Mechanism and Modulation of SidE Family Proteins in the Pathogenesis of Legionella pneumophila
Abstract
1. Introduction
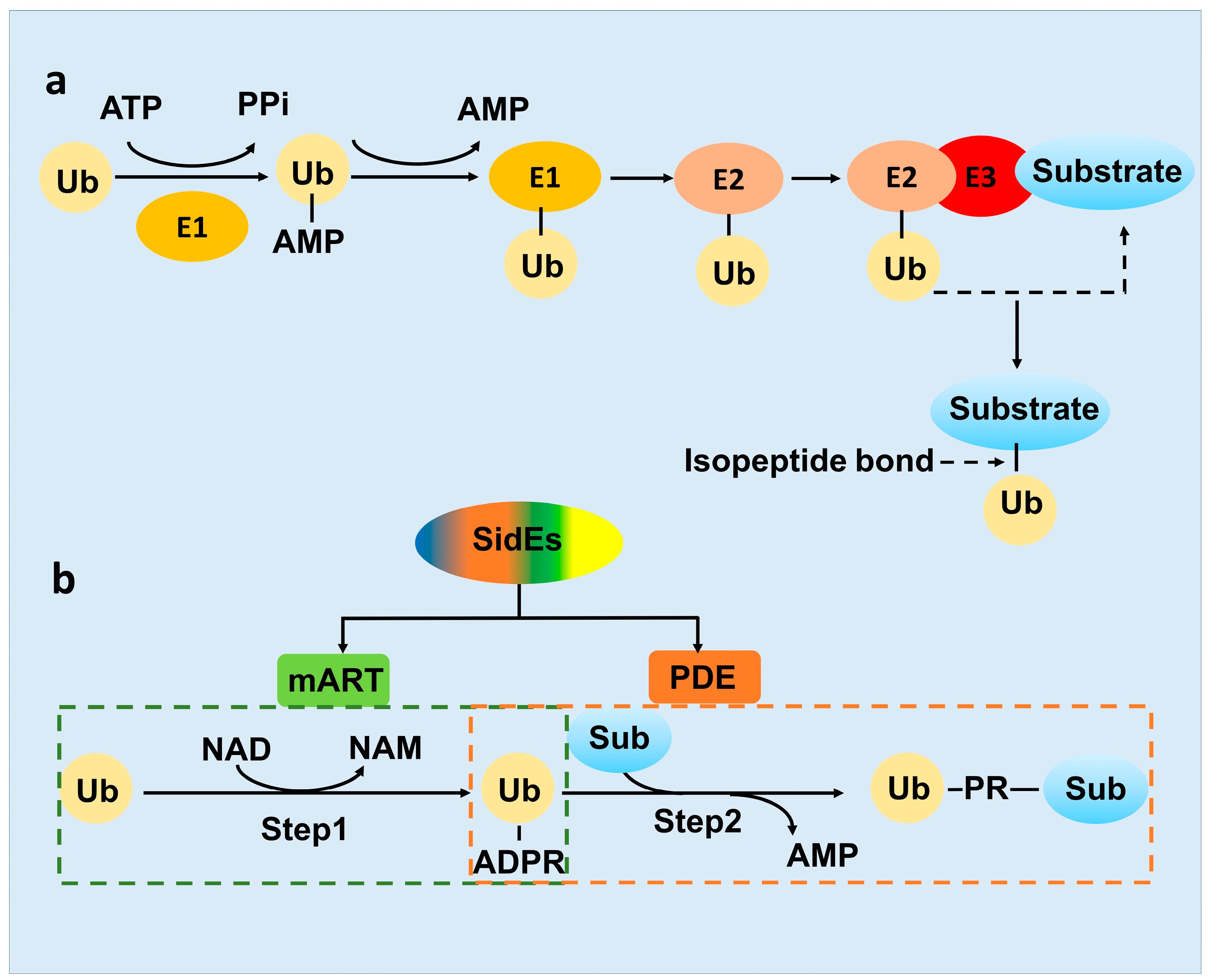
2. SidE Family Effectors Catalyze a Non-Canonical Ubiquitination Process
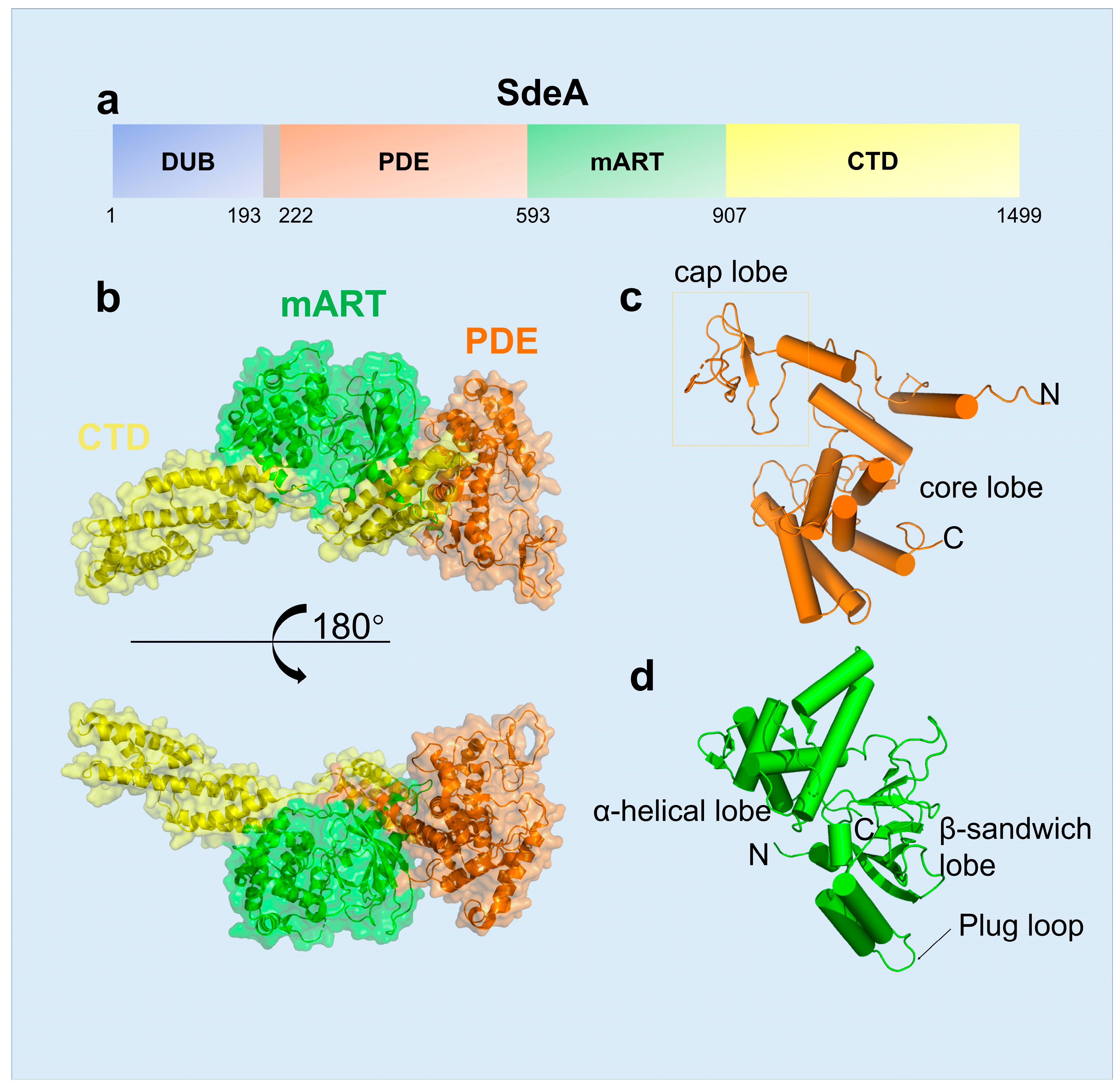
2.1. The Structural Features of SidE Family
2.2. The Novel-Ubiquitination Machinery of the SidE Family
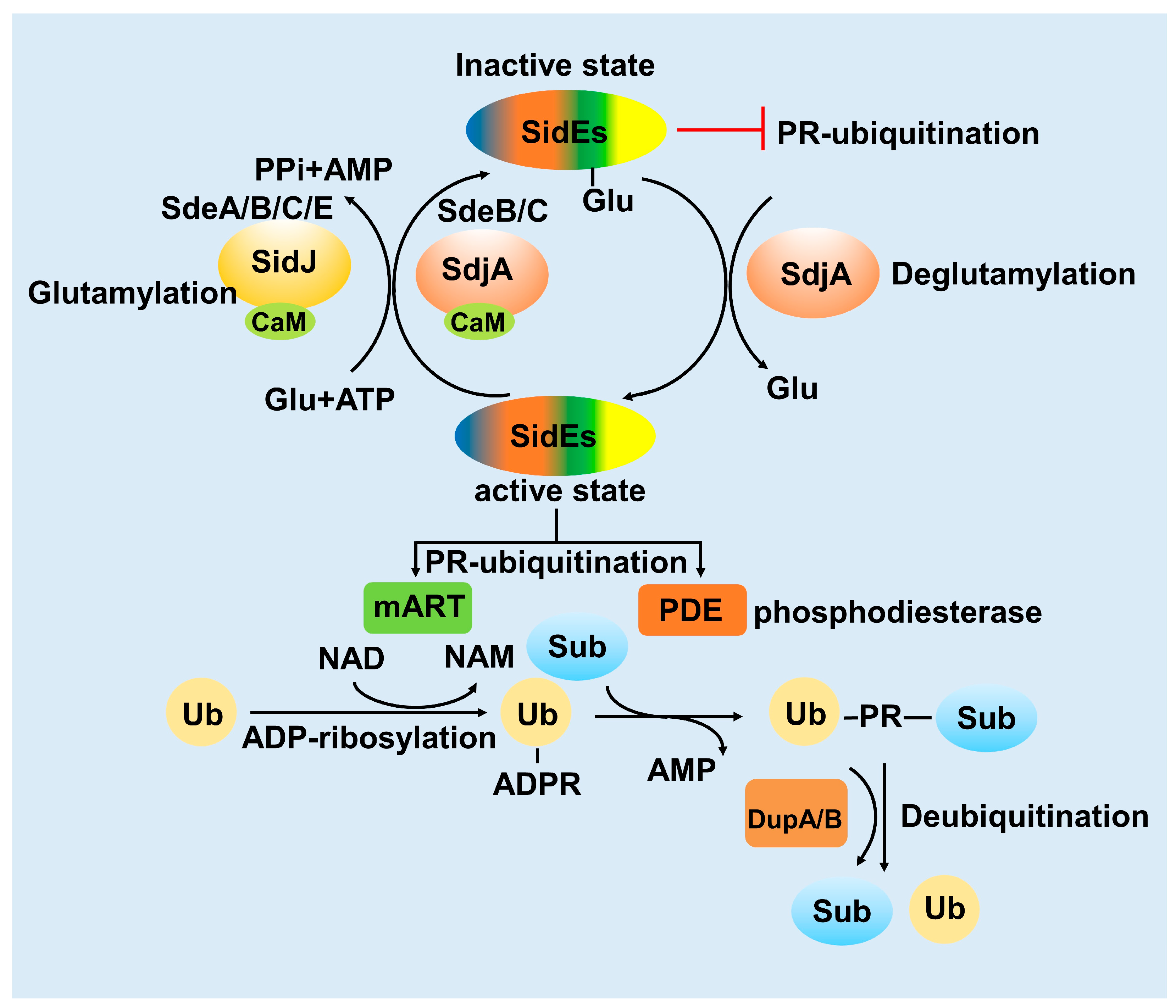
3. Activity of the SidE Family Was Strictly Modulated by Many Effectors
3.1. SidJ Interacts with Calmodulin to Modify SdeA
3.2. SdjA Reverses the Glutamylation Modification of SdeA
3.3. DupA and DupB Function as Deubiquitinases for PR-Ubiquitination
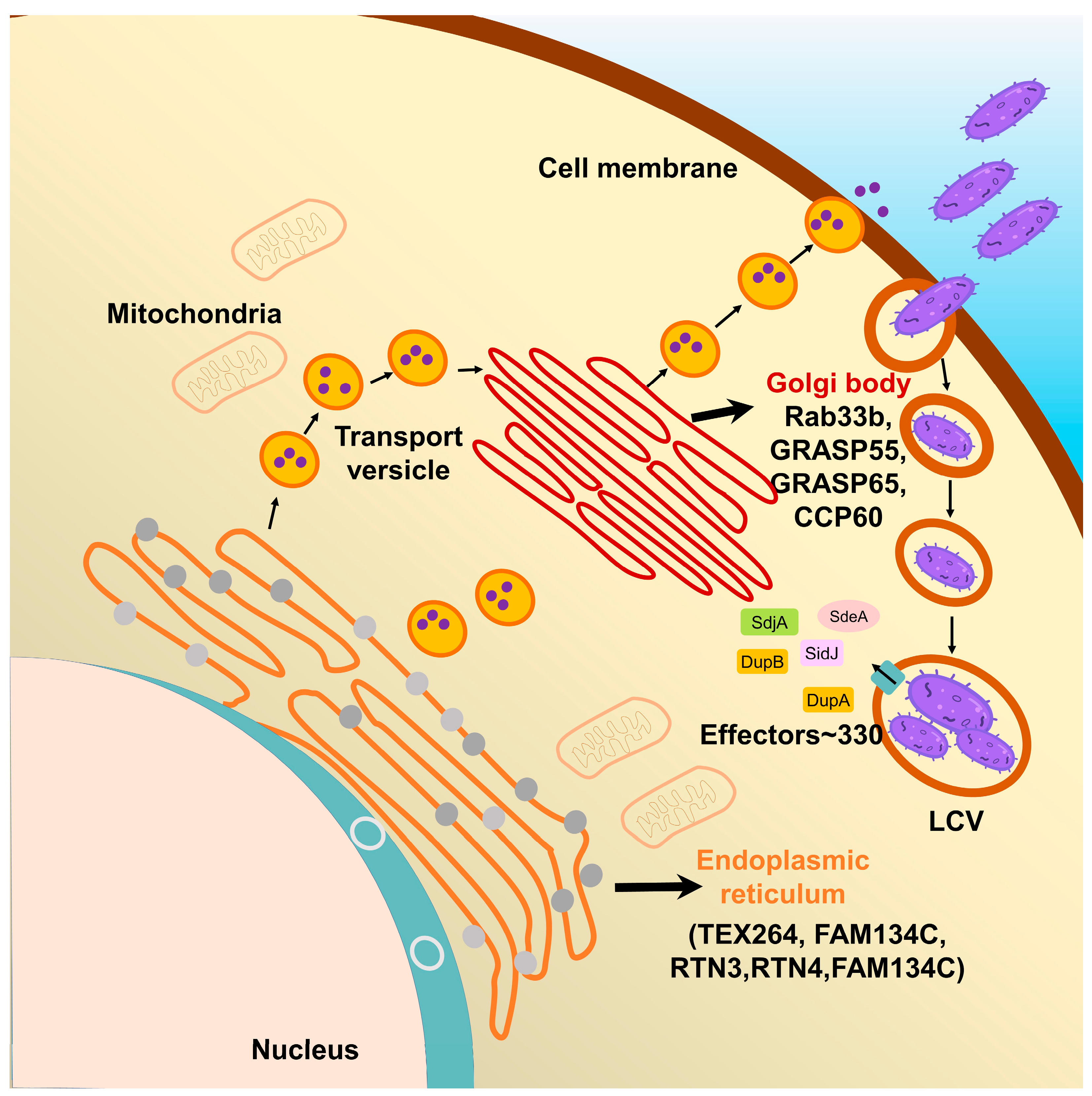
4. Multiple Host Proteins Targeted by SidE Family Effectors
4.1. The Effects of SidE Family Proteins on Endoplasmic Reticulum
4.2. The Effects of SidE Family Proteins on the Golgi Complex
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; Mcdade, J.E.; et al. Legionnaires’ Disease. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Q.; Isberg, R.R. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 2004, 101, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, A.W. Legionella pneumophila, armed to the hilt: Justifying the largest arsenal of effectors in the bacterial world. Curr. Opin. Microbiol. 2016, 29, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Isberg, R.R.; O’connor, T.J.; Heidtman, M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat. Rev. Microbiol. 2009, 7, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.G.; Roy, C.R. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell. Microbiol. 2006, 8, 793–805. [Google Scholar] [CrossRef]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, baab012. [Google Scholar] [CrossRef]
- Mann, M.; Jensen, O.N.J.N.B. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef]
- Olsen, J.V.; Mann, M.J.M.; Mcp, C.P. Status of Large-scale Analysis of Post-translational Modifications by Mass Spectrometry. Mol. Cell. Proteom. 2013, 12, 3444–3452. [Google Scholar] [CrossRef]
