Molecular and Serological Identification of Anaplasma marginale and Borrelia burgdorferi in Cattle and Ticks from Nuevo Leon, Northern Mexico
Abstract
1. Introduction
2. Materials and Methods
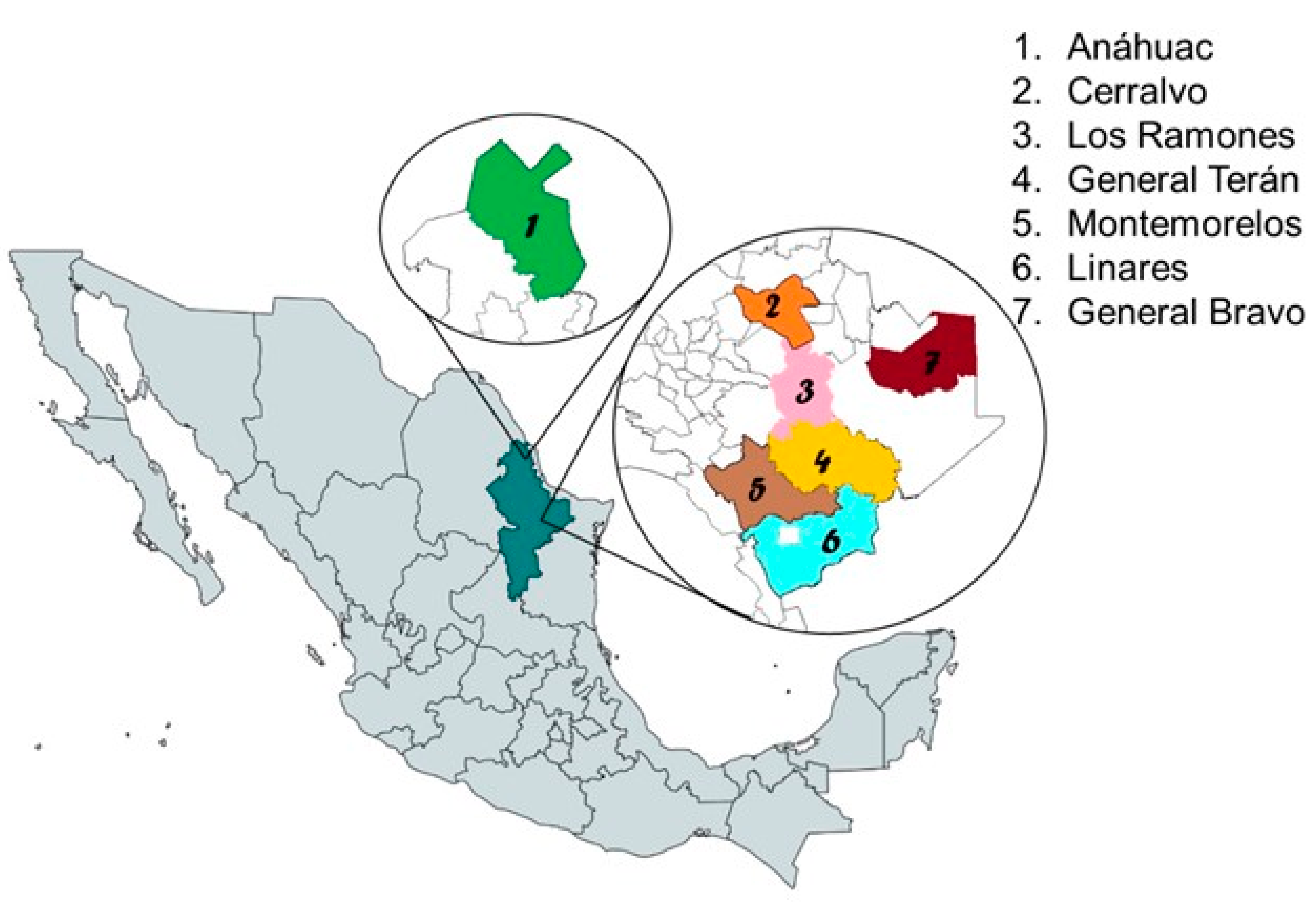
2.1. Study Areas
2.2. Ticks and Bovine Blood Collections
2.3. DNA Extraction and PCR Amplification
2.4. Serological Analysis
2.5. Statistical Analysis
3. Results
3.1. Ticks Collected from Cattle
3.2. Ticks Sample Size from Cattle for PCR Analysis
3.3. Ticks Analyzed Using PCR to Identify A. marginale
3.4. Bovine Blood Samples Analyzed Using PCR to Identify A. maginale
3.5. Ticks and Blood Bovine Samples Negative to B. burgdorferi s.l. by PCR and IFA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Schmid, G.P.; Hyde, F.W.; Steingerwalt, A.G.; Brenner, D.J. Borrelia burgdorferi sp. nov.: Etiological agent of Lyme disease. Int. J. Syst. Bacterio. 1984, 34, 496–497. [Google Scholar] [CrossRef]
- Parker, J.L.; White, K.K. Lyme borreliosis in cattle and horses: A review of the literature. Cornell. Vet. 1992, 82, 253–274. [Google Scholar] [PubMed]
- Colunga-Salas, P.; Sánchez-Montes, S.; Volkow, P.; Ruíz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef]
- Ferreira, G.C.M.; Canozzi, M.E.A.; Peripolli, V.; de Paula Moura, G.; Sánchez, J.; Martins, C.E.N. Prevalence of bovine Babesia spp., Anaplasma marginale, and their co-infections in Latin America: Systematic review-meta-analysis. Ticks Tick Borne Dis. 2022, 13, 101967. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; Ortiz, M.A.G.; Ocampo, R.J.; Murguía, C.A.V. Molecular epidemiology of bovine anaplasmosis with a particular focus in Mexico. Infect Genet Evol 2009, 9, 1092–1101. [Google Scholar] [CrossRef]
- Almazán, C.; Medrano, C.; Ortiz, M.; de la Fuente, J. Genetic diversity of Anaplasma marginale strains from an outbreak of bovine anaplasmosis in an endemic area. Vet. Parasitol. 2008, 158, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Pérez, G.; Vargas, M.; Solórzano-Santos, F.; Rivera, A.; Polaco, O.J.; Alvarado, L.; Muñoz, O.; Torres, J. Demonstration of Borrelia burgdorferi sensu stricto infection in ticks from the northeast of Mexico. Clin. Microbiol. Infect. 