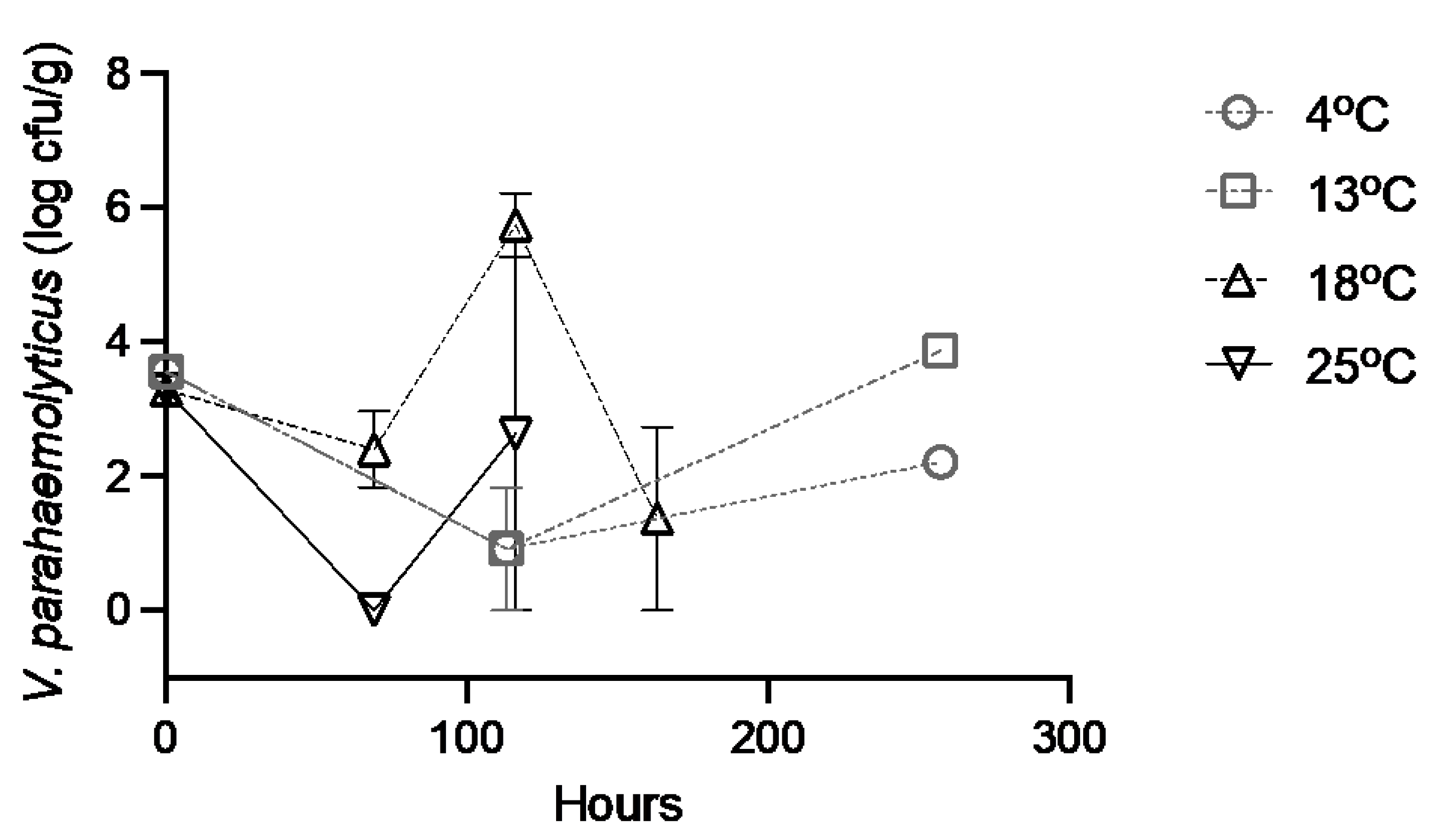
3.2. Control (Seawater Injected) Oysters
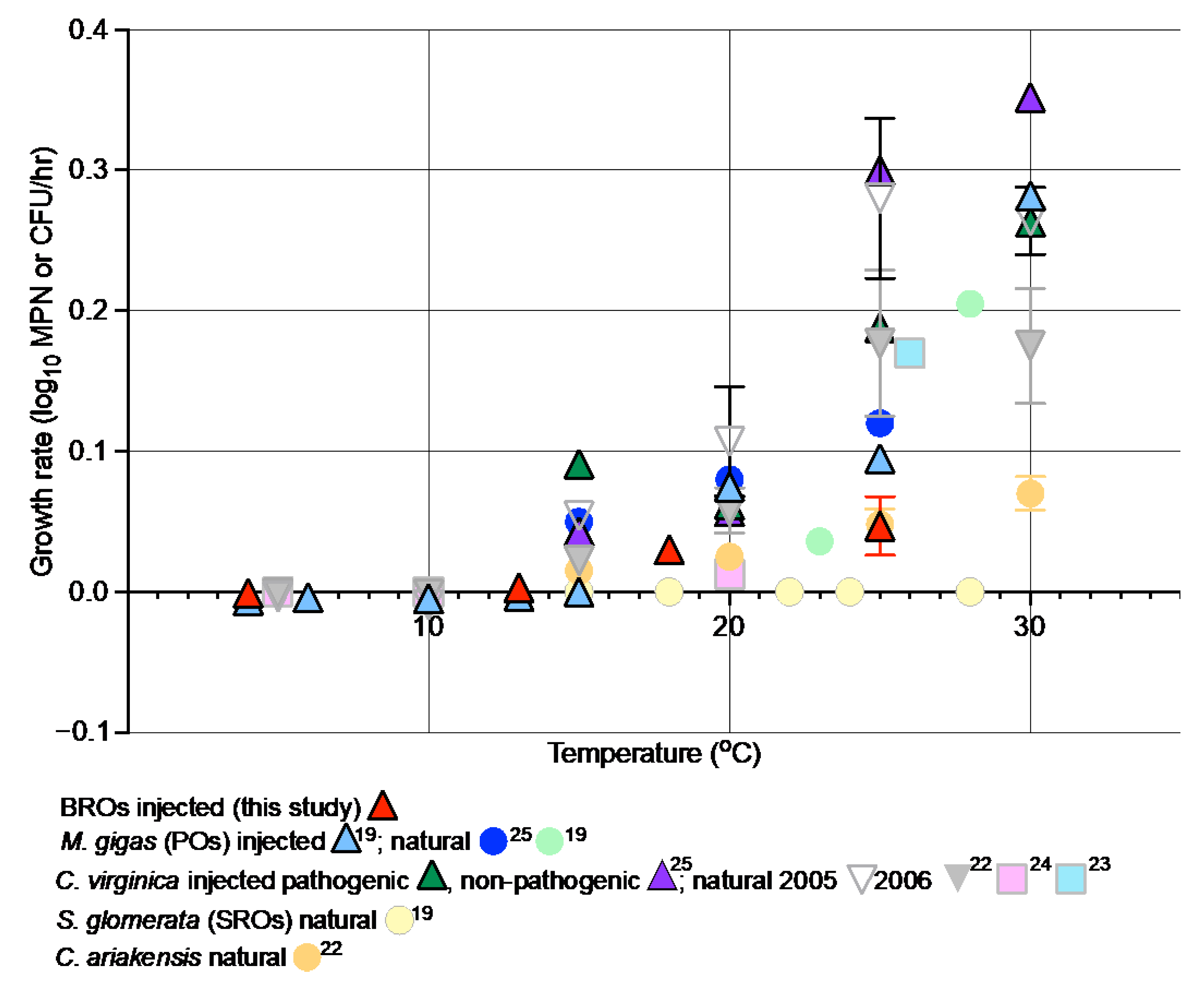
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Australian Bureau of Agricultural and Resource Economics and Sciences. Australian Fisheries and Aquaculture Statistics 2020; ABARES: Canberra, Australia, 2021. [Google Scholar]
- Oysters Australia Ltd. 2020–2025 Oysters Australia Strategic Plan; Project No. 2019-208–2020-25 Strategic Plan for the Australian Oyster Industry; Fisheries Research and Development Corporation: Canberra, Australia, 2020. [Google Scholar]
- Nowland, S.J.; O’Connor, W.A.; Osborne, M.W.J.; Southgate, P.C. Current Status and Potential of Tropical Rock Oyster Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 57–70. [Google Scholar] [CrossRef]
- Nowland, S.J.; O’Connor, W.A.; Elizur, A.; Southgate, P.C. Evaluating Spawning Induction Methods for the Tropical Black-Lip Rock Oyster, Saccostrea echinata. Aquac. Rep. 2021, 20, 100676. [Google Scholar] [CrossRef]
- Nowland, S.J.; O’Connor, W.A.; Southgate, P.C. Optimizing Stocking Density and Microalgae Ration Improves the Growth Potential of Tropical Black-lip Oyster, Saccostrea echinata, Larvae. J. World Aquac. Soc. 2019, 50, 728–737. [Google Scholar] [CrossRef]