- Sun, S.C. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 2008, 8, 501–511. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Huang, C.; Wang, X.; Tan, J.; Cheng, S.; Wan, M.; Wang, Z.; Wang, S.; Luo, S.; Li, A.; et al. Threonine ADP-Ribosylation of Ubiquitin by a Bacterial Effector Family Blocks Host Ubiquitination. Mol. Cell 2020, 78, 641–652.e649. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, Q.; Zhou, J.; Yin, W.; Yao, D.; Shao, Y.; Zhao, Y.; Guo, B.; Xia, Y.; Chen, Q.; et al. Phytophthora sojae effector Avr1d functions as an E2 competitor and inhibits ubiquitination activity of GmPUB13 to facilitate infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2018312118. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Nakayasu, E.S.; Hollenbeck, P.J.; Luo, Z.Q. Legionella pneumophila inhibits immune signalling via MavC-mediated transglutaminase-induced ubiquitination of UBE2N. Nat. Microbiol. 2019, 4, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mu, Y.; Xie, Y.; Zhang, Y.; Han, Y.; Zhou, Y.; Wang, W.; Liu, Z.; Wu, M.; Wang, H.; et al. Structural basis of ubiquitin modification by the Legionella effector SdeA. Nature 2018, 557, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wang, Y.; Huang, Y.; Li, D.; Han, Y.; Chang, M.; Fu, J.; Xie, Y.; Ren, J.; Wang, H.; et al. Structural insights into the mechanism and inhibition of transglutaminase-induced ubiquitination by the Legionella effector MavC. Nat. Commun. 2020, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Bardill, J.P.; Miller, J.L.; Vogel, J.P. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 2005, 56, 90–103. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Arvai, A.S.; Clancy, S.B.; Tainer, J.A. Crystal structure and novel recognition motif of Rho ADP-ribosylating C3 exoenzyme from Clostridium botulinum: Structural insights for recognition specificity and catalysis1 1Edited by D. Rees. J. Mol. Biol. 2001, 305, 95–107. [Google Scholar] [CrossRef]
- Qiu, J.; Sheedlo, M.J.; Yu, K.; Tan, Y.; Nakayasu, E.S.; Das, C.; Liu, X.; Luo, Z.Q. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 2016, 533, 120–124. [Google Scholar] [CrossRef]
- Zhang, M.; Mcewen, J.M.; Sjoblom, N.M.; Kotewicz, K.M.; Isberg, R.R.; Scheck, R.A. Members of the Legionella pneumophila Sde family target tyrosine residues for phosphoribosyl-linked ubiquitination. RSC Chem. Biol. 2021, 2, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Sheedlo, M.J.; Qiu, J.; Tan, Y.; Paul, L.N.; Luo, Z.Q.; Das, C. Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc. Natl. Acad. Sci. USA 2015, 112, 15090–15095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, M.; Feng, H.; Zhu, Y.; Liu, S.; Gao, A.; Gao, P. Structural Insights into Non-canonical Ubiquitination Catalyzed by SidE. Cell 2018, 173, 1231–1243.e1216. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, Y.; Yan, J.; Mehrens, J.; Yang, H.; Delucia, M.; Hao, C.; Gronenborn, A.M.; Skowronski, J.; Ahn, J.; et al. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat. Struct. Mol. Biol. 2013, 20, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The ubiquitin system. Trends Biochem. Sci. 1997, 22, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Bhogaraju, S.; Kalayil, S.; Liu, Y.; Bonn, F.; Colby, T.; Matic, I.; Dikic, I. Phosphoribosylation of Ubiquitin Promotes Serine Ubiquitination and Impairs Conventional Ubiquitination. Cell 2016, 167, 1636–1649.e1613. [Google Scholar] [CrossRef] [PubMed]
- Honjo, T.; Nishizuka, Y.; Hayaishi, O.; Kato, I. Diphtheria Toxin-dependent Adenosine Diphosphate Ribosylation of Aminoacyl Transferase II and Inhibition of Protein Synthesis. J. Biol. Chem. 1968, 243, 3553–3555. [Google Scholar] [CrossRef]
- Corda, D.; Di Girolamo, M. Functional aspects of protein mono-ADP-ribosylation. EMBO J. 2003, 22, 1953–1958. [Google Scholar] [CrossRef]
- Yang, C.S.; Jividen, K.; Spencer, A.; Dworak, N.; Ni, L.; Oostdyk, L.T.; Chatterjee, M.; Kuśmider, B.; Reon, B.; Parlak, M.; et al. Ubiquitin Modification by the E3 Ligase/ADP-Ribosyltransferase Dtx3L/Parp9. Mol. Cell 2017, 66, 503–516.e505. [Google Scholar] [CrossRef]
- Kotewicz, K.M.; Ramabhadran, V.; Sjoblom, N.; Vogel, J.P.; Haenssler, E.; Zhang, M.; Behringer, J.; Scheck, R.A.; Isberg, R.R. A Single Legionella Effector Catalyzes a Multistep Ubiquitination Pathway to Rearrange Tubular Endoplasmic Reticulum for Replication. Cell Host Microbe 2017, 21, 169–181. [Google Scholar] [CrossRef]
- Kalayil, S.; Bhogaraju, S.; Bonn, F.; Shin, D.; Liu, Y.; Gan, N.; Basquin, J.; Grumati, P.; Luo, Z.-Q.; Dikic, I. Insights into catalysis and function of phosphoribosyl-linked serine ubiquitination. Nature 2018, 557, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, S.; Krieglstein, J. Phosphorylation and dephosphorylation of histidine residues in proteins. Eur. J. Biochem. 2002, 269, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Akturk, A.; Wasilko, D.J.; Wu, X.; Liu, Y.; Zhang, Y.; Qiu, J.; Luo, Z.