Abstract
Changes in the cellular secretome are implicated in virus infection, malignancy, and anti-tumor immunity. We analyzed the association between transcriptional signatures (TS) from 24 different immune and stromal cell types on the prognosis of HPV-infected and HPV-free head and neck squamous carcinoma (HNSCC) patients from The Cancer Genome Atlas (TCGA) cohort. We found that HPV-positive HNSCC patients have tumors with elevated immune cell TS and improved prognosis, which was specifically associated with an increased tumor abundance of memory B and activated natural killer (NK) cell TS, compared to HPV-free HNSCC patients. HPV-infected patients upregulated many transcripts encoding secreted factors, such as growth factors, hormones, chemokines and cytokines, and their cognate receptors. Analysis of secretome transcripts and cognate receptors revealed that tumor expression of IL17RB and IL17REL are associated with a higher viral load and memory B and activated NK cell TS, as well as improved prognosis in HPV-infected HNSCC patients. The transcriptional parameters that we describe may be optimized to improve prognosis and risk stratification in the clinic and provide insights into gene and cellular targets that may potentially enhance anti-tumor immunity mediated by NK cells and memory B cells in HPV-infected HNSCC patients.
1. Introduction
Head and neck squamous cell carcinomas (HNSCC) are the most common type of malignancies that arise in the head and neck. Over 90% of head and neck cancers are HNSCC that develop from the mucosal epithelium in the oronasal cavity, pharynx, and larynx. In the past two decades, accumulating studies have revealed the link between prior infection with oncogenic human papillomavirus (HPV) strains and tumors that develop in the oropharynx. An estimated 30,000 oropharyngeal cancers were caused by HPV infection worldwide each year, and HPV has been detected in ~25% of all HNSCC patients [1]. Despite the progress made in treatments, the prognosis of HNSCC patients has not improved significantly and HNSCC patients frequently suffer complications, such as local relapses and metastases [2]. Modulating the secretome in HNSCC and by HPV infection has recently been implicated in tumor progression, cancer cell invasion and metastasis [3].
The interleukin 17 (IL17) cytokine family contains six structurally related members from IL17A to IL17F (encoded by IL17A–IL17F). Five relevant IL17 receptor (IL17R) proteins have also been described as IL17RA through to IL17RE (encoded by IL17RA–IL17RE, respectively). The IL17 family has pivotal roles in inflammation, autoimmune disease, and cancer. IL17A (IL17) is the prototypic member produced by RAR-related orphan receptor gamma t (RORγt)-expressing cells and predominantly expressed by TH17 cells [4]. IL17A binding and activation of the IL17RA/IL17RC heterodimer signaling complex induces the expression of cytokines and chemokines such as tumor necrosis factor (TNF), CXC-chemokine ligand 1 (CXCL1), CXCL2 and CXCL5, from macrophages and stromal cells that are critical for defense against extracellular pathogens [5,6]. In contrast, dysregulation of IL17A expression is implicated in inflammatory disorders, such as psoriasis, ankylosing spondylitis and psoriatic arthritis [7,8]. The role of IL17A in cancer also remains controversial [9]. IL17A usually indirectly shapes immune suppression and helps tumor cell proliferation during the early stages of cancer by upregulating phosphorylated ERK1/2, angiogenesis and self-renewal [10,11,12], but also shows clinical relevance in anti-tumor immunity since IL17-secreting cells also co-secrete anti-tumor factors, such as IFN-γ and TNF [9,13,14].
While the knowledge of IL17A has been well established, the immunological roles of other IL17 family members are less well known. Human IL17B is a monomer that only shares 21.3% homology with human IL17A in the amino acid sequence [15]. In contrast to IL17A, IL17B expression has been detected in naïve, memory, germinal center B cells and chondrocytes as well as neurons, but not in activated T helper (TH) cells [16,17,18]. IL17B binds to the IL17RB homodimer, which is expressed in the epithelial cells of various organs [19]. The expression of IL17RB has also been found in TH cells and innate lymphocytes [20,21]. IL17RB can combine with IL17RA to form a heterodimeric complex that recognizes IL17E and prevents the binding of IL17B to IL17RB. In inflammatory diseases, the IL17B/IL17RB pathway is protective and might restrict pro-inflammatory by the IL17E/IL17RA-IL17RB signaling complex [22].
Several reports have shown the association between the IL17B/IL17RB signaling pathway and tumor development in breast, gastric, lung, pancreas, prostate, brain and blood cancers, however the precise mechanisms involved remain unclear [23,24,25,26]. Like IL17B, IL17C is frequently detected in non-immune cells. The binding of IL17C and IL17RA/IL17RE complex plays an important role in inflammation by regulating the innate immune functions of epithelial cells [27,28,29]. However, IL17RE has also been found to be expressed on TH17 cells in addition to stromal cells and IL17RE signaling can amplify TH17 cell responses in autoimmune disease [30]. In intestinal malignancies, IL17C binding to IL17RE stimulated TH17 cells to produce pre-tumorigenic cytokines and deficiency of IL17RE dramatically decreased intestinal tumor growth [31]. Collectively, these studies implicate the IL17 family of cytokines and receptors in tumor-promoting inflammation.
Following the discovery of the IL17 cytokine family, a gene encoding a novel IL17RE-like protein (IL17REL) was defined by similarity searches of amino acid sequences [32,33]. Rare variants of IL17REL with minor allele frequency (MAF) of less than 0.01 have been associated with inflammatory bowel disease (IBD), ulcerative colitis (UC) and gout. However, the role of IL17REL in malignancies remains uncertain [33,34,35].
Accumulating evidence has revealed the role of the IL17 cytokine family members in regulating the migration and functions of germinal center (GC)-derived B cells [16,36,37,38]. The GC is a transitory structure consisting of proliferating B cells in primary follicles of secondary lymphoid organs. The construction of the GC is divided into light and dark zones, formed by B cells following different developmental and functional patterns. The centrocytes (CC) in the light zone can differentiate further into memory B cells and plasma cells, and will go through further selection based on the affinity of the antibodies they produce [39,40,41]. It has been demonstrated that IL17A and IL17B were both expressed in the GC microenvironment, dedicated to B cell recruitment and antibody production [16,36,37].
NK cells are innate lymphocytes with cytotoxic and cytokine-secreting functions that comprise approximately 10–15% of total lymphocytes in human peripheral blood. They are known to initiate potent anti-tumor immunity regulated by “missing-self” and “induced-self” recognition [42,43,44]. NK cell activation is regulated by the balance of signaling from an array of activating and inhibitory surface receptors that bind to extracellular ligands [44,45]. In addition to conventional ligands anchored on the target cell surface, secreted molecules may also play roles in NK cell anti-tumor immunity. For example, one study revealed the secreted platelet-derived growth factor (PDGF)-DD as a ligand for the activating NK cell receptor, NKp44. PDGF-DD binding to NKp44 induces NK cell activation and secretion of IFN-γ and TNF and the transcription of mRNAs encoding proinflammatory chemokines, such as CCL3, CCL4, XCL1 and XCL2 [46]. The secretion of IFN-γ and TNF induces tumor cell growth arrest as well as the expression of ligands for the activating NK cell receptors, DNAM-1 and CRTAM, which initiate NK cell tumor surveillance [47,48,49,50]. In contrast to a well established pro-tumorigenic role in angiogenesis, high vascular endothelial growth factor (VEGF) expression in tumor-associated macrophages (TAM)/stroma was found to be associated with better prognosis in primary colon carcinoma, [51]. These data show that the tumor secretome may influence anti-tumor immunity and cancer patient prognosis.