2009, 15, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística, Geografía e Informática. Encuesta Nacional Agropecuaria. Available online: https://www.inegi.org.mx/programas/ena/2019/ (accessed on 5 December 2022).
- Instituto Nacional de Estadística y Geografía. Available online: https://cuentame.inegi.org.mx/monografias/informacion/nl/territorio/clima.aspx?tema=me&e=19 (accessed on 5 December 2022).
- Guzmán-Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel-Parra, G.; Pérez, T.M. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa 2011, 2998, 16–38. [Google Scholar] [CrossRef]
- Guzmán-Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel-Parra, G.; Rivas, G.; Pérez, T.M. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: Hosts, geographical distribution and new records. Zookeys 2016, 569, 1–22. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. Available online: http://www.mapress.com/zootaxa/2010/f/z02528p028f.pdf (accessed on 8 August 2022). [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. Genus Rhipicephalus (Acari, Ixodidae). In A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Ferrer, C.; Colom, F.; Frasés, S.; Mulet, E.; Abad, J.L.; Alió, J.L. Detection and identification of fungal pathogens by PCR and ITS2 and 5.8 S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 2001, 39, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Jaulhac, B.; Heller, R.; Limbach, F.X.; Hansmann, Y.; Lipsker, D.; Monteil, H.; Sibilia, J.; Piémont, Y. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with Lyme arthritis. J. Clin. Microbiol. 2000, 38, 1895–1900. [Google Scholar] [CrossRef]
- Bilgiç, H.; Karagenç, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013, 133, 222–229. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Division of Health Informatics & Surveillance (DHIS), Center for Surveillance, Epidemiology & Laboratory Services (CSELS). Available online: http://www.cdc.gov/epiinfo (accessed on 9 January 2023).