-Q.; Reiter, K.H.; Brzovic, P.S.; Klevit, R.E.; et al. Mechanism of phosphoribosyl-ubiquitination mediated by a single Legionella effector. Nature 2018, 557, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Havey, J.C.; Roy, C.R. Toxicity and SidJ-Mediated Suppression of Toxicity Require Distinct Regions in the SidE Family of Legionella pneumophila Effectors. Infect. Immun. 2015, 83, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yu, K.; Fei, X.; Liu, Y.; Nakayasu, E.S.; Piehowski, P.D.; Shaw, J.B.; Puvar, K.; Das, C.; Liu, X.; et al. A unique deubiquitinase that deconjugates phosphoribosyl-linked protein ubiquitination. Cell Res. 2017, 27, 865–881. [Google Scholar] [CrossRef]
- Black, M.H.; Osinski, A.; Gradowski, M.; Servage, K.A.; Pawłowski, K.; Tomchick, D.R.; Tagliabracci, V.S. Bacterial pseudokinase catalyzes protein polyglutamylation to inhibit the SidE-family ubiquitin ligases. Science 2019, 364, 787–792. [Google Scholar] [CrossRef]
- Bhogaraju, S.; Bonn, F.; Mukherjee, R.; Adams, M.; Pfleiderer, M.M.; Galej, W.P.; Matkovic, V.; Lopez-Mosqueda, J.; Kalayil, S.; Shin, D.; et al. Inhibition of bacterial ubiquitin ligases by SidJ-calmodulin catalysed glutamylation. Nature 2019, 572, 382–386. [Google Scholar] [CrossRef]
- Gan, N.; Zhen, X.; Liu, Y.; Xu, X.; He, C.; Qiu, J.; Liu, Y.; Fujimoto, G.M.; Nakayasu, E.S.; Zhou, B.; et al. Regulation of phosphoribosyl ubiquitination by a calmodulin-dependent glutamylase. Nature 2019, 572, 387–391. [Google Scholar] [CrossRef]
- Sulpizio, A.; Minelli, M.E.; Wan, M.; Burrowes, P.D.; Wu, X.; Sanford, E.J.; Shin, J.H.; Williams, B.C.; Goldberg, M.L.; Smolka, M.B.; et al. Protein polyglutamylation catalyzed by the bacterial calmodulin-dependent pseudokinase SidJ. eLife 2019, 8, e51162. [Google Scholar] [CrossRef]
- Sulpizio, A.G.; Minelli, M.E.; Mao, Y. Glutamylation of Bacterial Ubiquitin Ligases by a Legionella Pseudokinase. Trends Microbiol. 2019, 27, 967–969. [Google Scholar] [CrossRef]
- Osinski, A.; Black, M.H.; Pawłowski, K.; Chen, Z.; Li, Y.; Tagliabracci, V.S. Structural and mechanistic basis for protein glutamylation by the kinase fold. Mol. Cell 2021, 81, 4527–4539.e4528. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Z.Q. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect. Immun. 2007, 75, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xie, Y.; Li, C.; Wang, L.; He, C.; Zhang, Y.; Yuan, J.; Luo, J.; Liu, X.; Xiu, Y.; et al. The Legionella Effector SdjA Is a Bifunctional Enzyme That Distinctly Regulates Phosphoribosyl Ubiquitination. mBio 2021, 12, e0231621. [Google Scholar] [CrossRef] [PubMed]