- Padovan, A.; Siboni, N.; Kaestli, M.; King, W.L.; Seymour, J.R.; Gibb, K. Occurrence and Dynamics of Potentially Pathogenic Vibrios in the Wet-Dry Tropics of Northern Australia. Mar. Environ. Res. 2021, 169, 105405. [Google Scholar] [CrossRef]
- Ralston, E.P.; Kite-Powell, H.; Beet, A. An Estimate of the Cost of Acute Health Effects from Food- and Water-Borne Marine Pathogens and Toxins in the USA. J. Water Health 2011, 9, 680–694. [Google Scholar] [CrossRef]
- EFSA, E.F.S.A.; Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G.D. Climate Change as a Driver of Emerging Risks for Food and Feed Safety, Plant, Animal Health and Nutritional Quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio Risk at High Latitudes in Response to Ocean Warming. Nat. Clim. Chang. 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Höfle, M.G.H.; Pruzzo, C. Long-Term Effects of Ocean Warming on the Prokaryotic Community: Evidence from the Vibrios. ISME J. 2011, 6, 21–30. [Google Scholar] [CrossRef]
- Froelich, B.A.; Daines, D.A. In Hot Water: Effects of Climate Change on Vibrio–Human Interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Bowers, J.C.; Trinanes, J.; DePaola, A. Climate Anomalies and the Increasing Risk of Vibrio parahaemolyticus and Vibrio vulnificus Illnesses. Food Res. Int. 2010, 43, 1780–1790. [Google Scholar] [CrossRef]
- Roux, F.L.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M.; et al. The Emergence of Vibrio Pathogens in Europe: Ecology, Evolution, and Pathogenesis (Paris, 11–12t March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [CrossRef]
- Harlock, M.; Quinn, S.; Turnbull, A.R. Emergence of Non-Choleragenic Vibrio Infections in Australia. Commun. Dis. Intell. 2022, 46, 1–7. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Safe Seafood Australia Guide to Standard 4.2.1 Primary Production and Processing Standard for Seafood; FSANZ: Canberra, Australia, 2005; p. 101. ISBN 0652346054.
- Australian Shellfish Quality Assurance Advisory Committee. The Australian Shellfish Quality Assurance Program; ASQAAC: Canberra, Australia, 2022. [Google Scholar]
- NSW-Food-Authority. NSW Shellfish Industry Manual; NSW-Food-Authority: Newington, Australia, 2018.