Tumor immune infiltration is largely linked to the survival of a variety of HPV-related cancers. It has been demonstrated that high volumes of tumor-infiltrating lymphocytes (TILs) is associated with good prognosis in HPV-related cancer [52,53]. In addition to CD8+ T cells, which are a well-established lymphocyte subset associated with improved clinical outcomes [54,55], B cell markers have also been reported to improve the overall survival (OS) of HNSCC patients [56] and HPV-specific B cell phenotypes have been defined in the HNSCC tumor microenvironment [57]. NK cells have also been shown to play a protective role in HPV-associated cervical cancer immunotherapies [58]. However, the infiltration of specific immune cell states and their prognostic values remain unclear.
In this study, we set out to test the prognostic values of HPV infection and the expression of transcripts encoding components of the cellular secretome, such as growth factors, cytokines, chemokines, hormones, and their cognate receptors in HNSCC patients. We have applied a 24-cell-type TS and provided an overview of the TIL profiles in HPV-negative and -positive HNSCC patients as well as their prognostic associations. We find that a high abundance of activated NK cell or memory B cell TS are associated with good prognosis in HPV-infected HNSC patient tumors. Moreover, we also find that two genes encoding IL17 receptor family members, IL17RB and IL17REL, are associated with improved prognosis and may modulate the anti-tumor activity of memory B, NK cells, and T cell subsets in HPV-infected HNSCC patients.
2. Materials and Methods
2.1. TCGA Data Collection and Viral Load Estimation
We first collected RNA-seq data and clinical results for 500 tumor biopsies in the GDC Data Portal [59]. According to the study published by Cantalupo’s group, HPV viral alignments were detected by either RNA-seq or DNA-seq in 88 TCGA-HNSCC tumor biopsies, which were considered HPV-infected patients, whilst 412 tumor samples were deemed uninfected without any aligned HPV viral sequences. We took the maximum aligned viral sequences from HNSCC patients as defined by the Cantalupo group [60] for measuring HPV viral load.
2.2. Generation of Transcriptional Signatures and Deconvolution
The processes of the generation and benchmark of 24 cell type transcriptional signatures were described in our former publications [61,62]. Briefly, we first collected 592 highly curated (i.e., for which identity was confirmed in the literature), non-redundant biological replicates for 24 different immune and stromal cell types. The expected value and variability of gene transcription abundance for each cell type was then estimated by a Bayesian statistical model known as CellSig (github: stemangiola/cellsig), based on a negative binomial data distribution [63]. Afterwards, the transcriptional markers were selected by the pairwise comparison of each cell type within cell type categories along the cell differentiation hierarchy, which together formed the transcriptional signature (TS) matrix, as described [61,62].
2.3. RNA-seq Data Manipulation and Differential Expression Analysis
Raw read counts from TCGA-HNSCC RNA-seq data were scaled with the trimmed mean of M values (TMM) method [64] to compensate for differences in sequencing depth. Differential expression analysis was performed through edgeR quasi-likelihood dispersion driven by Rstudio between HPV-infected and HPV-free groups according to the publication of Cantalupo’s group [60] without other covariates (Supplementary Figure S1). The threshold log fold change (logFC) ≥ 1.5 or log fold change ≤ −1.5 and false discovery rate (FDR) ≤ 0.05 defined the differentially significant genes. The significant genes were further classified and labeled by growth factor, cytokine, chemokine, hormone and their relevant receptors downloaded from the KEGG database [65].
Single-cell RNA-seq (scRNA-seq) analysis has also been performed to understand the cell type expressing IL17RB and IL17REL. Four open-access HNSCC single-cell RNA sequencing datasets from GEO: GSE139324 [66], GSE103322 [67], GSE164190 [68] and GSE173647. All analysis was performed based on the Seurat [69,70,71,72] package under the R environment.
2.4. Statistical Analysis
We estimated the cell type relative fractions for each biological replicate with our reference RNA-seq-derived transcriptional signature and the RNA-seq data from TCGA-HNSCC based on CIBERSORT [73]. Then, Kaplan–Meier (KM) survival curves were estimated from the median split CIBERSORT-inferred cell type fractions through the R framework tidybulk [74], with progression-free survival information as the measure of outcomes for HNSCC patients. The quantity percent survival versus time-to-event statistics was produced by the log-rank (Mantel–Cox) test [75]. The statistics of KM curves were adjusted using the Benjamini–Hochberg (BH) procedure. Further, the correlation analysis was performed by Pearson’s correlation test with default adjusted p-values.
Data analysis and visualization were performed using the R environment in RStudio. Packages include tidybulk [74], tidyHeatmap [76], survminer [77], survival [78], foreach [79], org.Hs.eg.db [80], cowplot [81], ggsci [82], GGally [83], gridExtra [84], reshape [85], Hmisc [86], and scales [87].
3. Results
3.1. HPV-Infected HNSCC Patients Have Increased Immune Cell TS Expression in HNSCC Tumors and More Favorable Survival
A previous study has shown that HNSCC patients have significantly lower rates of metastases with HPV infections, suggesting that viral infection may enhance cancer immune surveillance and influence HNSCC patient prognosis [88]. To understand whether HPV-infected HNSCC patients have improved prognosis, we compared the survival of HPV-positive and HPV-free HNSCC patients from 500 HNSCC tumors with both OS and last contact day information in the TCGA-HNSCC cohort (TCGA-HNSCC) (Figure 1). We found that HPV-positive patients (n = 88) had significantly better clinical outcomes than HPV-free patients (n = 412, Figure 1A).
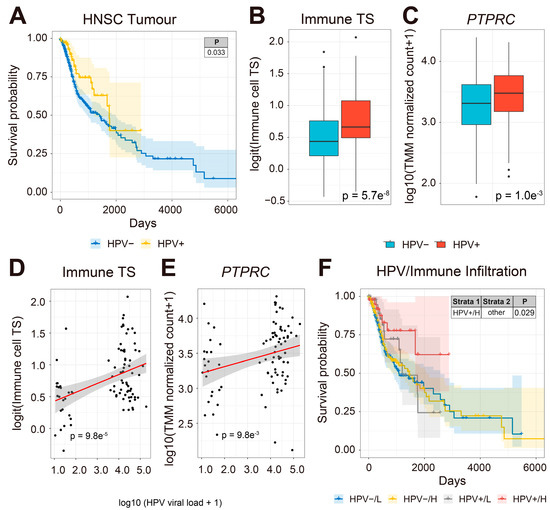
Figure 1.
HPV infection and immune cell transcriptional signature are associated with improved prognosis in HNSCC patients. (A) KM survival curve comparing HPV-infected (yellow line) and HPV-free (blue line) HNSCC patients (y-axis, survival probability; x-axis, days). HPV-infected patients have improved survival compared to HPV-free HNSCC patients. (B) Box plots comparing HNSCC tumor expression of immune cell TS and (C) PTPRC (CD45). (D) Scatter plots of the correlation (red line) between HPV viral load (x-axis) and the expression of immune cell TS (y-axis) and (E) PTPRC (y-axis) in HPV-infected HNSCC patients. The abundance of immune cell TS and PTPRC is positively correlated with viral load in HPV-infected HNSCC tumors. (F) Combined KM survival plot (y-axis, survival probability; x-axis, days) of HPV-infection status and tumor expression of total immune cell TS. The KM curve represents HNSCC patient survival plotted in all four combinations for each stratum (HPV%#x2212;/L, HPV-%#x2212;/H, HPV+/L, and HPV+/H) with total tumor expression of immune cell TS split by the median into L (low) and H (high) patient groups. HPV-infected HNSCC patients with high expression of immune cell TS have significantly better clinical outcomes compared to all other groups.