- Valleau, D.; Quaile, A.T.; Cui, H.; Xu, X.; Evdokimova, E.; Chang, C.; Cuff, M.E.; Urbanus, M.L.; Houliston, S.; Arrowsmith, C.H.; et al. Discovery of Ubiquitin Deamidases in the Pathogenic Arsenal of Legionella pneumophila. Cell Rep. 2018, 23, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Guan, H.; Huang, Y.; Yu, T.; Fu, J.; Nakayasu, E.S.; Puvar, K.; Das, C.; Wang, D.; Ouyang, S.; et al. Legionella pneumophila regulates the activity of UBE2N by deamidase-mediated deubiquitination. EMBO J. 2020, 39, e102806. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Sulpizio, A.G.; Akturk, A.; Beck WH, J.; Lanz, M.; Faça, V.M.; Smolka, M.B.; Vogel, J.P.; Mao, Y. Deubiquitination of phosphoribosyl-ubiquitin conjugates by phosphodiesterase-domain-containing Legionella effectors. Proc. Natl. Acad. Sci. USA 2019, 116, 23518–23526. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Liu, Y.; Gonzalez, A.; Bonn, F.; Liu, Y.; Rogov, V.V.; Heinz, M.; Stolz, A.; Hummer, G.; et al. Regulation of Phosphoribosyl-Linked Serine Ubiquitination by Deubiquitinases DupA and DupB. Mol. Cell 2020, 77, 164–179.e166. [Google Scholar] [CrossRef]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef]
- Swanson, M.S.; Isberg, R.R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 1995, 63, 3609–3620. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. CMLS 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Nixon-Abell, J.; Obara, C.J.; Weigel, A.V.; Li, D.; Legant, W.R.; Xu, C.S.; Pasolli, H.A.; Harvey, K.; Hess, H.F.; Betzig, E.; et al. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science 2016, 354, aaf3928. [Google Scholar] [CrossRef] [PubMed]
- Grumati, P.; Morozzi, G.; Hölper, S.; Mari, M.; Harwardt, M.I.; Yan, R.; Müller, S.; Reggiori, F.; Heilemann, M.; Dikic, I. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. eLife 2017, 6, e25555. [Google Scholar] [CrossRef]
- Khaminets, A.; Heinrich, T.; Mari, M.; Grumati, P.; Huebner, A.K.; Akutsu, M.; Liebmann, L.; Stolz, A.; Nietzsche, S.; Koch, N.; et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 2015, 522, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tukachinsky, H.; Romano, F.B.; Rapoport, T.A. Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. eLife 2016, 5, e18605. [Google Scholar] [CrossRef] [PubMed]