- Madigan, T. A Critical Evaluation of Supply-Chain Temperature Profiles to Optimise Food Safety and Quality of Australian Oysters; Project No. 2007/700; South Australian Research and Development Institute: Urrbrae, Australia, 2008. [Google Scholar]
- Fernandez-Piquer, J.; Bowman, J.P.; Ross, T.; Tamplin, M.L. Predictive Models for the Effect of Storage Temperature on Vibrio parahaemolyticus Viability and Counts of Total Viable Bacteria in Pacific Oysters (Crassostrea gigas). Appl. Environ. Microbiol. 2011, 77, 8687–8695. [Google Scholar] [CrossRef]
- Eyles, M.; Davey, G.; Arnold, G. Behavior and Incidence of Vibrio Parahaemolyticus in Sydney Rock Oysters (Crassostrea commercialis). Int. J. Food Microbiol. 1985, 1, 327–334. [Google Scholar] [CrossRef]
- Bird, P.; Arnold, G.; Holliday, J.; Boronovsky, A. Effect of Storage on the Quality of Purified Live Pacific and Sydney Rock Oysters. In Proceedings of the Conference Internationale sur la Purification des Coquillages, Rennes, France, 6–8 April 1992; IFREMER: Brest, France, 1992; pp. 315–322. [Google Scholar]
- Parveen, S.; DaSilva, L.; DePaola, A.; Bowers, J.; White, C.; Munasinghe, K.A.; Brohawn, K.; Mudoh, M.; Tamplin, M. Development and Validation of a Predictive Model for the Growth of Vibrio parahaemolyticus in Post-Harvest Shellstock Oysters. Int. J. Food Microbiol. 2013, 161, 1–6. [Google Scholar] [CrossRef]
- Gooch, J.A.; DePaola, A.; Bowers, J.; Marshall, D.L. Growth and Survival of Vibrio parahaemolyticus in Postharvest American Oysters. J. Food Prot. 2016, 65, 970–974. [Google Scholar] [CrossRef]
- Mudoh, M.F.; Parveen, S.; Schwarz, J.; Rippen, T.; Chaudhuri, A. The Effects of Storage Temperature on the Growth of Vibrio parahaemolyticus and Organoleptic Properties in Oysters. Front. Public Health 2014, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Ellett, A.N.; Rosales, D.; Jacobs, J.M.; Paranjpye, R.; Parveen, S. Growth Rates of Vibrio parahaemolyticus Sequence Type 36 Strains in Live Oysters and in Culture Medium. Microbiol. Spectr. 2022, 10, e02112-22. [Google Scholar] [CrossRef]
- Nordstrom, J.L.; Vickery, M.C.L.; Blackstone, G.M.; Murray, S.L.; DePaola, A. Development of a Multiplex Real-Time PCR Assay with an Internal Amplification Control for the Detection of Total and Pathogenic Vibrio parahaemolyticus Bacteria in Oysters. Appl. Environ. Microbiol. 2007, 73, 5840–5847. [Google Scholar] [CrossRef]
- Kim, Y.B.; Okuda, J.; Matsumoto, C.; Takahashi, N.; Hashimoto, S.; Nishibuchi, M. Identification of Vibrio parahaemolyticus Strains at the Species Level by PCR Targeted to the toxR Gene. J. Clin. Microbiol. 1999, 37, 1173–1177. [Google Scholar] [CrossRef]
- Goh, S.H.; Potter, S.; Wood, J.O.; Hemmingsen, S.M.; Reynolds, R.P.; Chow, A.W. HSP60 Gene Sequences as Universal Targets for Microbial Species Identification: Studies with Coagulase-Negative Staphylococci. J. Clin. Microbiol. 1996, 34, 818–823. [Google Scholar] [CrossRef]
