1. Introduction
Equine Herpesvirus type 1 (EHV-1) is a double-stranded DNA virus that typically causes mild respiratory disease. However, viremia can lead to late-term abortion, neonatal foal death and neurologic disease (equine herpesvirus myeloencephalopathy or EHM) [
1,
2,
3]. The virus is ubiquitous in equine populations and affects horses of every breed and discipline. Once a horse is infected, the virus localizes to trigeminal ganglia or lymphoid tissues for latent genome maintenance [
4,
5].
While the virus is latent, the animals are not infectious and are clinically normal. However, the virus can be reactivated during times of stress, such as transport or competition. The recrudescence of EHV-1 infections is sporadic but has also been attributed to the initiation of outbreaks, which have proven to be damaging for multiple facets of the equine industry. Recrudescence is likely responsible for the spread of EHV-1 to susceptible horses, resulting in outbreaks [
6].
One of the most devastating EHV-1 outbreaks occurred in Europe, starting in February 2021 during an international show jumping competition. Ten countries were involved with at least 118 horses infected, 18 of which died. Equestrian events across Europe were halted until the middle of April 2021, having profound economic effects across the industry [
7].
Considering the role that latency, and the reactivation thereof, plays in maintaining the virus within a population and initiating outbreaks, respectively, the mechanisms of recrudescence, including its environmental triggers, are of key importance for disease control. These facets of EHV-1 are currently poorly understood. Combined with a lack of feasibility of the ante mortem detection of latently infected horses, there is a profound paucity in well-rounded EHV-1 disease management strategies.
Understanding the carriage rate of latent EHV-1 in different regions is essential for the management of the disease. Currently, there are few studies regarding the prevalence of latent EHV-1 in horses, and none specifically in Virginia. The objective of the current study was to estimate the prevalence of latent EHV-1 and compare the frequency of each variant in the submandibular lymph nodes of horses in Virginia.
2. Materials and Methods
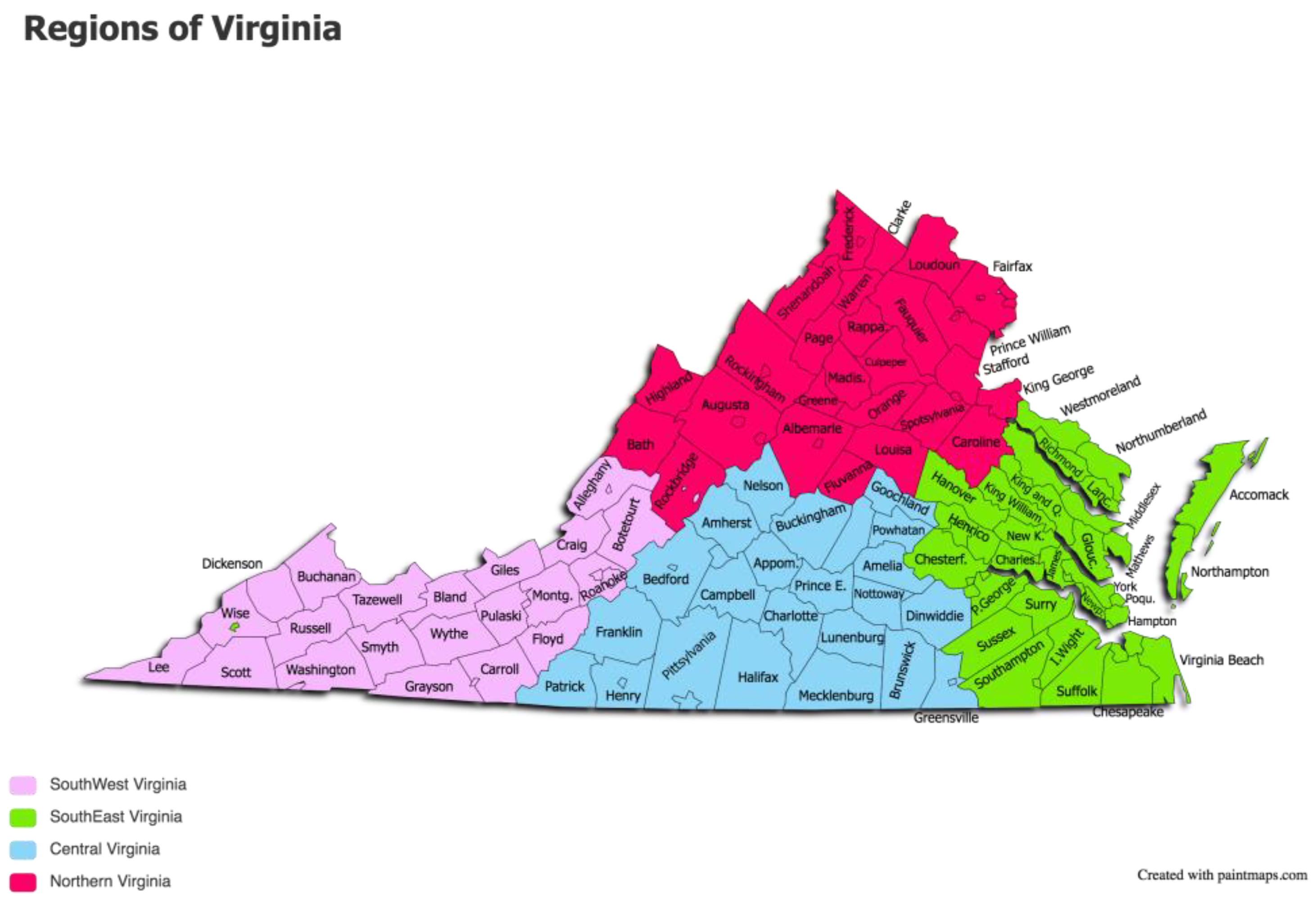
The study sample consisted of a total of 63 equids submitted for necropsy to diagnostic labs in 5 different pre-determined regions of Virginia from January 2020 to March 2021. The pre-determined regions of Virginia were Northern Virginia (NVA), Southwest Virginia (SWVA), Central Virginia, Southeast Virginia (SEVA), and the Outskirts (OVA) (see
Figure 1). Inclusion criteria included horses and mules over the age of 2 years that did not have signs of acute respiratory or neurologic disease that could have been due to EHV-1.
2.1. SMLN Collection