- Urade, T.; Yamamoto, Y.; Zhang, X.; Ku, Y.; Sakisaka, T. Identification and characterization of TMEM33 as a reticulon-binding protein. Kobe J. Med. Sci. 2014, 60, E57–E65. [Google Scholar] [PubMed]
- Yamamoto, Y.; Yoshida, A.; Miyazaki, N.; Iwasaki, K.; Sakisaka, T. Arl6IP1 has the ability to shape the mammalian ER membrane in a reticulon-like fashion. Biochem. J. 2014, 458, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Reggio, A.; Buonomo, V.; Berkane, R.; Bhaskara, R.M.; Tellechea, M.; Peluso, I.; Polishchuk, E.; Di Lorenzo, G.; Cirillo, C.; Esposito, M.; et al. Role of FAM134 paralogues in endoplasmic reticulum remodeling, ER-phagy, and Collagen quality control. EMBO Rep. 2021, 22, e52289. [Google Scholar] [CrossRef]
- An, H.; Ordureau, A.; Paulo, J.A.; Shoemaker, C.J.; Denic, V.; Harper, J.W. TEX264 Is an Endoplasmic Reticulum-Resident ATG8-Interacting Protein Critical for ER Remodeling during Nutrient Stress. Mol. Cell 2019, 74, 891–908.e810. [Google Scholar] [CrossRef]
- Chino, H.; Hatta, T.; Natsume, T.; Mizushima, N. Intrinsically Disordered Protein TEX264 Mediates ER-phagy. Mol. Cell 2019, 74, 909–921.e906. [Google Scholar] [CrossRef]
- Starr, T.; Sun, Y.; Wilkins, N.; Storrie, B. Rab33b and Rab6 are functionally overlapping regulators of Golgi homeostasis and trafficking. Traffic 2010, 11, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, R.M.; Ganley, I.G.; Barr, F.A.; Lambright, D.G.; Pfeffer, S.R. RUTBC1 protein, a Rab9A effector that activates GTP hydrolysis by Rab32 and Rab33B proteins. J. Biol. Chem. 2011, 286, 33213–33222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seemann, J. Rapid degradation of GRASP55 and GRASP65 reveals their immediate impact on the Golgi structure. J. Cell Biol. 2021, 220, e202007052. [Google Scholar] [CrossRef] [PubMed]
- Grond, R.; Veenendaal, T.; Duran, J.M.; Raote, I.; Van Es, J.H.; Corstjens, S.; Delfgou, L.; El Haddouti, B.; Malhotra, V.; Rabouille, C. The function of GORASPs in Golgi apparatus organization in vivo. J. Cell Biol. 2020, 219, e202004191. [Google Scholar] [CrossRef] [PubMed]