- Caburlotto, G.; Gennari, M.; Ghidini, V.; Tafi, M.; Lleo, M.M. Presence of T3SS2 and Other Virulence-related Genes in tdh-negative Vibrio parahaemolyticus Environmental Strains Isolated from Marine Samples in the Area of the Venetian Lagoon, Italy. FEMS Microbiol. Ecol. 2009, 70, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.W.; Ruple, A.D. Indicator Bacteria and Vibrionaceae Multiplication in Post-Harvest Shellstock Oysters. J. Food Prot. 2016, 52, 343–349. [Google Scholar] [CrossRef]
- Wang, D.; Yu, S.; Chen, W.; Zhang, D.; Shi, X. Enumeration of Vibrio parahaemolyticus in Oyster Tissues Following Artificial Contamination and Depuration. Lett. Appl. Microbiol. 2010, 51, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Tamplin, M.; Fernandez-Piquer, J.; Ross, T. Protecting the Safety and Quality of Australian Oysters with Integrated Predictive Tools; Project No. 2007/719; Fisheries Research and Development Corporation: Canberra, Australia, 2007. [Google Scholar]
- Meng, J.; Wang, T.; Li, L.; Zhang, G. Inducible Variation in Anaerobic Energy Metabolism Reflects Hypoxia Tolerance across the Intertidal and Subtidal Distribution of the Pacific Oyster (Crassostrea gigas). Mar. Environ. Res. 2018, 138, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, L.; Meng, J.; Qi, H.; Qu, T.; Xu, F.; Zhang, L. Molecular Basis for Adaptation of Oysters to Stressful Marine Intertidal Environments. Annu. Rev. Anim. Biosci. 2016, 4, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, B.J.; Wells, R.M.G.; Jeffs, A.G. Oxygen Consumption and Enzyme Activity of the Subtidal Flat Oyster (Ostrea chilensis) and Intertidal Pacific Oyster (Crassostrea gigas): Responses to Temperature and Starvation. N. Z. J. Mar. Freshw. Res. 2006, 40, 149–158. [Google Scholar] [CrossRef]
- Dudognon, T.; Soudant, P.; Seguineau, C.; Quéré, C.; Auffret, M.; Kraffe, E. Functional Capacities of Gill Mitochondria in Oyster Crassostrea gigas during an Emersion/Immersion Tidal Cycle. Aquat. Living Resour. 2013, 26, 249–256. [Google Scholar] [CrossRef][Green Version]
- Allen, S.M.; Burnett, L.E. The Effects of Intertidal Air Exposure on the Respiratory Physiology and the Killing Activity of Hemocytes in the Pacific Oyster, Crassostrea gigas (Thunberg). J. Exp. Mar. Biol. Ecol. 2008, 357, 165–171. [Google Scholar] [CrossRef]
- Macey, B.M.; Achilihu, I.O.; Burnett, K.G.; Burnett, L.E. Effects of Hypercapnic Hypoxia on Inactivation and Elimination of Vibrio campbellii in the Eastern Oyster, Crassostrea virginica. Appl. Environ. Microbiol. 2008, 74, 6077–6084. [Google Scholar] [CrossRef]
