Molecular Detection of Toxoplasma gondii in Blood Samples of Domestic Livestock in the Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Collection
2.2. DNA Extraction, Genomic Detection, and Sequencing
2.3. Sequencing and Phylogenetic Tree
2.4. Statistical Analysis
3. Results
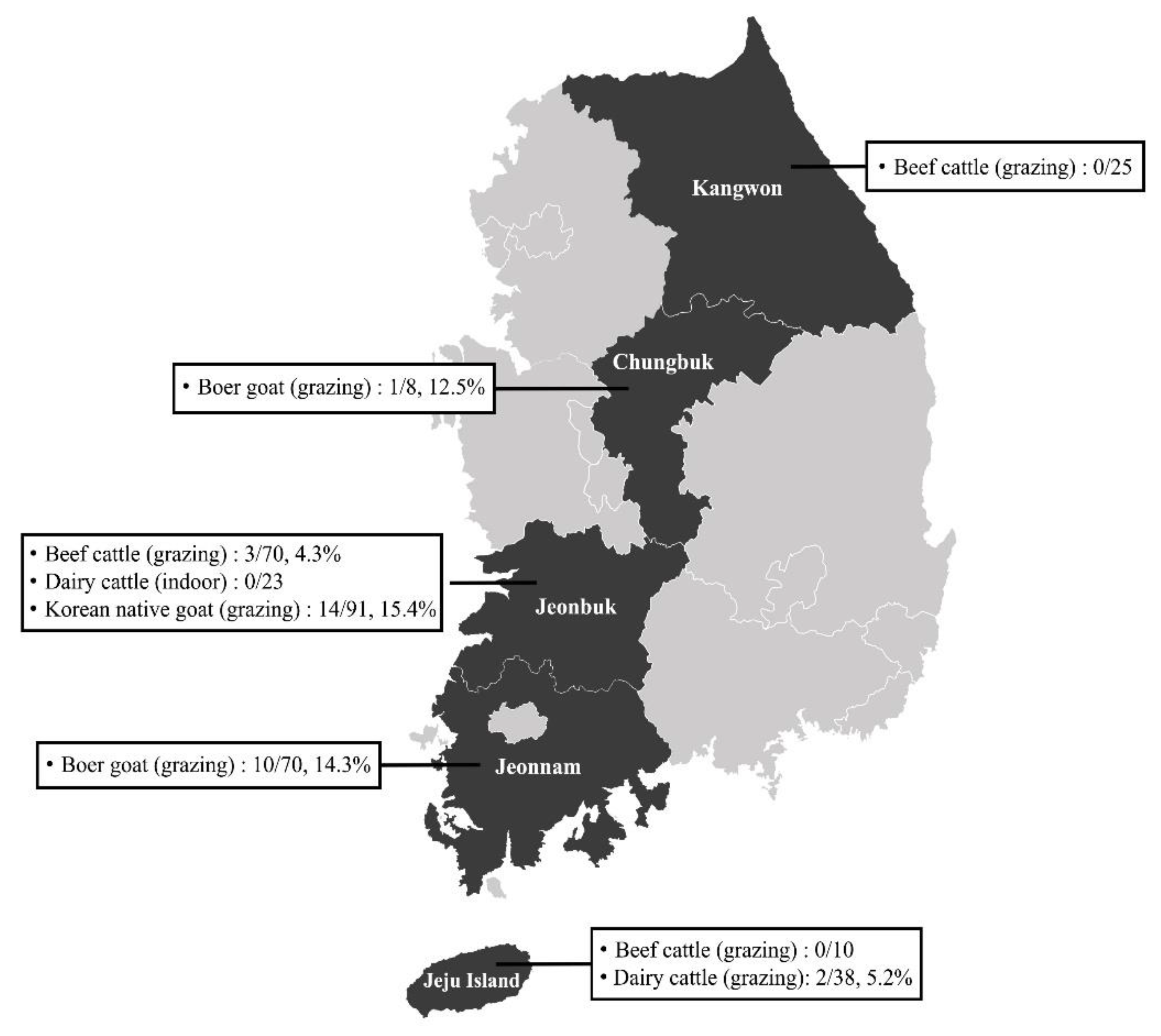
3.1. Prevalence of T. gondii in Ruminants
3.2. Sequence and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| PCR | polymerase chain reaction |
| ROK | Republic of Korea |
| T. gondii | Toxoplasma gondii |
References
- Dubey, J.P. Toxoplasmosis–A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fevre, E.M.; Sripa, B.; et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Jilo, K.; Tegegne, D.; Kasim, S.; Dabasa, G.; Zewdei, W. Seroprevalence and public health significance of toxoplasmosis in small ruminants of pastoral community in Yabello district, Borana zone, southern Ethiopia. Vet. Med. Int. 2021, 2021, 6683797. [Google Scholar] [CrossRef] [PubMed]
- Fazel, R.; Rezanezhad, H.; Solhjoo, K.; Kalantari, M.; Erfanian, S.; Armand, B.; Jahromi, M.E. PCR-based detection of Toxoplasma gondii from cattle in southern Iran. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101677. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P.; Baroch, J.A.; Swafford, S.R.; Fournet, V.F.; Hawkins-Cooper, D.; Pyburn, D.G.; Schmit, B.S.; Gamble, H.R.; Pedersen, K.; et al. Surveillance of feral swine for Trichinella spp. and Toxoplasma gondii in the USA and host-related factors associated with infection. Vet. Parasitol. 2014, 205, 653–665. [Google Scholar] [CrossRef]