- Sohda, M.; Misumi, Y.; Yamamoto, A.; Yano, A.; Nakamura, N.; Ikehara, Y. Identification and Characterization of a Novel Golgi Protein, GCP60, That Interacts with the Integral Membrane Protein Giantin*. J. Biol. Chem. 2001, 276, 45298–45306. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, Y.; Zhu, H.; Ahmed, K.; Fu, C.; Yao, X. Human Yip1A specifies the localization of Yif1 to the Golgi apparatus. Biochem. Biophys. Res. Commun. 2005, 334, 16–22. [Google Scholar] [CrossRef]
- Van Weering, J.R.; Sessions, R.B.; Traer, C.J.; Kloer, D.P.; Bhatia, V.K.; Stamou, D.; Carlsson, S.R.; Hurley, J.H.; Cullen, P.J. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 2012, 31, 4466–4480. [Google Scholar] [CrossRef]
- Kawabata, M.; Matsuo, H.; Koito, T.; Murata, M.; Kubori, T.; Nagai, H.; Tagaya, M.; Arasaki, K. Legionella hijacks the host Golgi-to-ER retrograde pathway for the association of Legionella-containing vacuole with the ER. PLoS Pathog. 2021, 17, e1009437. [Google Scholar] [CrossRef]
- Kulkarni-Gosavi, P.; Makhoul, C.; Gleeson, P.A. Form and function of the Golgi apparatus: Scaffolds, cytoskeleton and signalling. FEBS Lett. 2019, 593, 2289–2305. [Google Scholar] [CrossRef]
- Li, J.; Ahat, E.; Wang, Y. Golgi Structure and Function in Health, Stress, and Diseases. Results Probl. Cell Differ. 2019, 67, 441–485. [Google Scholar]
- Rabouille, C.; Linstedt, A.D. GRASP: A Multitasking Tether. Front. Cell Dev. 2016, 4, 1. [Google Scholar] [CrossRef]
- Shorter, J.; Watson, R.; Giannakou, M.E.; Clarke, M.; Warren, G.; Barr, F.A. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999, 18, 4949–4960. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, T.N.; Linstedt, A.D. GRASP55 regulates Golgi ribbon formation. Mol. Biol. Cell 2008, 19, 2696–2707. [Google Scholar] [CrossRef]
- Liu, Y.; Mukherjee, R.; Bonn, F.; Colby, T.; Matic, I.; Glogger, M.; Heilemann, M.; Dikic, I. Serine-ubiquitination regulates Golgi morphology and the secretory pathway upon Legionella infection. Cell Death Differ. 2021, 28, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Abrams, C.; Wang, L.; Gizzi, A.; He, L.; Lin, R.; Chen, Y.; Loll Patrick, J.; Pascal John, M.; Zhang, J.-F. Structural Basis for Calmodulin as a Dynamic Calcium Sensor. Structure 2012, 20, 911–923. [Google Scholar] [CrossRef] [PubMed]
| Gene ID | Species | Aliases | Function | Reference |
|---|---|---|---|---|
| lpg2157 | L. pneumophila | SdeA | PR-ubiquitination | [20] |
| lpg2156 | L. pneumophila | SdeB | PR-ubiquitination | |
| lpg2153 | L. pneumophila | SdeC | PR-ubiquitination | |