Submandibular lymph nodes (SMLNs) were collected from each study animal during routine postmortem examination at each of 5 regional diagnostic laboratories in Virginia. The entirety of the SMLNs were retrieved from each study animal using sterile, disposable instruments. The samples were placed into sterile whirlpacks and immediately stored at −80 °C until further processing. Fresh, disposable instruments were used for sample collections from each animal to avoid cross-contamination. Sample collection procedures were approved by the Institutional Animal Care and Use Committee of Virginia-Maryland College of Veterinary Medicine prior to collection.
2.2. DNA Isolation
DNA extraction was performed under a biosafety cabinet with sterile labware to reduce environmental contamination and DNA carryover between samples. DNA was extracted from all samples using a Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Based on the manufacturer’s recommendations, a 25 mg piece of SMLN from each horse was placed in individual Petri dishes and minced. The minced tissue was transferred to a clean microcentrifuge tube containing Buffer ATL (Qiagen, Valencia, CA, USA) and 40 µL proteinase K, then digested overnight at 56 °C. A mixture of the tissue lysate, buffer AL and ethanol was pipetted into a DNeasy Mini spin column (Qiagen), then centrifuged for 1 min at 13,300 rpm. The supernatant was discarded, and Buffer AW1 (Qiagen) was added to the spin column, and then centrifuged again. The spin column was then washed with Buffer AW2 (Qiagen), which was centrifuged for 3 min. The DNA was eluted in 100 µL of Buffer AE (Qiagen, Valencia, CA, USA) via incubation at room temperature for 1 min before centrifugation for 1 min at 13,300 rpm. The DNA was stored at −20 °C until downstream processing took place.
2.3. Polymerase Chain Reaction
Each sample of purified nucleic acid underwent a PCR preamplification step as previously described [
7]. Briefly, a reaction was performed using Advantage 2 Polymerase Mix (Takara Bio USA, San Jose, CA, USA) containing the target primers for the
gB gene and the
ORF30 gene of EHV-1 in a 50 µL total volume. The primer mix was amplified in a thermocycler (Biometra, Gottingen, Germany) under the following conditions: 1 min at 94 °C, followed by 25 cycles of 15 s at 94 °C, 15 s at 55 °C and 45 s at 70 °C, followed by 5 min at 70 °C.
All samples were tested for the presence of the gB and 2 variants (A and G) of the DNA polymerase (
ORF30) gene of EHV-1 as previously described [
8]. In total, 9 of the 63 samples were also tested for the C variant of the
ORF30 gene as previously described [
9]. Nine samples were also tested for the presence of the C variant of EHV-1.
Four positive controls were used in this study as follows:
An SMLN from a horse with confirmed natural latent infection with the G2254 strain of EHV-1 was used as a positive control of SMLN; DNA from a horse with confirmed, natural infection with the C2254 strain of EHV-1; DNA from wild-type EHV-1 (National Veterinary Services Laboratory, Ames, IA, USA) as a control for A2254, and DNA from EHV-1 vaccine (Rhinomune, Boehringer Ingelheim Vetmedica, St. Joseph, MO, USA) as a control for G2254.