- Castañeda-Ortiz, E.J.; Ueti, M.W.; Camacho-Nuez, M.; Mosqueda, J.J.; Mousel, M.R.; Johnson, W.C.; Palmer, G.H. Association of Anaplasma marginale strain superinfection with infection prevalence within tropical regions. PLoS ONE 2015, 10, e0120748. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Mata-Mendez, Y.; Pérez-Gutierrez, E.; Wagner, G. The effect of management factors on the seroprevalence of Anaplasma marginale in Bos indicus cattle in the Mexican tropics. Trop. Anim. Health Prod. 2004, 36, 135–143. [Google Scholar] [CrossRef]
- Vega, L.E.O.; Rodríguez, S.D.; Alarcón, G.J.C.; Flores, R.L.; Ocampo, R.J.; Ortiz, M.Á.G.; Preciado de la Torre, J.F.; Ramírez, E.E.R. Anaplasma marginale field challenge: Protection by an inactivated immunogen that shares partial sequence of msp1α variable region with the challenge strain. Vaccine 2007, 25, 519–525. [Google Scholar] [CrossRef]
- López-Sánchez, F.; Canto-Alarcón, G.J.; Falcón-Neri, A.; Aboytes-Torres, R. Anaplasmosis and babesiosis prevalence in the Experimental Station of Aldama, Tamaulipas. Técnica Pecu. México 1984, 46, 88–92. [Google Scholar]
- Fragoso, S.G.; Suazo, F.M. Prevalencia de anticuerpos contra Anaplasma marginale y Babesia spp. en la zona centro del estado de Guerrero. Rev. Mex Cienc. Pecu 1984, 47, 133–136. Available online: https://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/viewFile/3313/2733 (accessed on 8 August 2022).
- Cossio-Bayugar, R.; Rodriguez, S.D.; Garcia-Ortiz, M.A.; Garcia-Tapia, D.; Aboytes-Torres, R. Bovine anaplasmosis prevalence in northern Veracruz state, Mexico. Prev. Vet. Med. 1997, 32, 165–170. [Google Scholar] [CrossRef] [PubMed]
- García-Tapia, D.G.; Rojas, M.L.; Bayugar, R.C.; Vazquez, Z.G.; Ortiz, M.A.G.; Jalil, P.D.; Torres, R.A. Seroprevalencia de anaplasmosis en explotaciones bovinas de 18 municipios de la zona norte de Veracruz. Rev. Mex Cienc. Pecu 1996, 34, 38–45. Available online: https://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/viewFile/698/696 (accessed on 10 August 2022).
- Osorno, M.B.; Ristic, M. Anaplasmosis bovina con énfasis en control, diagnostico, distribución de la enfermedad en México y uso de una vacuna atenuada de Anaplasma marginale. Vet. México 1977, 8, 85–98. [Google Scholar]
- Estrada-Peña, A.; García, Z.; Sánchez, H.F. The distribution and ecological preferences of Boophilus microplus (Acari: Ixodidae) in Mexico. Exp. Appl. Acarol. 2006, 38, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-de la Fuente, V.; Merino-Charrez, O.; Tovar-Carman, E.; Rodríguez-Camarillo, S.D.; Lagunes-Quintanilla, R.E.; Muñoz-Tenería, F.A.; Contreras, M.; de la Fuente, J. Differential expression analysis for subolesin in Rhipicephalus microplus infected with Anaplasma marginale. Exp. Appl. Acarol. 2018, 76, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Szabó, M.; Labruna, M.; Mosqueda, J.; Merino, O.; Tarragona, E.; Venzal, J.M.; de la Fuente, J. Towards an effective, rational and sustainable approach for the control of cattle ticks in the Neotropics. Vaccines 2020, 8, 9. [Google Scholar] [CrossRef]
- Arroyave, C.M.; Tamez-González, R. Enfermedad de Lyme. Informe de dos casos. Bol. Med. Hosp. Infant. Mex 1994, 51, 117–121. [Google Scholar]
- Gordillo-Pérez, G.; Torres, J.; Solórzano-Santos, F.; Garduño-Bautista, V.; Tapia-Conyer, R.; Muñoz, O. Estudio seroepidemiológico de borreliosis de Lyme en la Ciudad de México y el noreste de la República Mexicana. Salud. Publ. Mex 2003, 45, 351–355. [Google Scholar] [CrossRef]
- Skinner-Taylor, C.M.; Flores-González, M.S.; Esquivel-Valerio, J.A.; Salinas-Meléndez, J.A.; Salinas-Palacios, C.K.; Rodríguez-Amado, J.; Garza, E.M.A. Evidencia de la enfermedad de Lyme en una población de alto riesgo del noreste de México. Med. Univer. 2007, 9, 105–111. Available online: https://www.medigraphic.com/pdfs/meduni/mu-2007/mu073c.pdf (accessed on 8 August 2022).