Previous studies have reported that viral infection may enhance anti-tumor immunity in cancer patients by activating type-I interferon signaling [89,90,91]. To estimate the effect of viral load on tumor immune infiltration, we compared tumor expression of immune cell transcriptional signatures (TS) [61,62] between 88 HPV-infected and 412 HPV-free tumors in 500 primary HNSCC tumor biopsies (Figure 1B). Interestingly, the expression of immune cell TS and the gene encoding the common leukocyte antigen, CD45 (PTPRC), were significantly upregulated in HPV-infected compared to HPV-free HNSCC tumors, respectively (Figure 1B,C). Next, we asked whether the abundance of immune cell TS and PTPRC correlate with HPV viral load in HNSCC patient samples. The expression of immune TS and PTPRC was positively correlated with HPV viral load in HNSCC tumors (Figure 1D,E). Moreover, HPV-infected HNSCC patients with high viral load and high tumor abundance of immune TS had significantly improved prognosis compared to the rest of the cohort (Figure 1F). Our results show that HPV-positive HNSCC patient tumors have more abundant immune cell TS, which is associated with improved prognosis compared to HPV-negative HNSCC patients. We conclude that HPV-positive tumors are more immunogenic than HPV-free tumors, which is associated with an improved clinical outcome in HNSCC patients.
3.2. Increased Tumor Abundance of Memory B and NK Cell TS Are Associated with Improved Prognosis in HPV-Infected HNSCC Patients
To further understand the immune cell types associated with improved prognosis in HPV-infected HNSCC tumors, we compared the expression of TS from 24 different immune and stromal cell types in HPV-positive and HPV-free patients (Figure 2A–C). We found that TS of monocytes, immature dendritic cells (iDC), memory B cells, resting NK cells (ReNK) and activated NK cells (aNK) were more abundant in HPV-infected patient tumors (Figure 2B,C). Memory B cells and NK cells play important roles in anti-tumor immunity [46,92,93,94,95,96,97]. We wanted to understand the prognostic values of the cell-type-specific TS in HNSCC patients. Intriguingly, HNSCC patients with higher abundance of either memory B or aNK TS had improved prognosis compared to HPV-free patients (Figure 2D), but not for other immune cell TS (Supplementary Figure S2). NK cells are known to be regulated by the missing-self recognition [44]. To understand whether the downregulation of MHC-I molecules at the transcriptional level was associated with the aNK TS, we compared the expression and combined survival of six MHC-I molecules (encoded by HLA-A, -B, -C, -E, -F, and -G) with the aNK TS (Supplementary Figure S3). Interestingly, we found HLA-E was significantly downregulated, whilst its relevant receptors CD94 (encoded by KLRD1) and NKG2A (encoded by KLRC1) were significantly upregulated in HPV-infected patients (Supplementary Figure S3A). The combined KM survival plot showed that HPV-infected patients with both low MHC-I and high aNK TS expression had improved prognosis for HLA-A, -C and -F (Supplementary Figure S3B). Similar trends were also observed for HLA-B, -E and -G. These results indicate that HPV infection may boost anti-tumor immunity by increasing tumor infiltration of memory B cells and activated NK cells in HNSCC patients.
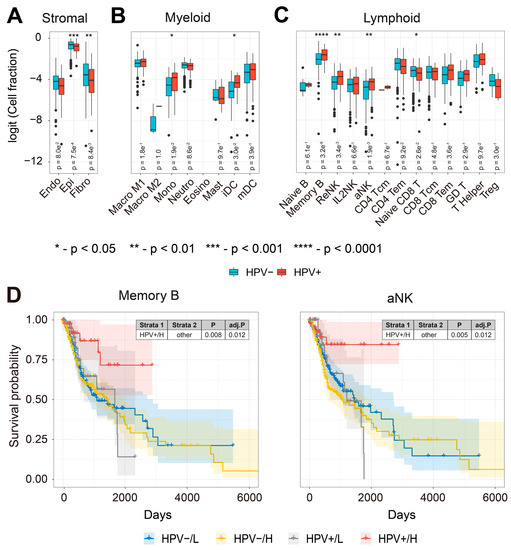
Figure 2.
TS of Memory B and activated NK cells are associated with improved prognosis in HPV-positive HNSCC patients. (A) Box plots comparing the abundance of stromal cell TS (endothelial cells, epithelial cells, and fibroblasts); (B) myeloid cell TS (M1 and M2 macrophages, monocytes, neutrophils, eosinophils, mast cells, immature and mature dendritic cells); (C) lymphoid cell TS (naïve B cells, memory B cells, resting NK cells (ReNK), IL2-primed NK cells (IL2NK), activated NK cells (aNK), central (Tcm) and effector memory (Tem) CD4+ T cells, naïve CD8 T cells, Tcm and Tem CD8+ T cells, γδ T cells, Helper T cells and regulatory T cells (Treg) (* refers to p-value < 0.05, ** refers to p-value < 0.01, *** refers to p-value < 0.001, and **** refers to p-value < 0.0001); (D) KM survival curves (y-axis, survival probability; x-axis, days) constructed for combinations of HPV infection and memory B cell and aNK TS expression in HNSCC patient tumors. Each cell’s TS expression was split by the median into L and H groups. HNSCC patients with both HPV infection and high expression of either memory B cell TS or aNK TS had significantly improved prognosis compared to other groups.
3.3. Secretome Genes and Cognate Receptors Are Differentially Expressed in HPV-Infected HNSCC Patients
Given that growth factor pathways may contribute to anti-tumor immunity [46,62], we were interested in discovering other potential biomarkers in the secretomes of HPV-infected patients that may contribute to anti-tumor immunity. Among all the genes that differentially expressed (logFC ≥ 1.5 or logFC ≤ −1.5 and FDR ≤ 0.05) in HPV-infected HNSCC patients (Supplementary Table S4), growth factor or relevant receptor genes NGF, CSF2, EREG, IL1F10, FGF19, and FGFBP2 were downregulated, whereas CSPG5, IGBPL1, IGFALS, and BMP3 were upregulated in HPV-infected tumors (Figure 3A). As for cytokine, chemokine, and relevant receptor genes, NGF, IL1RL1, CSF2, IL1F10, CXCL5, and IFNK were downregulated, and IL17RB, IL17REL, CCL25, and BMP3 were upregulated with HPV infection (Figure 3B). The hormone and related receptor genes GAL, RXFP1, and CGB5 were downregulated, whilst EHNO, NPPG and TG were upregulated in HPV-infected HNSCC patients (Figure 3C). In addition, we tested the correlation between the upregulated secretome genes and immune- and stromal-cell-type TS and HPV infection status to provide insights into possible pathways or cell responses in HPV-positive and HPV-negative HNSCC patients (Figure 3D, Supplementary Figure S5). Apart from BMP3, which was positively correlated with the fibroblast TS in HPV-infected HNSCC patients, most upregulated secretome genes analyzed were positively associated with immune cells TS in HPV-infected patients compared to uninfected HNSCC patients (Figure 3D). Notable positive correlations for growth factors and receptors existed between: IGFBPL1 (encoding insulin-like growth factor-binding protein 1)/Helper T, γδ T cell (GD) T and monocyte TS; BMP3 (encoding bone morphogenetic protein 3)/fibroblast (Fibro) TS; IGFALS (encoding acid labile subunit)/T helper cell and GD T TS; CSPG5 (encoding chondroitin sulfate proteoglycan 5)/naive CD8 T and M2 macrophage TS; and cytokines/chemokines and receptors: IL17REL/Treg, T Helper, aNK, monocyte, mast cell and memory B TS; CCL25 (encoding chemokine C-C motif ligand 25)/Treg and GD T, CD8 Tem, IL2NK, monocyte, Mast cells and memory B cells TS; IL17RB/Treg, GD T, naïve CD8 T, CD4 Tem, aNK, monocyte, M2 macrophage and memory B TS; and hormones and receptors: ENHO (encoding adropin)/naïve CD8 T. CD8 Tcm, CD4 Tem, aNK, Macro M2 TS; NPPC (encoding natriuretic peptide precursor C)/Treg, GD T cell, aNK, monocytes and memory B TS; and TG (encoding thyroglobulin)/GD T cell, CD4 Tem, monocyte, mast cell and memory B TS in HPV-infected HNSCC tumors (Figure 3D). Overall, the upregulation of secretome genes was correlated with immune cell TS rather than stromal cell TS in HPV-infected HNSCC patients compared to HPV-free HNSCC patients, suggesting that secretome-encoded transcripts may play a role in anti-tumor immunity and improved prognosis in HPV-infected HNSCC patients.