| lpg0234 | L. pneumophila | SidE | PR-ubiquitination | |
| lpg2155 | L. pneumophila | SidJ | Inhibits SdeA, SdeB, SdeC and SidE ubiquitinating activity by Glutamylation. | [35,36,38] |
| lpg2508 | L. pneumophila | SdjA | Reverses the SidJ-CaM modification of SdeA. | [43] |
| lpg2154 | L. pneumophila | DupA | Regulates Phosphoribosyl-Linked Serine Ubiquitination by Deubiquitination. | [47,48] |
| lpg2509 | L. pneumophila | DupB | ||
| 10313 | Homo sapiens | RTN3 | Induces the formation of ER | [56] |
| 57142 | Homo sapiens | RTN4 | Stabilization of endoplasmic reticulum (ER) | [55,56,57] |
| 162427 | Homo sapiens | FAM134C | Endoplasmic reticulum remodeling, ER-phagy, and Collagen quality control. | [58] |
| 51368 | Homo sapiens | TEX264 | ATG8-Interacting Protein Critical for ER Remodeling.ER-phage. | [59,60] |
| 83452 | Homo sapiens | Rab33b | ER-associated Rab small GTPases.Regulators of Golgi homeostasis and trafficking. | [61,62] |
| 26003 | Homo sapiens | GRASP55 | Function in the connection of Golgi stack and the maintenance of Golgi structure | [63,64] |
| 64689 | Homo sapiens | GRASP65 | ||
| 64746 | Homo sapiens | GCP60 | Affecting protein transport between the endoplasmic reticulum and Golgi. | [65] |
| 10897 | Homo sapiens | YIF1A | Role in transport between endoplasmic reticulum and Golgi. | [66] |
| 27131 | Homo sapiens | SNX5 | Mediates retrograde transport of cargo proteins from endosomes to the trans-Golgi network. | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhang, Y.; Wang, Y.; Feng, Y. Mechanism and Modulation of SidE Family Proteins in the Pathogenesis of Legionella pneumophila. Pathogens 2023, 12, 629. https://doi.org/10.3390/pathogens12040629
Xie Y, Zhang Y, Wang Y, Feng Y. Mechanism and Modulation of SidE Family Proteins in the Pathogenesis of Legionella pneumophila. Pathogens. 2023; 12(4):629. https://doi.org/10.3390/pathogens12040629
Chicago/Turabian StyleXie, Yongchao, Yi Zhang, Yong Wang, and Yue Feng. 2023. "Mechanism and Modulation of SidE Family Proteins in the Pathogenesis of Legionella pneumophila" Pathogens 12, no. 4: 629. https://doi.org/10.3390/pathogens12040629
APA StyleXie, Y., Zhang, Y., Wang, Y., & Feng, Y. (2023). Mechanism and Modulation of SidE Family Proteins in the Pathogenesis of Legionella pneumophila. Pathogens, 12(4), 629. https://doi.org/10.3390/pathogens12040629





