Detection of HEV RNA Using One-Step Real-Time RT-PCR in Farrow-to-Finish Pig Farms in Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Virus Concentration and RNA Isolation
2.3. HEV Specific One Step Real-Time RT-PCR
2.4. Limit of Detection (LOD)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emerson, S.U.; Anderson, D.; Arankalle, A.V.; Meng, X.J.; Purdy, M.; Schlauder, G.G.; Tsarev, S.A. Hepevirus. In Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier/Academic Press: London, UK, 2004; pp. 851–855. [Google Scholar]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 97, 537–542. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Prim. 2017, 3, 17086. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Tedder, R.S.; Ijaz, S. Persistent Carriage of Hepatitis E Virus in Patients with HIV Infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Capobianchi, M.R.; Garbuglia, A.R. Epidemiology of Hepatitis E Virus in European Countries. Int. J. Mol. Sci. 2015, 16, 25711–25743. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Pischke, S.; Manns, M.P. Pathogenesis and Treatment of Hepatitis E Virus Infection. Gastroenterology 2012, 142, 1388–1397.e1. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Mai, L.; Zhu, F.-Q.; Pan, X.-F.; Sun, H.-X.; Cao, H.; Shu, X.; Ke, W.-M.; Li, G.; Xu, Q.-H. Influence of chronic HBV infection on superimposed acute hepatitis E. World J. Gastroenterol. 2013, 19, 5904–5909. [Google Scholar] [CrossRef]
- Damiris, K.; Meybodi, M.A.; Niazi, M.; Pyrsopoulos, N. Hepatitis E in immunocompromised individuals. World J. Hepatol. 2022, 14, 482–494. [Google Scholar] [CrossRef]
- Navaneethan, U.; Al Mohajer, M.; Shata, M.T. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int. 2008, 28, 1190–1199. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fer-nandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; et al. Scientific opinion on the public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, 4886–4889. [Google Scholar]
- Baymakova, M.; Terzieva, K.; Popov, R.; Grancharova, E.; Kundurzhiev, T.; Pepovich, R.; Tsachev, I. Seroprevalence of Hepatitis E Virus Infection among Blood Donors in Bulgaria. Viruses 2021, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Teoharov, P.; Kevorkyan, A.; Raycheva, R.; Golkocheva-Markova, E.; Trandeva-Bankova, D.; Andonov, A. Data on the Prevalence of Hepatitis e virus in Bulgaria. Comptes Rendus l’Académie Bulg Sci. 2014, 67, 1427–1432. [Google Scholar]
- Chelli, E.; Suffredini, E.; De Santis, P.; De Medici, D.; Di Bella, S.; D’amato, S.; Gucciardi, F.; Guercio, A.; Ostanello, F.; Perrone, V.; et al. Hepatitis E Virus Occurrence in Pigs Slaughtered in Italy. Animals 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Feurer, C.; Le Roux, A.; Rossel, R.; Barnaud, E.; Dumarest, M.; Garry, P.; Pavio, N. High load of hepatitis E viral RNA in pork livers but absence in pork muscle at French slaughterhouses. Int. J. Food Microbiol. 2018, 264, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pintó, R.M.; Costafreda, M.I.; Bosch, A. Risk Assessment in Shellfish-Borne Outbreaks of Hepatitis A. Appl. Environ. Microbiol. 2009, 75, 7350–7355. [Google Scholar] [CrossRef]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of Hepatitis E Virus Infection in Pigs at the Time of Slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396–1401. [Google Scholar] [CrossRef]