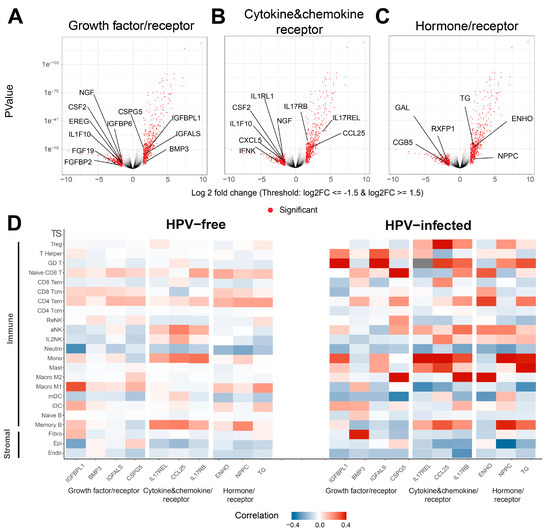
Figure 3.
Figure 3. Identification of differentially expressed secretome genes in HPV-infected HNSCC tumors. (A) Volcano plots showing significantly downregulated or upregulated growth factor, (B) cytokine and chemokine, and (C) hormone and relevant receptor genes in HPV-infected HNSCC tumors. (D) Correlation heatmap of significantly upregulated secretome genes and all cell TS in HPV-free and -infected HNSCC patients.
3.4. Expression of IL17RB and IL17REL Are Associated with Improved Prognosis in HPV-Positive HNSCC Patients
We wanted to understand the relationship between the expression of genes encoding growth factors (Figure 4A, Supplementary Figure S6A), cytokines and chemokines (Figure 4B, Supplementary Figure S6B), hormones (Figure 4C, Supplementary Figure S6C) and their cognate receptors on the prognosis of HNSCC patients and viral load. In HPV-free HNSCC patients, the expression of all selected secretome genes did not influence prognosis except for CCL25 (Figure 4B). In contrast, higher expression of IL17REL was associated with improved prognosis in HPV-positive HNSCC patients, while higher expression of CSPG5, IL17RB, CCL25, NPPC and TG trended towards improved prognosis and ENHO trended towards poor prognosis in HPV-positive HNSCC patients (Figure 4B). Moreover, consistent with upregulation in HPV infection, expression of all secretome genes was positively correlated with HPV viral load (Figure 4A–C).
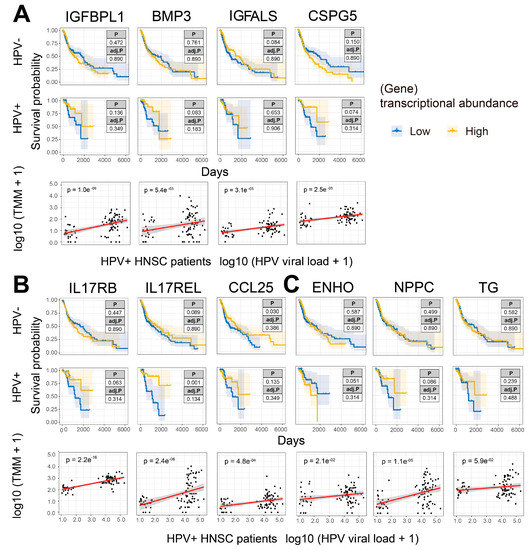
Figure 4.
Upregulated secretome (and receptor) genes are associated with improved HNSCC prognosis and are positively correlated with viral load. (A) KM curves (y-axis, survival probability; x-axis, days) constructed for growth factor, (B) cytokine and chemokine, (C) hormone and relevant receptor genes that were upregulated in HPV-infected HNSCC patients and having a significant correlation with viral loads in HPV-infected patients. Each gene expression was split by the median into L and H groups. The scatter plots were constructed to correlate the above genes and HPV viral loads in HPV-infected patients. High tumor expression of IL17REL was significantly associated with improved prognosis, while another IL17 family member IL17RB showed the same trend but without significance. The expression of both IL17REL and IL17RB was significantly positively correlated with HPV viral loads in infected HNSCC patients.
We next performed a combined survival analysis using all curated secretome genes upregulated in HPV-infected HNSCC tumors (Supplementary Table S4) with HPV viral load in HNSCC patients (Figure 5 and Supplementary Figure S7). Of the secretome genes analyzed, only higher expression of IL17RB and IL17REL was associated with improved prognosis in HPV-infected HNSCC patients, which was more marked in those patients with higher HPV viral loads (Figure 5 and Supplementary Figure S7) compared to HNSCC patients with lower HPV loads. Finally, higher expression of IL17A, which encodes the known anti-tumoral cytokine IL17A [9,13,14], was associated with improved prognosis (Supplementary Figure S8). These results show that higher expression of IL17RB and IL17REL are associated with higher viral load and improved prognosis of HPV-positive HNSCC patients.
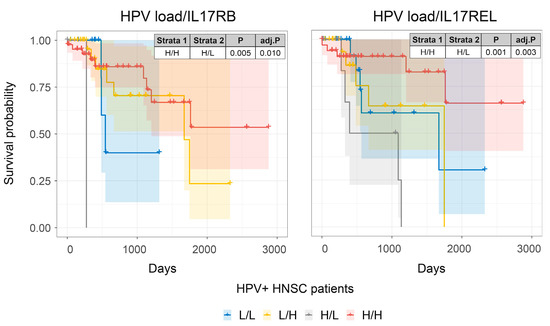
Figure 5.
The association of IL17 family receptors and survival in HPV-infected HNSCC patients. Combined HNSCC patient survival analysis stratified for HPV viral loads and IL17 family members, IL17RB and IL17REL, respectively. KM curves (y-axis, survival probability; x-axis, days) display HPV-infected HSNC patient survival plotted in all four combinations for each stratum (L/L, L/H, H/L, and H/H, both L and H groups were split by the median viral load or gene expression). For patients with higher HPV viral loads, high expression of either IL17RB or IL17REL resulted in enhanced prognosis.
3.5. High Expression of IL17RB and IL17REL and TIL Subset TS Are Associated with Improved Prognosis in HPV-Infected HNSCC Patients
Since higher expression of IL17RB, IL17REL, and the memory B and aNK TS are associated with improved prognosis in HPV-infected HNSCC patients, we aimed to understand whether these IL17 receptor family genes may cooperate with certain immune or stromal cell types for improved prognosis in HNSCC patients by carrying out a combined survival analysis based on tumor expression of either IL17RB or IL7REL and our immune and stromal cell TS in HPV-infected HNSCC patients (Figure 6). HNSCC patients with higher tumor expression of IL17RB combined with high expression of either the CD4 Tem, memory B cell, or aNK TS had improved prognosis compared to patients with lower expression of IL17RB and high expression of either the CD4 Tem, memory B cell, or aNK TS (Figure 6, top panel). Moreover, HNSCC patients with higher tumor expression of IL17REL combined with high expression of either the CD8 Tcm, memory B, M1 macrophages, or Helper T cell TS also had improved prognosis compared to those HNSCC patients with lower tumor expression of IL17RB and high expression of either CD8 Tcm, memory B, M1 macrophages or Helper T cell TS (Figure 6, bottom panel). These trends were not observed for any other immune or stromal cell TS in either HPV-positive or HPV-negative HNSCC patients (Supplementary Figure S9).