- Skinner-Taylor, C.M.; Flores, M.S.; Salinas, J.A.; Arevalo-Nińo, K.; Galán-Wong, L.J.; Maldonado, G.; Garza-Elizondo, M.A. Antibody profile to Borrelia burgdorferi in veterinarians from Nuevo León, Mexico, a non-endemic area of this zoonosis. Rheumatology 2016, 54, 97–102. [Google Scholar] [CrossRef]
- Salinas-Meléndez, J.A.; Tamez-González, R.; Welsh-Lozano, O.; Barrera-Saldaña, H.A. Detection of Borrelia burgdorferi DNA in human skin biopsies and dog synovial fluid by the polymerase chain reaction. Rev. Latinoam. Microbiol. 1995, 37, 7–10. [Google Scholar] [PubMed]
- Salinas-Meléndez, J.A.; Avalos-Ramírez, R.; Riojas-Valdez, V.M.; Martínez-Muñoz, A. Serological survey of canine borreliosis. Rev. Latinoam. Microbiol. 1999, 41, 1–4. [Google Scholar]
- Rodríguez-Rojas, J.J.; Rodríguez-Moreno, Á.; Sánchez-Casas, R.M.; Hernández-Escareño, J.J. Molecular detection of Leptospira interrogans and Borrelia burgdorferi in wild rodents from Mexico. Vector Borne Zoonotic Dis. 2020, 20, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Galaviz-Silva, L.; Pérez-Treviño, K.C.; Molina-Garza, Z.J. Distribution of ixodid ticks on dogs in Nuevo León, Mexico, and their association with Borrelia burgdorferi sensu lato. Exp. Appl. Acarol. 2013, 61, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ocampo, R.; Vega y Murguía, C.A.; Oviedo-Ortega, N.; Rojas-Ramírez, E.E.; García-Ortiz, M.A.; Preciado de la Torre, J.F.; Rodríguez-Camarillo, S.D. Diversidad genética de la región variable de los genes msp1a y msp4 en cepas de Anaplasma marginale de México. Rev. Mex Cienc. Pecu 2012, 3, 373386. Available online: https://www.scielo.org.mx/scielo.php?pid=S2007-11242012000300008&script=sci_arttext (accessed on 8 August 2022).
- Martínez-Ocampo, F.; Quiroz-Castañeda, R.E.; Amaro-Estrada, I.; Dantán-González, E.; de la Torre, J.F.; Rodríguez-Camarillo, S. Whole-genome sequencing of mexican strains of Anaplasma marginale: An approach to the causal agent of bovine anaplasmosis. Int. J. Genom. 2020, 2020, 5902029. [Google Scholar] [CrossRef]
| Localities | Rhipicephalus microplus (F:M) | Amblyomma spp. (F:M) | Dermacentor variabilis (F:M) | Total (F:M) |
|---|---|---|---|---|
| Linares | 860 (734:126) | 1 (0:1) | 1 (1:0) | 862 (735:127) |
| General Teran | 653 (584:69) | 7 (7:0) | 0 | 660 (591:69) |
| General Bravo | 256 (182:74) | 21 (3:18) | 0 | 277 (185:92) |
| Cerralvo | 523 (469:54) | 10 (8:2) | 0 | 533 (477:56) |
| Montemorelos | 355 (297:58) | 53 (32:21) | 0 | 408 (329:79) |
| Anahuac | 134 (120:14) | 0 | 0 | 134 (120:14) |
| Los Ramones | 5 (5:0) | 1 (1:0) | 0 | 6 (6:0) |
| Total (F:M) | 2786 (2391:395) | 93 (51:42) | 1 (1:0) | 2880 (2443:437) |