- Di Pasquale, S.; De Santis, P.; La Rosa, G.; Di Domenico, K.; Iaconelli, M.; Micarelli, G.; Martini, E.; Bilei, S.; De Medici, D.; Suffredini, E. Quantification and genetic diversity of Hepatitis E virus in wild boar (Sus scrofa) hunted for domestic consumption in Central Italy. Food Microbiol. 2019, 82, 194–201. [Google Scholar] [CrossRef]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Marutsov, P.; Gospodinova, K.; Kundurzhiev, T.; Petrov, V.; Pepovich, R. Seroprevalence of Hepatitis E Virus Infection Among Wild Boars in Western Bulgaria. Vector-Borne Zoonotic Dis. 2021, 21, 441–445. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Lo, K.H.Y.; Wu, S.; Situ, J.; Chew, N.F.S.; Leung, K.H.; Chan, H.S.Y.; Wong, S.C.Y.; Leung, A.W.S.; et al. Hepatitis E Virus Species C Infection in Humans, Hong Kong. Clin. Infect. Dis. 2021, 75, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Monini, M.; Ostanello, F.; Dominicis, A.; Tagliapietra, V.; Vaccari, G.; Rizzoli, A.; Trombetta, C.M.; Montomoli, E.; Di Bartolo, I. Seroprevalence of Hepatitis E Virus in Forestry Workers from Trentino-Alto Adige Region (Northern Italy). Pathogens 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Baymakova, M.; Sakem, B.; Plochev, K.; Popov, G.; Mihaylova-Garnizova, R.; Kovaleva, V.; Kundurdjiev, T. Epidemiological characteristics and clinical manifestations of hepatitis E virus infection in Bulgaria: A report on 20 patients. Srp. Arh. za Celok. Lek. 2016, 144, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Pishmisheva, M.; Baymakova, M.; Golkocheva-Markova, E.; Kundurzhiev, T.; Pepovich, R.; Popov, G.T.; Tsachev, I. First serological study of hepatitis E virus infection in pigs in Bulgaria. Comptes Rendus l’Académie Bulg Sci. 2018, 71, 1001–1008. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Ciccozzi, M.; Pepovich, R.; Kundurzhiev, T.; Marutsov, P.; Dimitrov, K.K.; Gospodinova, K.; Pishmisheva, M.; Pekova, L. Seroprevalence of Hepatitis E Virus Infection in Pigs from Southern Bulgaria. Vector-Borne Zoonotic Dis. 2019, 19, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Tsachev, I.; Baymakova, M.; Dimitrov, K.K.; Gospodinova, K.; Marutsov, P.; Pepovich, R.; Kundurzhiev, T.; Ciccozzi, M.; Dalton, H.R. Serological evidence of hepatitis E virus infection in pigs from Northern Bulgaria. Vet. Ital. 2021, 57, 155–159. [Google Scholar] [CrossRef]
- European Food Safety Authority. Analysis of the baseline survey on the prevalence of Salmonella in holdings with breeding pigs in the EU, 2008—Part A: Salmonella prevalence estimates. EFSA J. 2009, 7, 1377. [Google Scholar] [CrossRef]
- Powell, B.J.; Mandell, D.S.; Hadley, T.R.; Rubin, R.M.; Evans, A.C.; Hurford, M.O.; Beidas, R.S. Are general and strategic measures of organizational context and leadership associated with knowledge and attitudes toward evidence-based practices in public behavioral health settings? A cross-sectional observational study. Implement. Sci. 2017, 12, 64. [Google Scholar] [CrossRef]
- Sergeant ESG. Epitools Epidemiological Calculators, Ausvet. 2018. Available online: http://epitools.ausvet.com.au (accessed on 16 February 2018).
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Valcarce, M.D.; Kovač, K.; Cook, N.; Rodriguez-Lazaro, D.; Hernández, M. Construction and Analytical Application of Internal Amplification Controls (IAC) for Detection of Food Supply Chain-Relevant Viruses by Real-Time PCR-Based Assays. Food Anal. Methods 2011, 4, 437–445. [Google Scholar] [CrossRef]
- ISO 15216-1:2017; Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A virus and No-Rovirus Using Real-Time RT-PCR—Part 1: Method for Quantification. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 15216-2:2019; Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A virus and No-Rovirus Using Real-Time RT-PCR—Part 2: Method for Detection. International Organization for Standardization: Geneva, Switzerland, 2019.