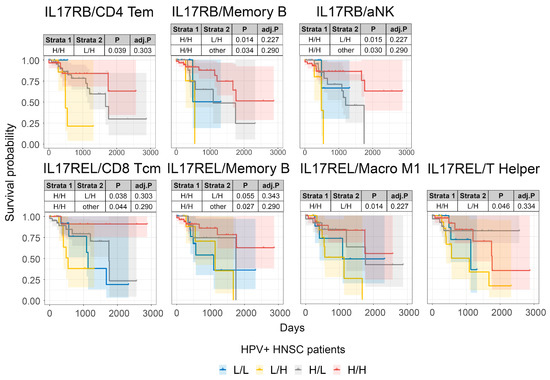
Figure 6.
The combined survival analysis of IL17RB, IL17REL and immune cell TS in HPV-infected HNSCC patients. Combined HNSCC patient survival analysis stratified for IL17RB and IL17REL and TIL TS expression in HPV-infected HNSCC tumors. KM curves (y-axis, survival probability; x-axis, days) display HPV-infected HSNC patient survival plotted in all four combinations for each stratum (L/L, L/H, H/L, and H/H, both L and H groups were split by the median viral load or gene expression). For patients with high TIL TS expression, higher IL17RB or IL17REL expression was associated with an improved prognosis.
In addition to our investigation, we analyzed four publicly available HNSCC scRNA-seq datasets, namely GSE139324 [66], GSE103322 [67], GSE164190 [68] and GSE173647, but were unable to detect significant read counts for IL17RB or IL17REL (Supplementary Figure S10). Our results suggest that the expression of IL17RB may influence the anti-tumor functions of CD4 Tem, memory B, and activated NK cells, and IL17REL may influence the anti-tumor functions of CD8Tcm, memory B cells, M1 macrophages, and Helper T cells in HPV-positive HNSCC tumors.
4. Discussion
Head and neck squamous cell carcinomas (HNSCC) are among the most common cancers worldwide, with over 870,000 new cases and 440,000 deaths in 2020 [98]. Smoking, chewing tobacco, alcohol and HPV infection are the main risk factors for HNSCC. The prognosis of HNSCC is known to be implicated by a group of host and tumor characteristics, including pathological differentiation grading, performance status, and tumor node metastasis (TNM) staging. HPV infection has been described to alleviate distant metastases and is associated with improved survival of HNSCC patients [88,99,100,101,102,103]. In this study, we aimed to determine the impact of transcripts encoding secreted factors, such as growth factors, hormones, chemokines and cytokines, and cognate receptors, and immune cell TS on the prognosis of HPV-infected and uninfected HNSCC patients to provide insights for biomarkers and future therapies that may target the secretome in HNSCC.
The immune response has been implicated in the development of HNSCC and HPV infection [104,105]. We hypothesized that the improved prognosis of HPV-infected HNSCC patients resulted from enhanced immune responses induced by viral infection at the tumor site. Tumor abundance of TS representing immune cells and the gene (PTPRC) encoding the common leukocyte antigen, CD45, were associated with improved prognosis in HPV-infected HNSCC patients compared to uninfected patients and positively correlated with HPV viral loads, suggesting HPV infection may enhance anti-tumor immunity in HNSCC patients. Analysis of the tumor abundance of 24 different cell-type-specific TS [61,62] showed that activated NK cells and memory B cells are associated with the improved prognosis of HPV-infected HNSCC patients. It has been reported that the gene expression profiles and tumor microenvironment (TME) in HPV-infected tumors are different from HPV-free malignancies [106]. Interestingly, we have found both resting NK cell and activated NK cell TS to be upregulated in HPV-infected patients. Since the reduction of MHC class I gene expression was identified as a hallmark of HPV-infected biopsies [107], these results may suggest increased recruitment and activation of NK cells in HNSCC tumors after HPV infection. Indeed, we found significant downregulation of transcripts encoding HLA-E in HPV-infected compared to HPV-free patients, and combined survival analysis showed that HPV-infected patients with low expression of HLA-A, -C, and -F and high tumor abundance of activated NK cells were associated with improved prognosis (Supplementary Figure S3). Memory B cells that initiate rapid immune responses were enriched in HNSCC tumor sites [57,108,109]. It has been reported that the genital HPV vaccine may help prevent cancers that develop in the oral cavity [110] and induce long-term protection by developing high-affinity memory B cells [111,112], indicating the importance of memory B cells in HPV-related cancers.
Interestingly, our study revealed no increase in tumor abundance of T cell subsets, in HPV-positive HNSCC patients, contrary to previous reports suggesting that favorable prognosis in HNSCC patients was mediated by CD8+ GZMA+ PRF1+ T cells [113]. Moreover, consistent with T cell exhaustion due to prolonged antigen stimulation in viral infections and cancer [114], HPV-infected HNSCC tumors showed significant upregulation of T cell exhaustion markers in the TCGA-HNSCC dataset [115]. However, our T cell transcriptional signatures were based on normal T cell subsets, which may have limited our ability to detect exhausted T cells enriched in HPV-positive HNSCC tumor sites. Proteomic approaches to identify candidate secreted protein biomarkers of prognostic significance in HNSCC have been reported, but are limited by small patient cohorts [3,116,117]. In our study, we chose a computational approach focused on understanding the association of transcripts encoding secreted molecules and their cognate receptors and the immune system on the prognosis of HPV-infected patients from the TCGA-HNSCC cohort comprising 500 patient samples. Taking this approach, we have clarified the prognostic values of two genes encoding proteins from the IL17 receptor family, IL17RB and IL17REL, in HNSCC that were significantly upregulated in HPV-infected compared to uninfected HNSCC patients. The IL17 signaling pathway has previously been found to be associated with poor prognosis in cancers, whilst the role of IL17REL in cancers remains unknown. Interestingly, expression of the gene for the current known ligand for IL17RB, IL17B, did not show any correlation with immune cell TS or survival (Supplementary Figure S11).
IL17REL encodes an IL17RE-like protein originally discovered using a similarity search for novel members of the IL17 receptor family. All IL17 receptor family sequences share the same conservative intracellular signaling motif named SEF/IL17R (SEFIR) [32]. However, IL17RE-like proteins lack the SEFIR motif but instead resembles the extracellular domain of IL17RE [32]. Though IL17REL was found to be highly associated with gout and ulcerative colitis in genome-wide association studies (GWAS) [33,35], the expression and function of IL17REL in different cell types remain largely unknown, especially in human cancers. In contrast, IL17RE is expressed on both epithelial and TH17 cells and recognizes IL17C as ligand. IL17C binding to IL17RE induces IL17 production from TH17 cells, which can enhance host innate immunity [118]. We conducted single-cell RNA sequencing (scRNA-seq) analysis on four publicly available HNSCC datasets to identify the cells expressing IL17RB or IL17REL. However, in contrast to bulk RNA-seq data, scRNA-seq data had a lower resolution for detecting IL17RB or IL17REL (Supplementary Figure S10). Thus, it awaits to be seen whether IL17RE-like has similar immune functions to IL17RE and how these might influence prognosis in HPV-infected HNSCC patients.
We have also found a positive correlation between IL17RB, IL17REL and memory B cell and aNK TS expression (Figure 3D). It has been previously studied that in germinal centers, CXCR4+ or CXCR5+ GC B cells were induced to migrate to CXCL12 or CXCL13 through the IL17RB signaling pathway [16]. Strikingly, CXCR4 has also been defined as a critical molecule in NK cell trafficking, and reduced CXCR4 expression significantly impaired NK cell migration in vitro [119,120,121]. It may be possible that increased expression of IL17RB and IL17REL may participate in the recruitment of memory B and activated NK cells in HPV-infected HNSCC patients.