- Yoon, K.S.; Min, K.J.; Jung, Y.J.; Kwon, K.Y.; Lee, J.K.; Oh, S.W. A Model of the Effect of Temperature on the Growth of Pathogenic and Nonpathogenic Vibrio parahaemolyticus Isolated from Oysters in Korea. Food Microbiol. 2008, 25, 635–641. [Google Scholar] [CrossRef]
- Saito, S.; Iwade, Y.; Tokuoka, E.; Nishio, T.; Otomo, Y.; Araki, E.; Konuma, H.; Nakagawa, H.; Tanaka, H.; Sugiyama, K.; et al. Epidemiological Evidence of Lesser Role of Thermostable Direct Hemolysin (TDH)–Related Hemolysin (TRH) than TDH on Vibrio parahaemolyticus Pathogenicity. Foodborne Pathog. Dis. 2015, 12, 131–138. [Google Scholar] [CrossRef]
- Park, K.; Ono, T.; Rokuda, M.; Jang, M.; Iida, T.; Honda, T. Cytotoxicity and Enterotoxicity of the Thermostable Direct Hemolysin-deletion Mutants of Vibrio parahaemolyticus. Microbiol. Immunol. 2004, 48, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.P.; Chao, M.C.; Abel, S.; Blondel, C.J.; Wiesch, P.A.Z.; Zhou, X.; Davis, B.M.; Waldor, M.K. Genetic Analysis of Vibrio parahaemolyticus Intestinal Colonization. Proc. Natl. Acad. Sci. USA 2016, 113, 6283–6288. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.A.; Ness, G.E.; Rodrick, G.E.; Blake, N.J. Effects of Storage on Microbial Loads of Two Commercially Important Shellfish Species, Crassostrea virginica and Mercenaria campechiensis. Appl. Environ. Microbiol. 1983, 45, 1221–1228. [Google Scholar] [CrossRef]
- Elvira, F.A.; Sedas, V.T.P.; Herrera, D.M.; Castro, R.Q.; Ros, R.M.O.; Hernández, K.L.; Primo, A.F.; Elvira, K.R. Comparative Survival and the Cold-Induced Gene Expression of Pathogenic and Nonpathogenic Vibrio parahaemolyticus from Tropical Eastern Oysters during Cold Storage. Int. J. Environ. Res. Public Health 2020, 17, 1836. [Google Scholar] [CrossRef]
- Burks, D.J.; Norris, S.; Kauffman, K.M.; Joy, A.; Arevalo, P.; Azad, R.K.; Wildschutte, H. Environmental Vibrios Represent a Source of Antagonistic Compounds That Inhibit Pathogenic Vibrio cholerae and Vibrio parahaemolyticus Strains. Microbiologyopen 2017, 6, e00504. [Google Scholar] [CrossRef]
- Froelich, B.; Oliver, J. Increases in the Amounts of Vibrio spp. in Oysters upon Addition of Exogenous Bacteria. Appl. Environ. Microbiol. 2013, 79, 5208–5213. [Google Scholar] [CrossRef]
- Kaufman, G.; Bej, A.; Bowers, J.; DePaola, A. Oyster-to-Oyster Variability in Levels of Vibrio parahaemolyticus. J. Food Prot. 2016, 66, 125–129. [Google Scholar] [CrossRef]
- Lewis, T.; Brown, M.; Abell, G.; McMeekin, T.; Sumner, J. Pathogenic Vibrio Parahaemolyticus in Australian Oysters; Project No. 2002/409; Fisheries Research and Development Corporation: Canberra, Australia, 2002; pp. 1–29. [Google Scholar]
- Williams, N.L.R.; Siboni, N.; King, W.L.; Balaraju, V.; Bramucci, A.; Seymour, J.R. Latitudinal Dynamics of Vibrio along the Eastern Coastline of Australia. Water 2022, 14, 2510. [Google Scholar] [CrossRef]