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Blümel, J.; Mizusawa, S.; Matsubayashi, K.; Sakata, H.; Okada, Y.; Nübling, C.M.; Hanschmann, K.-M.O.; The HEV Collaborative Study Group. World Health Organization International Standard to Harmonize Assays for Detection of Hepatitis E Virus RNA. Emerg. Infect. Dis. 2013, 19, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Martelli, F.; Inglese, N.; Pourshaban, M.; Caprioli, A.; Ostanello, F.; Ruggeri, F.M. Widespread diffusion of genotype 3 hepatitis E virus among farming swine in Northern Italy. Vet. Microbiol. 2008, 132, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Der Honing, R.W.H.-V.; Van Coillie, E.; Antonis, A.F.G.; Van Der Poel, W.H.M. First Isolation of Hepatitis E Virus Genotype 4 in Europe through Swine Surveillance in the Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef]
- Widén, F.; Sundqvist, L.; Matyi-Toth, A.; Metreveli, G.; Belák, S.; Hallgren, G.; Norder, H. Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiol. Infect. 2010, 139, 361–371. [Google Scholar] [CrossRef]
- Anita, A.; Gorgan, L.; Aniță, D.C.; Oslobanu, E.L.L.; Pavio, N.; Savuţa, G. Evidence of hepatitis E infection in swine and humans in the East Region of Romania. Int. J. Infect. Dis. 2014, 29, 232–237. [Google Scholar] [CrossRef]
- Steyer, A.; Naglič, T.; Močilnik, T.; Poljšak-Prijatelj, M.; Poljak, M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: Prevalence and molecular characterization of a novel genotype 3 lineage. Infect. Genet. Evol. 2011, 11, 1732–1737. [Google Scholar] [CrossRef]
- Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Palombieri, A.; Tsachev, I.; Sarchese, V.; Fruci, P.; Di Profio, F.; Pepovich, R.; Baymakova, M.; Marsilio, F.; Martella, V.; Di Martino, B. A Molecular Study on Hepatitis E Virus (HEV) in Pigs in Bulgaria. Vet. Sci. 2021, 8, 267. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Pepovich, R.; Palova, N.; Marutsov, P.; Gospodinova, K.; Kundurzhiev, T.; Ciccozzi, M. High Seroprevalence of Hepatitis E Virus Infection Among East Balkan Swine (Sus scrofa) in Bulgaria: Preliminary Results. Pathogens 2020, 9, 911. [Google Scholar] [CrossRef]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent Hepatitis E Virus Contamination in Food Containing Raw Pork Liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Nakai, I.; Ikeda, H.; Li, T.