3. Results
Samples were received from a total of 62 horses and 1 mule from five pre-determined regions of Virginia: 17 from NVA, 30 from SWVA, 11 central, 1 SEVA, 4 OVA, and the rest of the samples were from an unknown region of Virginia. There were 35 geldings and 18 mares, and the remainder were undefined. The age of the horses and mule ranged from the age of 3 years to 25 years; 11 were 5–7 years, and 8 were over 20 years (median 12.0 years).
Several breeds were represented, most of which were quarter horses (17 horses), thoroughbreds (14), warmbloods (5) and unlisted (14), but these also included American paint horses (2), standardbreds (2) a saddlebred (1), a Percheron (1), a Morgan (1), a mustang (1), a Tennessee walking horse (1), ponies (2), a Gypsy Vanner (1), a Norwegian fjord (1), an Arabian (1), a Haflinger (1), a spotted saddle horse (1) and a mule (1).
The control positive SMLN consistently tested PCR positive for the
gB gene of the EHV-1 and G variants of the
ORF30 with a Ct of 25.54 and 25.92, respectively. The A
2254 and C
2254 controls had a Ct of 26.08 and 25.90, respectively. All of the 63 SMLN samples were qPCR negative for the
gB gene of EHV-1. In total, 63/63 were negative for the
ORF30 gene for the A, G and C variants with a 95% confidence interval of 0–5.75 [
10].
4. Discussion
This study represents the first evaluation of the prevalence of EHV-1 latency in Virginia with an attempt to evaluate throughout the region. We were anticipating a frequency of detection somewhere between 3.3 percent and 54 percent based on studies by Pusterla and others [
11] and Allen and others [
12], respectively. In this current study, we found a low estimated prevalence. The Californian study, which was in a sample population of 147 horses, 4 mules and 3 donkeys, had low prevalence in submandibular lymph nodes as well [
11]. Our findings were dissimilar to those of the study in Kentucky where they assessed the prevalence of EHV-1 in SMLN of 132 thoroughbred mares in Kentucky [
12]. Although we did not find any positives in this population of horses, we were able to detect EHV-1 in samples that were confirmed positive. Furthermore, the confidence interval of our current study indicates a similar estimated prevalence in submandibular lymph nodes to the Californian study. This does not equate to the zero prevalence of EHV-1 in horses, as our findings are an estimate of the prevalence in horses in the study area. For instance, the confidence interval indicates the range that the prevalence might have been, given that we had zero detections out of 63 equids sampled. Additionally, we cannot rule out latency in other tissues, such as the retropharyngeal lymph nodes or trigeminal ganglia, that have been shown to carry EHV-1 in latency [
13]. It is plausible that the overall prevalence of EHV-1 of this population of horses in Virginia is low; however, follow-up studies in a larger population of horses and multiple sampling sites is likely warranted to make a more accurate prevalence conclusion in this region. Based on a recent affinity study, samples from retropharyngeal lymph nodes, pharyngeal roofs and trigeminal ganglion should be included with submandibular lymph node samples for future prevalence studies [
13]. Thus, the estimated prevalence of EHV-1 in, specifically, the SMLNs of horses in Virginia is low.
Differences in prevalence in this sample type could be related to the variability in disciplines or horse use across the country. For instance, in 2003, 39% of California’s horse population was used for showing or racing [
14]. This is compared to a report from 2001 indicating that 24.5% of Virginia horses were used for showing or racing [
15]. The population of horses used in the Allen paper were specifically thoroughbred broodmares. These horses are required to endure live cover breeding and thus are typically transported frequently and interact with horses from a wide geographic area. These discipline differences should be considered when looking at the variable prevalences reported.
While the lymph node sample size in this current study (25 mg) is small compared to the entire organ, the PCR results from the Pusterla 2010 study showed that EHV-1 is evenly distributed in the tissue during latency. Our detection method consisted of PCR pre-amplification followed by traditional qPCR, which appropriately detected EHV-1 in our positive control lymph node. This is also true considering our sample size of 25 mg compared to previous studies using 500 mg [
11,
12].
The low estimated prevalence in this population of horses indicates that the focus of outbreak mitigation should be on preventing exogenous infection with consistent vaccination, prompt diagnosis and diligent biosecurity. Current vaccine recommendations for EHV-1 are bi-annual intramuscular inoculations [
16]. It is also prudent to encourage horse care-takers to be meticulous in their monitoring and timely in their outreach for veterinary intervention when early clinical signs are noted. In horses that develop EHV-1 infection and recover, care should be taken to abate the likelihood of recrudescence. This can be achieved by minimizing stress and providing prudent biosecurity, particularly with those horses; continuing every 6-month vaccination; and remaining diligent with monitoring and prompt diagnosis and appropriate treatment.
In conclusion, the results of the current study demonstrated a low estimated prevalence of latent EHV-1 DNA in, specifically, submandibular lymph nodes in this population of horses in Virginia. Despite this, the mainstay for outbreak prevention and mitigation continues to focus on minimizing risks and using appropriate and diligent biosecurity.