To summarize, our study set out to uncover a possible role for genes encoding secretome factors or their receptors and their association with immune and stromal cell TS and HPV infection on the prognosis of HNSCC patients. We have uncovered a novel association between a higher tumor expression of IL17RB with CD4 Tem, memory B, and activated NK TS and IL17REL with CD8 Tcm, memory B, M1 macrophages and T Helper cell TS and the improved prognosis of HPV-infected HNSCC patients. Our results have important consequences for anti-tumor immunity and reveal potential new biomarkers or targets for immunotherapy in HPV-infected HNSCC patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12040572/s1, Figure S1: The correlation between principal components (PCs) of gene expression and all covariates; Figure S2: The KM survival curves (y-axis, survival probability; x-axis, days) constructed for combinations of HPV infection and 24 different cell TS expression in HNSCC patient tumors; Figure S3: Abundance and survival analysis of MHC-I molecules and cognate NK cell receptors; Table S4: All differentially expressed genes (logFC ≥ 1.5 or logFC ≤ −1.5 and FDR ≤ 0.05) in HPV-infected HNSCC patients; Figure S5: The correlation heatmap of significantly upregulated secretome genes and all cell TS in all HNSCC patients (both HPV-infected and HPV uninfected patients); Figure S6: Survival analysis and box plots of selected secretome genes in all HNSCC patients; Figure S7: Combined HNSCC patient survival analysis stratified for HPV viral loads and all the upregulated secret protein genes; Figure S8: Survival analysis, box plot and correlation test of IL17 family members; Figure S9: survival analysis of IL17RB, IL17REL and all cell TS; Figure S10: scRNA-seq analysis of IL17RB and IL17REL expression cell clusters; Figure S11: Survival analysis and correlation test of IL17 family members.
Author Contributions
Methodology, S.M., A.D.B., Y.S. and M.A.A.K.K.; data collection, Y.S. and M.A.A.K.K.; analysis performing, Y.S.; writing, figure editing, original draft preparation, Y.S. and A.D.B.; review and editing, A.D.B. and S.M.; supervision, A.D.B. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by an MRFF research acceleration grant APP1162217 awarded to A.B., and a University of Melbourne PhD scholarship awarded to Y.S. and M.A.A.K.K. S.M. is funded by a Galli Early Career Research Fellowship.
Data Availability Statement
The codes for all figures (including Supplementary Figures) are available at https://github.com/RAGG3D/HPV-TCGA-HNSCC (accessed on 16 March 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Cohen, E.E. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, L.; Savage, S.R.; Eguez, R.V.; Dou, Y.; Li, Y.; da Veiga Leprevost, F.; Jaehnig, E.J.; Lei, J.T.; Wen, B.; et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 2021, 39, 361–379.e16. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 Family Members and Inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef]
- Chung, S.-H.; Ye, X.-Q.; Iwakura, Y. Interleukin-17 family members in health and disease. Int. Immunol. 2021, 33, 723–729. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2019, 217, e20190456. [Google Scholar] [CrossRef]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.-M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar] [CrossRef]
- Chung, A.S.; Wu, X.; Zhuang, G.; Ngu, H.; Kasman, I.; Zhang, J.; Vernes, J.-M.; Jiang, Z.; Meng, Y.G.; Peale, F.V.; et al. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013, 19, 1114–1123. [Google Scholar] [CrossRef]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.; Liang, X.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.; Wang, Q.; Li, Y.; Chen, S.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60. [Google Scholar] [CrossRef]
- Jain, P.; Javdan, M.; Feger, F.K.; Chiu, P.Y.; Sison, C.; Damle, R.N.; Bhuiya, T.A.; Sen, F.; Abruzzo, L.V.; Burger, J.A.; et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: Delineation, distribution, and clinical relevance. Haematologica 2012, 97, 599–607. [Google Scholar] [CrossRef]
- Shi, Y.; Ullrich, S.J.; Zhang, J.; Connolly, K.; Grzegorzewski, K.J.; Barber, M.C.; Wang, W.; Wathen, K.; Hodge, V.; Fisher, C.L.; et al. A Novel Cytokine Receptor-Ligand Pair: Identification, Molecular Characterization, and in vivo Immunomodulatory Activity. J. Biol. Chem. 2000, 275, 19167–19176. [Google Scholar] [CrossRef]
- Ferretti, E.; Ponzoni, M.; Doglioni, C.; Pistoia, V. IL-17 superfamily cytokines modulate normal germinal center B cell migration. J. Leukoc. Biol. 2016, 100, 913–918. [Google Scholar] [CrossRef]
- Kokubu, T.; Haudenschild, D.R.; Moseley, T.A.; Rose, L.; Reddi, A.H. Immunolocalization of IL-17A, IL-17B, and Their Receptors in Chondrocytes during Fracture Healing. J. Histochem. Cytochem. 2008, 56, 89–95. [Google Scholar] [CrossRef]
- Moore, E.E.; Presnell, S.; Garrigues, U.; Guilbot, A.; LeGuern, E.; Smith, D.; Yao, L.; Whitmore, T.E.; Gilbert, T.; Palmer, T.D.; et al. Expression of IL-17B in neurons and evaluation of its possible role in the chromosome 5q-linked form of Charcot–Marie–Tooth disease. Neuromuscul. Disord. 2002, 12, 141–150. [Google Scholar] [CrossRef]
- Lee, J.; Ho, W.-H.; Maruoka, M.; Corpuz, R.T.; Baldwin, D.T.; Foster, J.S.; Goddard, A.D.; Yansura, D.G.; Vandlen, R.L.; Wood, W.I.; et al. IL-17E, a Novel Proinflammatory Ligand for the IL-17 Receptor Homolog IL-17Rh1 *. J. Biol. Chem. 2001, 276, 1660–1664. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.J.; Dong, C.; Homey, B.; et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC–activated Th2 memory cells. J. Exp. Med. 2007, 204, 1837–1847. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Ota, N.; Sambandam, A.; Wong, K.; Hackney, J.; Martinez-Martin, N.; Ouyang, W.; Pappu, R. Cutting Edge: IL-17B Uses IL-17RA and IL-17RB to Induce Type 2 Inflammation from Human Lymphocytes. J. Immunol. 2019, 202, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.M.; Lee, Y.-H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C.; Flaherty, S.; Chang, S.H.; Watarai, H.; Dong, C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 2015, 42, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Bastid, J.; Dejou, C.; Docquier, A.; Bonnefoy, N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ji, B.; Jiang, C.; Chen, Z.; Yao, N.; Mukaida, N.; Huang, H. IL17RB expression might predict prognosis and benefit from gemcitabine in patients with resectable pancreatic cancer. Pathol.-Res. Pract. 2019, 215, 152650. [Google Scholar] [CrossRef]
- Alinejad, V.; Hossein Somi, M.; Baradaran, B.; Akbarzadeh, P.; Atyabi, F.; Kazerooni, H.; Samadi Kafil, H.; Aghebati Maleki, L.; Siah Mansouri, H.; Yousefi, M. Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed. Pharmacother. 2016, 83, 229–240. [Google Scholar] [CrossRef]
- Alinejad, V.; Dolati, S.; Motallebnezhad, M.; Yousefi, M. The role of IL17B-IL17RB signaling pathway in breast cancer. Biomed. Pharmacother. 2017, 88, 795–803. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nambu, A.; Numata, T.; Yoshizaki, T.; Narushima, S.; Shimura, E.; Hiraishi, Y.; Arae, K.; Morita, H.; Matsumoto, K.; et al. The roles of IL-17C in T cell-dependent and -independent inflammatory diseases. Sci. Rep. 2018, 8, 15750. [Google Scholar] [CrossRef]