- Machado, A.; Bordalo, A.A. Detection and Quantification of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in Coastal Waters of Guinea-Bissau (West Africa). EcoHealth 2016, 13, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Montaño, A.M.; Luis-Villasenor, I.E.; Pina-Valdez, P.; Gomez-Gil, B.; Lizarraga-Partida, M.L. Spatiotemporal Distribution of Vibrio parahaemolyticus in Relation to Environmental Parameters in a Coastal Lagoon on the Pacific Coast of Northwestern Mexico. Cienc. Mar. 2018, 44, 141–153. [Google Scholar] [CrossRef]
- Robles, A.L.; Félix, E.A.; Gomez-Gil, B.; Ramírez, E.I.Q.; Nevárez-Martínez, M.; Noriega-Orozco, L. Relationship of Aquatic Environmental Factors with the Abundance of Vibrio cholerae, Vibrio parahaemolyticus, Vibrio mimicus and Vibrio vulnificus in the Coastal Area of Guaymas, Sonora, Mexico. J. Water Health 2013, 11, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Villalba, J.; Arreola-Lizárraga, A.; Burrola-Sánchez, S.; Hoyos-Chairez, F. Growth, Condition, and Survival of the Pacific Oyster Crassostrea gigas Cultivated within and Outside a Subtropical Lagoon. Aquaculture 2010, 300, 128–136. [Google Scholar] [CrossRef]
- Nowland, S.J.; O’Connor, W.A.; Penny, S.S.; Southgate, P.C. Monsoonally Driven Reproduction in the Tropical Black-Lip Rock Oyster Saccostrea echinata (Quoy & Gaimard, 1835) in Northern Australia. J. Shellfish Res. 2019, 38, 89–100. [Google Scholar] [CrossRef]
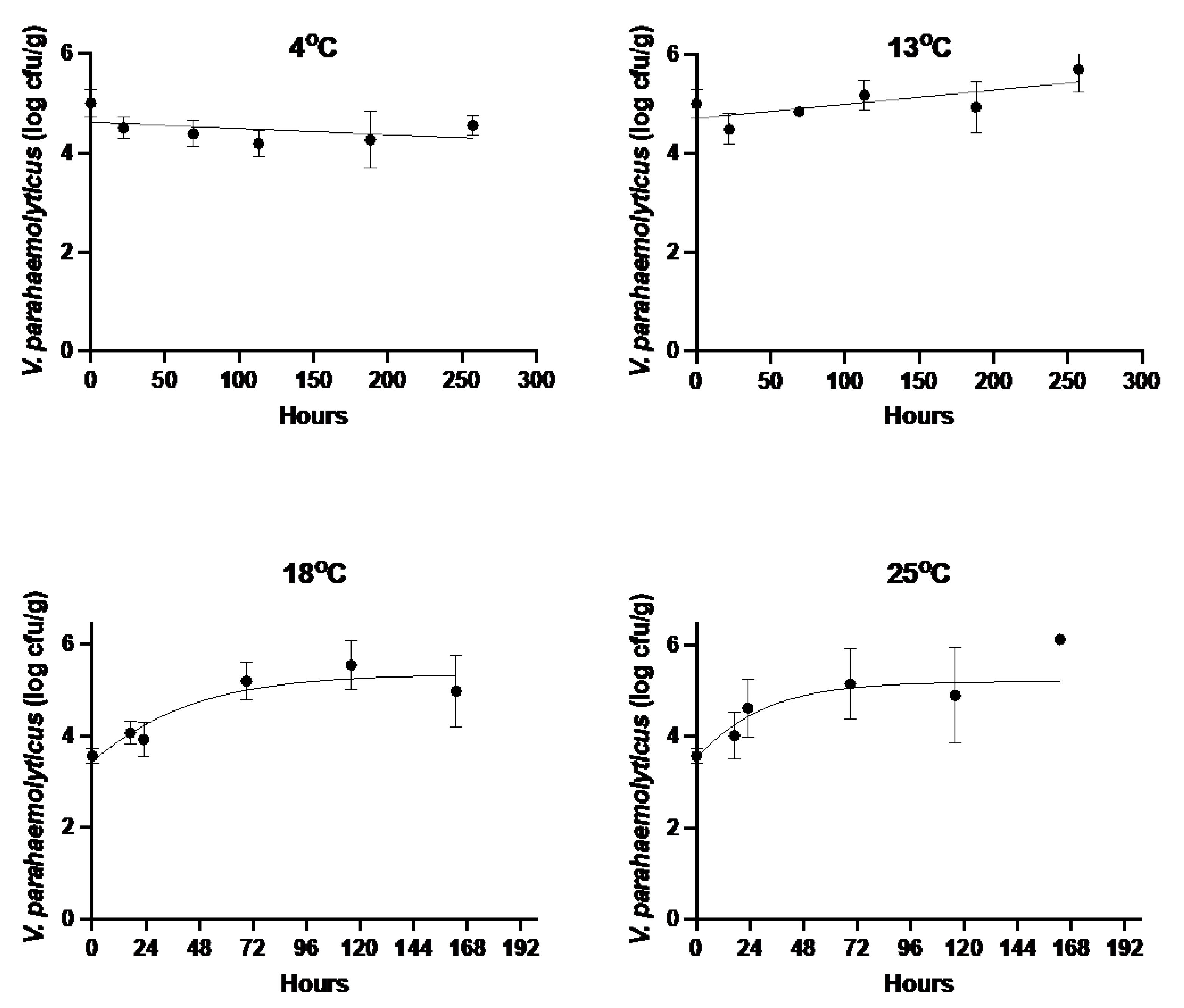
| Storage Temperature (°C) | Growth Rate (Log CFU/h ± SE) | Maximum Population Density (log10 CFU/g ± SE) | Goodness of Fit (RMSE) |
|---|---|---|---|
| 4 | −0.0013 ± 0.0007 | ND | 0.390 |
| 13 | 0.0029 ± 0.0009 | ND | 0.408 |
| 18 | 0.032 ± 0.011 | 5.31 ± 0.245 | 0.463 |
| 25 | 0.047 ± 0.021 | 5.14 ± 0.394 | 0.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padovan, A.C.; Turnbull, A.R.; Nowland, S.J.; Osborne, M.W.J.; Kaestli, M.; Seymour, J.R.; Gibb, K.S. Growth of V. parahaemolyticus in Tropical Blacklip Rock Oysters. Pathogens 2023, 12, 834. https://doi.org/10.3390/pathogens12060834
Padovan AC, Turnbull AR, Nowland SJ, Osborne MWJ, Kaestli M, Seymour JR, Gibb KS. Growth of V. parahaemolyticus in Tropical Blacklip Rock Oysters. Pathogens. 2023; 12(6):834. https://doi.org/10.3390/pathogens12060834
Chicago/Turabian StylePadovan, Anna C., Alison R. Turnbull, Samantha J. Nowland, Matthew W. J. Osborne, Mirjam Kaestli, Justin R. Seymour, and Karen S. Gibb. 2023. "Growth of V. parahaemolyticus in Tropical Blacklip Rock Oysters" Pathogens 12, no. 6: 834. https://doi.org/10.3390/pathogens12060834
APA StylePadovan, A. C., Turnbull, A. R., Nowland, S. J., Osborne, M. W. J., Kaestli, M., Seymour, J. R., & Gibb, K. S. (2023). Growth of V. parahaemolyticus in Tropical Blacklip Rock Oysters. Pathogens, 12(6), 834. https://doi.org/10.3390/pathogens12060834