Author Contributions
Conceptualization, S.P. and N.S.; methodology, N.S.; validation, N.S., R.F. and K.L.; formal analysis, N.S.; investigation, N.S.; resources, K.L.; data curation, N.S.; writing—original draft preparation, N.S.; writing—review and editing, K.L., N.S. and R.F.; visualization, N.S. and S.P.; supervision, R.F. and K.L.; project administration, K.L.; funding acquisition, N.S., R.F. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Virginia Horse Industry Board, 467015.
Institutional Review Board Statement
Sample collection procedures were approved by the Division of Scholarly Integrity and Research Compliance; Institutional Animal Care and Use Committee of Virginia-Maryland College of Veterinary Medicine (Approval Code: 19-216; Approval Date: 18 October 2019) prior to collection.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is provided within this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kohn, C.W.; Fenner, W.R. Equine Herpes Myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 1987, 3, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.; Davis-Poynter, N.; Flaminio, M.; Horohov, D.; Osterrieder, N.; Pusterla, N.; Townsend, H. Equine Herpesvirus-1 Consensus Statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef]
- Patel, J.; Heldens, J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—Epidemiology, disease and immunoprophylaxis: A brief review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef]
- Welch, H.M.; Bridges, C.G.; Lyon, A.M.; Griffiths, L.; Edington, N. Latent equid herpesviruses 1 and 4: Detection and distinction using the polymerase chain reaction and co-cultivation from lymphoid tissues. J. Gen. Virol. 1992, 73, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.D.; Borchers, K.; Thackray, A.M.; Field, H.J. The trigeminal ganglion is a location for equine herpesvirus 1 latency and reactivation in the horse. J. Gen. Virol. 1994, 75, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Dunowska, M. A review of equid herpesvirus 1 for the veterinary practitioner. Part B: Pathogenesis and epidemiology. N. Z. Vet. J. 2014, 62, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Termine, C.; Akerstrom, G.; Paixao, G. Management of an EHV-1 outbreak at FEI events and its international impact. Vet. Rec. 2021, 189, 1–3. [Google Scholar] [CrossRef]
- Smith, K.L.; Li, Y.; Breheny, P.; Cook, R.F.; Henney, P.J.; Sells, S.; Pronost, S.; Lu, Z.; Crossley, B.M.; Timoney, P.J.; et al. New Real-Time PCR Assay Using Allelic Discrimination for Detection and Differentiation of Equine Herpesvirus-1 Strains with A 2254 and G 2254 Polymorphisms. J. Clin. Microbiol. 2012, 50, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.; Thieulent, C.; Fortier, C.; Hue, E.S.; Marcillaud-Pitel, C.; Pléau, A.; Deslis, A.; Guitton, E.; Paillot, R.; Pronost, S. Identification of a New Equid Herpesvirus 1 DNA Polymerase (ORF30) Genotype with the Isolation of a C2254/H752 Strain in French Horses Showing no Major Impact on the Strain Behaviour. Viruses 2020, 12, 1160. [Google Scholar] [CrossRef] [PubMed]
- OpenEpi. Available online: https://www.openepi.com/Menu/OE_Menu.htm (accessed on 1 April 2023).
- Pusterla, N.; Mapes, S.; Wilson, W.D. Prevalence of equine herpesvirus type 1 in trigeminal ganglia and submandibular lymph nodes of equids examined postmortem. Vet. Rec. 2010, 167, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Bolin, D.C.; Bryant, U.; Carter, C.N.; Giles, R.C.; Harrison, L.R.; Hong, C.B.; Jackson, C.B.; Poonacha, K.; Wharton, R.; et al. Prevalence of latent, neuropathogenic equine herpesvirus-1 in the Thoroughbred broodmare population of central Kentucky. Equine Vet. J. 2008, 40, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Samoilowa, S.; Giessler, K.S.; Torres, C.E.M.; Hussey, G.S.; Allum, A.; Fux, R.; Jerke, C.; Kiupel, M.; Matiasek, K.; Sledge, D.G.; et al. Equid herpesvirus-1 Distribution in Equine Lymphoid and Neural Tissues 70 Days Post Infection. Pathogens 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Kilby, E.R. The demographics of the U.S. equine population. In The State of the Animals 2007; Salem, D.J., Rowan, A.N., Eds.; Humane Society Press: Washington, DC, USA, 2007; pp. 175–205. [Google Scholar]
- Manheimer, S.; Mueller, D.; Foard, K. 2001 Virginia Equine Report. Virginia Agricultural Statistics Service: Richmond, VA, USA, 2002; pp. 1–19. [Google Scholar]
- American Association of Equine Practitioners Guidelines on Vaccination. Available online: http://www.aaep.org/ (accessed on 1 April 2023).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).