- Song, X.; Zhu, S.; Shi, P.; Liu, Y.; Shi, Y.; Levin, S.D.; Qian, Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat. Immunol. 2011, 12, 1151–1158. [Google Scholar] [CrossRef]
- Chang, S.H.; Reynolds, J.M.; Pappu, B.P.; Chen, G.; Martinez, G.J.; Dong, C. Interleukin-17C Promotes Th17 Cell Responses and Autoimmune Disease via Interleukin-17 Receptor E. Immunity 2011, 35, 611–621. [Google Scholar] [CrossRef]
- Song, X.; Gao, H.; Lin, Y.; Yao, Y.; Zhu, S.; Wang, J.; Liu, Y.; Yao, X.; Meng, G.; Shen, N.; et al. Alterations in the Microbiota Drive Interleukin-17C Production from Intestinal Epithelial Cells to Promote Tumorigenesis. Immunity 2014, 40, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Jin, M.; Zhang, Y.; Wei, T.; Bai, Z. Evolution of the IL17 receptor family in chordates: A new subfamily IL17REL. Immunogenetics 2011, 63, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Balschun, T.; Sina, C.; Ellinghaus, D.; Häsler, R.; Mayr, G.; Albrecht, M.; Wittig, M.; Buchert, E.; Nikolaus, S.; et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat. Genet. 2010, 42, 292–294. [Google Scholar] [CrossRef]
- Sasaki, M.M.; Skol, A.D.; Hungate, E.A.; Bao, R.; Huang, L.; Kahn, S.A.; Allan, J.M.; Brant, S.R.; McGovern, D.P.B.; Peter, I.; et al. Whole-exome Sequence Analysis Implicates Rare Il17REL Variants in Familial and Sporadic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 20–27. [Google Scholar] [CrossRef]
- Dong, Z.; Li, Y.; Zhou, J.; Jiang, S.; Wang, Y.; Chen, Y.; Zhao, D.; Yang, C.; Qian, Q.; Ma, Y.; et al. Copy number variants of ABCF1, IL17REL, and FCGR3A are associated with the risk of gout. Protein Cell 2017, 8, 467–470. [Google Scholar] [CrossRef]
- Ferretti, E.; Di Carlo, E.; Ognio, E.; Fraternali-Orcioni, G.; Corcione, A.; Belmonte, B.; Ravetti, J.L.; Tripodo, C.; Ribatti, D.; Pistoia, V. IL-25 dampens the growth of human germinal center-derived B-cell non Hodgkin Lymphoma by curtailing neoangiogenesis. OncoImmunology 2018, 7, e1397249. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.D.; Lantier, L.; Reynolds, J.; Wang, J.; Re, F. Critical role of IL-25-ILC2-IL-5 axis in the production of anti-Francisella LPS IgM by B1 B cells. PLoS Pathog. 2021, 17, e1009905. [Google Scholar] [CrossRef]
- Shibui, A.; Shimura, E.; Nambu, A.; Yamaguchi, S.; Leonard, W.J.; Okumura, K.; Sugano, S.; Sudo, K.; Nakae, S. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine 2012, 59, 108–114. [Google Scholar] [CrossRef]
- Basso, K.; Dalla-Favera, R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 2015, 15, 172–184. [Google Scholar] [CrossRef]
- MacLennan, I.C.M. Germinal Centers. Annu. Rev. Immunol. 1994, 12, 117–139. [Google Scholar] [CrossRef]
- Klein, U.; Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008, 8, 22–33. [Google Scholar] [CrossRef]
- Ljunggren, H.-G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Shifrin, N.; Raulet, D.H.; Ardolino, M. NK cell self tolerance, responsiveness and missing self recognition. Semin. Immunol. 2014, 26, 138–144. [Google Scholar] [CrossRef]
- Barrow, A.D.; Colonna, M. Exploiting NK Cell Surveillance Pathways for Cancer Therapy. Cancers 2019, 11, 55. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172, 534–548.e19. [Google Scholar] [CrossRef]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Boles, K.S.; Barchet, W.; Diacovo, T.; Cella, M.; Colonna, M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005, 106, 779–786. [Google Scholar] [CrossRef]
- Okumura, G.; Iguchi-Manaka, A.; Murata, R.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J. Exp. Med. 2020, 217, 1. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, N.; Lu, Y.; Davidson, D.; Colonna, M.; Veillette, A. DNAM-1 controls NK cell activation via an ITT-like motif. J. Exp. Med. 2015, 212, 2165–2182. [Google Scholar] [CrossRef]
- Khorana, A.A.; Ryan, C.K.; Cox, C.; Eberly, S.; Sahasrabudhe, D.M. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma. Cancer 2003, 97, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Saber, C.N.; Grønhøj Larsen, C.; Dalianis, T.; von Buchwald, C. Immune cells and prognosis in HPV-associated oropharyngeal squamous cell carcinomas: Review of the literature. Oral Oncol. 2016, 58, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M.L. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Kissick, H.T.; Patel, M.R.; Cardenas, M.A.; Prokhnevska, N.; Obeng, R.C.; Nasti, T.H.; Griffith, C.C.; Im, S.J.; Wang, X.; et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef]
- Kim, S.S.; Shen, S.; Miyauchi, S.; Sanders, P.D.; Franiak-Pietryga, I.; Mell, L.; Gutkind, J.S.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. B Cells Improve Overall Survival in HPV-Associated Squamous Cell Carcinomas and Are Activated by Radiation and PD-1 Blockade. Clin. Cancer Res. 2020, 26, 3345–3359. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Cantalupo, P.G.; Katz, J.P.; Pipas, J.M. Viral Sequences in Human Cancer. Virology 2018, 513, 208–216. [Google Scholar] [CrossRef]
- Sun, Y.; Sedgwick, A.J.; Khan, M.A.-A.-K.; Palarasah, Y.; Mangiola, S.; Barrow, A.D. A Transcriptional Signature of IL-2 Expanded Natural Killer Cells Predicts More Favorable Prognosis in Bladder Cancer. Front. Immunol. 2021, 12, 724107. [Google Scholar] [CrossRef]
- Sun, Y.; Sedgwick, A.J.; Palarasah, Y.; Mangiola, S.; Barrow, A.D. A Transcriptional Signature of PDGF-DD Activated Natural Killer Cells Predicts More Favorable Prognosis in Low-Grade Glioma. Front. Immunol. 2021, 12, 668391. [Google Scholar] [CrossRef]
- Pillow, J.; Scott, J. Fully Bayesian inference for neural models with negative-binomial spiking. Adv. Neural Inf. Process. Syst. 2012, 25, 1898–1906. Available online: https://dl.acm.org/doi/10.5555/2999325.2999347 (accessed on 1 July 2019). [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucl. Acids Res. 2022, gkac963. [Google Scholar] [CrossRef]
- Cillo, A.R.; Kürten, C.H.; Tabib, T.; Qi, Z.; Onkar, S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.J.; et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 2020, 52, 183–199.e9. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Kürten, C.H.L.; Kulkarni, A.; Cillo, A.R.; Santos, P.M.; Roble, A.K.; Onkar, S.; Reeder, C.; Lang, S.; Chen, X.; Duvvuri, U.; et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat. Commun. 2021, 12, 7338. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M.; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, S.; Molania, R.; Dong, R.; Doyle, M.A.; Papenfuss, A.T. Tidybulk: An R tidy framework for modular transcriptomic data analysis. Genome Biol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Lunde, A.; Timmermann, A.; Blake, D. The hazards of mutual fund underperformance: A Cox regression analysis. J. Empir. Financ. 1999, 6, 121–152. [Google Scholar] [CrossRef]
- Mangiola, S.; Papenfuss, A.T. TidyHeatmap: An R package for modular heatmap production based on tidy principles. J. Open Source Softw. 2020, 5, 2472. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ’ggplot2’. R Package Version 0.3 2017, 1. Available online: https://rpkgs.datanovia.com/survminer/reference/ggsurvplot.html (accessed on 1 July 2019).