-C.; Kato, K.; Miyazaki, A.; Tsunemitsu, H.; Takeda, N.; Yoshii, M. Different Fecal Shedding Patterns of Two Common Strains of Hepatitis e virus at Three Japanese Swine Farms. Am. J. Trop. Med. Hyg. 2006, 75, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Walachowski, S.; Dorenlor, V.; Lefevre, J.; Lunazzi, A.; Eono, F.; Merbah, T.; Eveno, E.; Pavio, N.; Rose, N. Risk factors associated with the presence of hepatitis E virus in livers and seroprevalence in slaughter-age pigs: A retrospective study of 90 swine farms in France. Epidemiol. Infect. 2013, 142, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Galipó, E.; Zoche-Golob, V.; Sassu, E.L.; Prigge, C.; Sjölund, M.; Tobias, T.; Rzeżutka, A.; Smith, R.P.; Burow, E. Prioritization of pig farm biosecurity for control of Salmonella and hepatitis E virus infections: Results of a European expert opinion elicitation. Porc. Health Manag. 2023, 9, 8. [Google Scholar] [CrossRef]
- Zani, L.; Dietze, K.; Dimova, Z.; Forth, J.H.; Denev, D.; Depner, K.; Alexandrov, T. African Swine Fever in a Bulgarian Backyard Farm—A Case Report. Vet. Sci. 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Chelli, E.; De Sabato, L.; Monini, M.; Ostanello, F.; Di Bartolo, I. Long-term surveillance for hepatitis E virus in an Italian two-site farrow-to-finish swine farm. Zoonoses Public Health 2021, 68, 474–482. [Google Scholar] [CrossRef] [PubMed]
| Administrative District | Number of Sampled Farms | Total Pooled Samples per Districts |
|---|---|---|
| Montana | 2 | 40 |
| Vratsa | 1 | 20 |
| Lovech | 2 | 40 |
| Gabrovo | 1 | 20 |
| Ruse | 5 | 100 |
| Razgrad | 4 | 80 |
| Shumen | 2 | 40 |
| Varna | 3 | 60 |
| Pazardzhik | 4 | 70 * |
| Stara Zagora | 4 | 80 |
| Yambol | 4 | 80 |
| In total | 32 | 630 |
| Administrative District | Farms | ||
|---|---|---|---|
| Total Tested | Number of Positive Farms | % of Positive Farms | |