| Localities | Rhipicephalus microplus (%) | Amblyomma mixtum (%) | Amblyomma tenellum (%) | Dermacentor variabilis (%) | Total (%) |
|---|---|---|---|---|---|
| Linares | 20/105 (9.5) | 0 | 0 | 1/1 (100) | 21/106 (19.8) |
| General Teran | 5/110 (4.5) | 0 | 0/4 (0) | 0 | 5/114 (4.4) |
| General Bravo | 3/60 (5.0) | 0/4 (0) | 0 | 0 | 3/64 (4.7) |
| Cerralvo | 1/65 (1.5) | 0/5 (0) | 0 | 0 | 1/70 (1.4) |
| Montemorelos | 9/52 (17.3) | 0 | 5/23 (21.7) | 0 | 14/75 (18.7) |
| Anahuac | 0/10 (0) | 0 | 0 | 0 | 0/10 (0) |
| Los Ramones | 0/2 (0) | 0/1 (0) | 0 | 0 | 0/3 (0) |
| Total (%) | 38/404 (9.4) | 0/10 (0) | 5/27 (18.5) | 1/1 (100) | 44/442 (9.9) |
| Localities | Total Samples | + Samples | Total (%) |
|---|---|---|---|
| Linares | 95 | 61 | 64.21 |
| General Teran | 90 | 34 | 37.78 |
| General Bravo | 45 | 32 | 71.11 |
| Cerralvo | 53 | 49 | 92.45 |
| Montemorelos | 44 | 36 | 81.82 |
| Anahuac | 5 | 1 | 20.00 |
| Los Ramones | 5 | 1 | 20.00 |
| Total | 337 | 214 | 63.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Ramírez, J.Á.; Rodríguez-Rojas, J.J.; Hernández-Escareño, J.J.; Galan-Huerta, K.-A.; Rebollar-Téllez, E.A.; Moreno-Degollado, G.; Medina-De la Garza, C.E.; Sánchez-Casas, R.M.; Fernández-Salas, I. Molecular and Serological Identification of Anaplasma marginale and Borrelia burgdorferi in Cattle and Ticks from Nuevo Leon, Northern Mexico. Pathogens 2023, 12, 784. https://doi.org/10.3390/pathogens12060784
Ortiz-Ramírez JÁ, Rodríguez-Rojas JJ, Hernández-Escareño JJ, Galan-Huerta K-A, Rebollar-Téllez EA, Moreno-Degollado G, Medina-De la Garza CE, Sánchez-Casas RM, Fernández-Salas I. Molecular and Serological Identification of Anaplasma marginale and Borrelia burgdorferi in Cattle and Ticks from Nuevo Leon, Northern Mexico. Pathogens. 2023; 12(6):784. https://doi.org/10.3390/pathogens12060784
Chicago/Turabian StyleOrtiz-Ramírez, José Ángel, Jorge Jesús Rodríguez-Rojas, Jesús Jaime Hernández-Escareño, Kame-A Galan-Huerta, Eduardo Alfonso Rebollar-Téllez, Gustavo Moreno-Degollado, Carlos E. Medina-De la Garza, Rosa María Sánchez-Casas, and Ildefonso Fernández-Salas. 2023. "Molecular and Serological Identification of Anaplasma marginale and Borrelia burgdorferi in Cattle and Ticks from Nuevo Leon, Northern Mexico" Pathogens 12, no. 6: 784. https://doi.org/10.3390/pathogens12060784
APA StyleOrtiz-Ramírez, J. Á., Rodríguez-Rojas, J. J., Hernández-Escareño, J. J., Galan-Huerta, K.-A., Rebollar-Téllez, E. A., Moreno-Degollado, G., Medina-De la Garza, C. E., Sánchez-Casas, R. M., & Fernández-Salas, I. (2023). Molecular and Serological Identification of Anaplasma marginale and Borrelia burgdorferi in Cattle and Ticks from Nuevo Leon, Northern Mexico. Pathogens, 12(6), 784. https://doi.org/10.3390/pathogens12060784