- Therneau, T.M. A package for survival analysis in S. R Package Version 1977, 2, 280. Available online: https://www.semanticscholar.org/paper/A-Package-for-Survival-Analysis-in-S-Therneau/aba73f6957bce1648f066935f7ea85a99119be5d (accessed on 1 July 2019).
- Weston, S. Foreach: Provides Foreach Looping Construct. R Package Version 1.5.1. 2019. Available online: https://CRAN.R-project.org/package=foreach (accessed on 15 November 2022).
- Carlson, M. Genome Wide Annotation for Human. 2019. Available online: https://bioconductor.org/packages/release/data/annotation/html/org.Hs.eg.db.html (accessed on 10 March 2020).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. Available online: https://wilkelab.org/cowplot (accessed on 31 January 2023).
- Xiao, N. Ggsci: Scientific Journal and Sci-Fi Themed Color Palettes for ‘ggplot2’. R Package Version 2.9. 2018. Available online: https://CRAN.R-project.org/package=ggsci (accessed on 12 June 2022).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofman, H.; et al. GGally: Extension to “ggplot2”. R Package Version 2.1.2. 2021. Available online: https://CRAN.R-project.org/package=GGally (accessed on 19 October 2021).
- Auguie, B. GridExtra: Miscellaneous Functions for “Grid” Graphics. R Package Version 2.3. 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 4 April 2022).
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Hmisc: Harrell Miscellaneous. R Package Version 0.4.7-0. 2022. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 1 July 2022).
- Wickham, H.; Seidel, D. Scales: Scale Functions for Visualization; 2022. R Package Version 1.2.1. Available online: https://CRAN.R-project.org/package=scales (accessed on 24 January 2023).
- Duprez, F.; Berwouts, D.; De Neve, W.; Bonte, K.; Boterberg, T.; Deron, P.; Huvenne, W.; Rottey, S.; Mareel, M. Distant metastases in head and neck cancer. Head Neck 2017, 39, 1733–1743. [Google Scholar] [CrossRef]
- Vitiello, G.A.F.; Ferreira, W.A.S.; de Lima, V.C.C.; da Silva Medina, T. Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment. Front. Immunol. 2021, 12, 782852. [Google Scholar] [CrossRef]
- Brown, M.C.; Mosaheb, M.M.; Mohme, M.; McKay, Z.P.; Holl, E.K.; Kastan, J.P.; Yang, Y.; Beasley, G.M.; Hwang, E.S.; Ashley, D.M.; et al. Viral infection of cells within the tumor microenvironment mediates antitumor immunotherapy via selective TBK1-IRF3 signaling. Nat. Commun. 2021, 12, 1858. [Google Scholar] [CrossRef]
- Jacqueline, C.; Tasiemski, A.; Sorci, G.; Ujvari, B.; Maachi, F.; Missé, D.; Renaud, F.; Ewald, P.; Thomas, F.; Roche, B. Infections and cancer: The “fifty shades of immunity” hypothesis. BMC Cancer 2017, 17, 257. [Google Scholar] [CrossRef]
- Downs-Canner, S.M.; Meier, J.; Vincent, B.G.; Serody, J.S. B Cell Function in the Tumor Microenvironment. Annu. Rev. Immunol. 2022, 40, 169–193. [Google Scholar] [CrossRef]
- Kinker, G.S.; Vitiello, G.A.F.; Ferreira, W.A.S.; Chaves, A.S.; Cordeiro de Lima, V.C.; da Silva Medina, T. B Cell Orchestration of Anti-tumor Immune Responses: A Matter of Cell Localization and Communication. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2022, 1–16. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Janecka-Widła, A.; Mucha-Małecka, A.; Majchrzyk, K.; Halaszka, K.; Przewoźnik, M.; Słonina, D.; Biesaga, B. Active HPV infection and its influence on survival in head and neck squamous-cell cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 1677–1692. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Grandis, J.R.; Ferris, R.L. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu. Rev. Med. 2016, 67, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Ragin, C.C.R.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.S.; Koldjær Sølling, A.S.; Ovesen, T.; Rusan, M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int. J. Cancer 2014, 134, 2755–2763. [Google Scholar] [CrossRef]
- Zeng, H.; Song, X.; Ji, J.; Chen, L.; Liao, Q.; Ma, X. HPV infection related immune infiltration gene associated therapeutic strategy and clinical outcome in HNSCC. BMC Cancer 2020, 20, 796. [Google Scholar] [CrossRef]
- Aggarwal, N.; Yadav, J.; Thakur, K.; Bibban, R.; Chhokar, A.; Tripathi, T.; Bhat, A.; Singh, T.; Jadli, M.; Singh, U.; et al. Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Näsman, A.; Andersson, E.; Nordfors, C.; Grün, N.; Johansson, H.; Munck-Wikland, E.; Massucci, G.; Dalianis, T.; Ramqvist, T. MHC class I expression in HPV positive and negative tonsillar squamous cell carcinoma in correlation to clinical outcome. Int. J. Cancer 2013, 132, 72–81. [Google Scholar] [CrossRef]
- Pretscher, D.; Distel, L.V.; Grabenbauer, G.G.; Wittlinger, M.; Buettner, M.; Niedobitek, G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 2009, 9, 292. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kürten, C.H.L.; et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef]
- D’Souza, G.; Dempsey, A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev. Med. 2011, 53, S5–S11. [Google Scholar] [CrossRef]
- Stanley, M. Immunobiology of HPV and HPV vaccines. Gynecol. Oncol. 2008, 109, S15–S21. [Google Scholar] [CrossRef]
- Scherer, E.M.; Smith, R.A.; Simonich, C.A.; Niyonzima, N.; Carter, J.J.; Galloway, D.A. Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity. PLoS Pathog. 2014, 10, e1004461. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Damasio, M.P.S.; Nascimento, C.S.; Andrade, L.M.; de Oliveira, V.L.; Calzavara-Silva, C.E. The role of T-cells in head and neck squamous cell carcinoma: From immunity to immunotherapy. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Ghasemi, F.; Barrett, J.W.; Koropatnick, J.; Nichols, A.C.; Mymryk, J.S.; Maleki Vareki, S. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018, 7, e1498439. [Google Scholar] [CrossRef]
- Mumtaz, M.; Bijnsdorp, I.V.; Böttger, F.; Piersma, S.R.; Pham, T.V.; Mumtaz, S.; Brakenhoff, R.H.; Akhtar, M.W.; Jimenez, C.R. Secreted protein markers in oral squamous cell carcinoma (OSCC). Clin. Proteom. 2022, 19, 4. [Google Scholar] [CrossRef]
- Hussein, M.R.; Cullen, K. Molecular biomarkers in HNSCC: Prognostic and therapeutic implications. Expert Rev. Anticancer. Ther. 2001, 1, 116–124. [Google Scholar] [CrossRef]
- Nies, J.F.; Panzer, U. IL-17C/IL-17RE: Emergence of a Unique Axis in TH17 Biology. Front. Immunol. 2020, 11, 341. [Google Scholar] [CrossRef]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection with Gain-of-Function Variant CXCR4R334X. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Mayol, K.; Biajoux, V.; Marvel, J.; Balabanian, K.; Walzer, T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood 2011, 118, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).