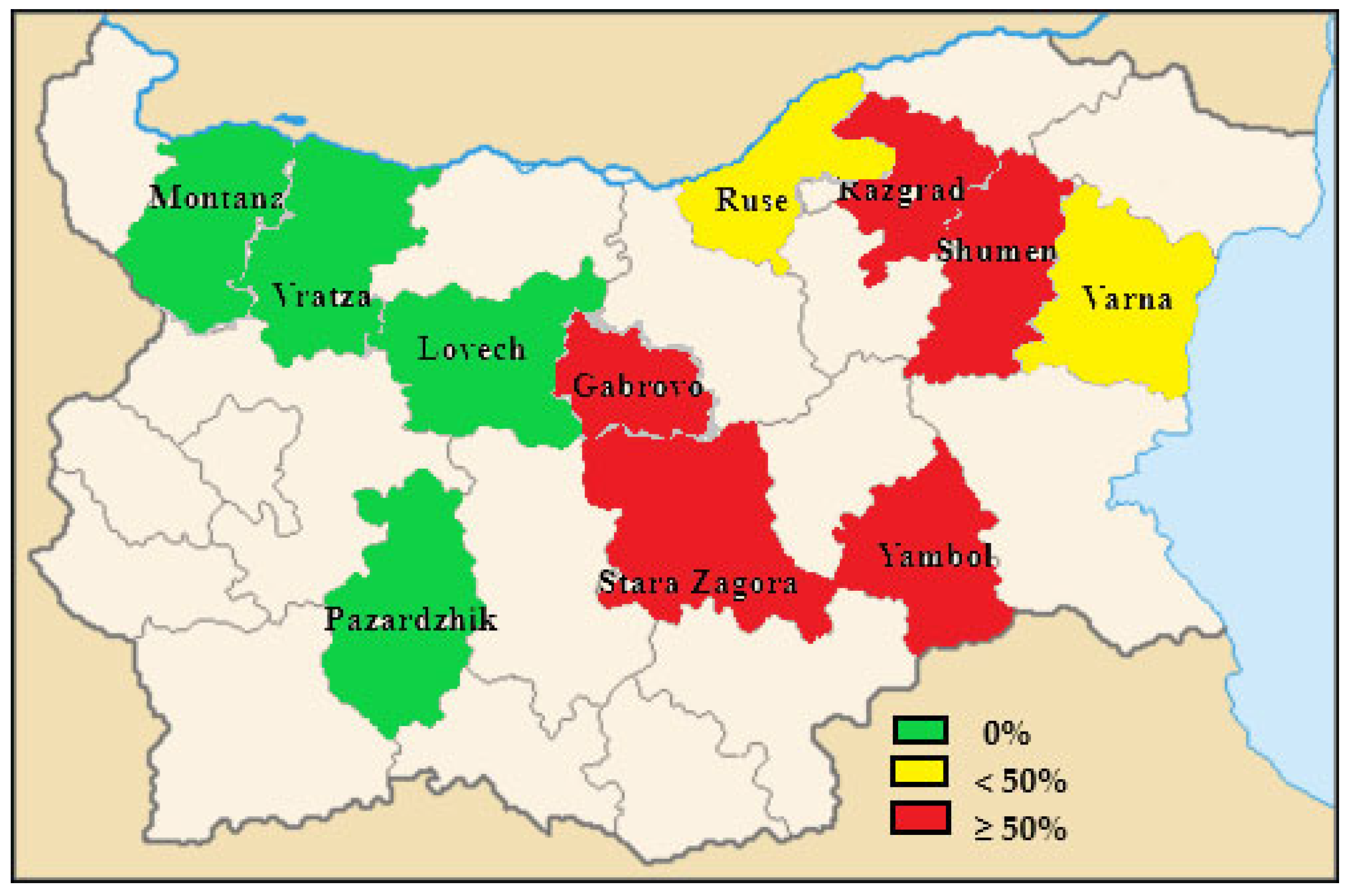
| Razgrad | 4 | 3 | 75 |
| Pazardzhik | 4 | 0 | 0 |
| Vratza | 1 | 0 | 0 |
| Montana | 2 | 0 | 0 |
| Ruse | 5 | 1 | 20 |
| Lovech | 2 | 0 | 0 |
| Gabrovo | 1 | 1 | 100 |
| Stara Zagora | 4 | 2 | 50 |
| Yambol | 4 | 2 | 50 |
| Shumen | 2 | 2 | 100 |
| Varna | 3 | 1 | 33.3 |
| Total | 32 | 12 | 37.5 |
| Production Stage | Total Number of Tested Pooled Fecal Samples | Number of Positive Pooled Fecal Samples | % of Positve Pooled Fecal Samples |
|---|---|---|---|
| Dry sows | 62 | 1 | 1.6 |
| Gilts | 248 | 1 | 0.4 |
| Total Breeding | 310 | 2 | 0.64 |
| Fattening pigs (60–120 days) | 197 | 48 | 24.4 |
| Fattening pigs (121–180 days) | 123 | 18 | 14.6 |
| Total fattening | 320 | 66 | 20.6 |
| Total (fattening and breeding) | 630 | 68 | 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumova-Valcheva, G.L.; Di Bartolo, I.; Smith, R.P.; Gyurova, E.; Mateva, G.; Milanov, M.; Dimitrova, A.; Burow, E.; Daskalov, H. Detection of HEV RNA Using One-Step Real-Time RT-PCR in Farrow-to-Finish Pig Farms in Bulgaria. Pathogens 2023, 12, 673. https://doi.org/10.3390/pathogens12050673
Krumova-Valcheva GL, Di Bartolo I, Smith RP, Gyurova E, Mateva G, Milanov M, Dimitrova A, Burow E, Daskalov H. Detection of HEV RNA Using One-Step Real-Time RT-PCR in Farrow-to-Finish Pig Farms in Bulgaria. Pathogens. 2023; 12(5):673. https://doi.org/10.3390/pathogens12050673
Chicago/Turabian StyleKrumova-Valcheva, Gergana Lyubomirova, Ilaria Di Bartolo, Richard Piers Smith, Eva Gyurova, Gergana Mateva, Mihail Milanov, Albena Dimitrova, Elke Burow, and Hristo Daskalov. 2023. "Detection of HEV RNA Using One-Step Real-Time RT-PCR in Farrow-to-Finish Pig Farms in Bulgaria" Pathogens 12, no. 5: 673. https://doi.org/10.3390/pathogens12050673
APA StyleKrumova-Valcheva, G. L., Di Bartolo, I., Smith, R. P., Gyurova, E., Mateva, G., Milanov, M., Dimitrova, A., Burow, E., & Daskalov, H. (2023). Detection of HEV RNA Using One-Step Real-Time RT-PCR in Farrow-to-Finish Pig Farms in Bulgaria. Pathogens, 12(5), 673. https://doi.org/10.3390/pathogens12050673