- Khattab, R.A.; Barghash, S.M.; Mostafa, O.M.S.; Allam, S.A.; Taha, H.A.; Ashour, A.A.E. Seroprevalence and molecular characterization of Toxoplasma gondii infecting ruminants in the north-west of Egypt. Acta Trop. 2022, 225, 106139. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in sheep--the last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef]
- Alemayehu, G.; Mamo, G.; Alemu, B.; Desta, H.; Tadesse, B.; Benti, T.; Bahiru, A.; Yimana, M.; Wieland, B. Causes and flock level risk factors of sheep and goat abortion in three agroecology zones in Ethiopia. Front. Vet. Sci. 2021, 8, 615310. [Google Scholar] [CrossRef]
- Engeland, I.V.; Waldeland, H.; Kindahl, H.; Ropstad, E.; Andresen, O. Effect of Toxoplasma gondii infection on the development of pregnancy and on endocrine foetal-placental function in the goat. Vet. Parasitol. 1996, 67, 61–74. [Google Scholar] [CrossRef]
- Suazo-Cortez, R.; Martinez-Herrera, D.I.; Pardio-Sedas, V.T.; Cruz-Vazquez, C.R.; Morales-Alvarez, J.F.; Sanchez-Viveros, G.; Galindo-Tovar, M.E. Seroprevalence and risk factors associated with Toxoplasma gondii infection in sheep of Veracruz state, southeast Mexico. Vet. Res. Forum 2020, 11, 77–81. [Google Scholar] [CrossRef]
- Taalay, I.; Iqbal, R.K.; Asif, M.; Ahmad, A.; Amjad, M.; Anwar, F.N.; Aktas, M.; Ben Said, M.; Iqbal, F. Molecular survey of Toxoplasma gondii in cattle and buffaloes and phylogenetic position of Pakistani isolates based on ITS-1 gene. Comp. Immunol. Microbiol. Infect. Dis. 2022, 84, 101782. [Google Scholar] [CrossRef]
- Opsteegh, M.; Dam-Deisz, C.; de Boer, P.; DeCraeye, S.; Fare, A.; Hengeveld, P.; Luiten, R.; Schares, G.; van Solt-Smits, C.; Verhaegen, B.; et al. Methods to assess the effect of meat processing on viability of Toxoplasma gondii: Towards replacement of mouse bioassay by in vitro testing. Int. J. Parasitol. 2020, 50, 357–369. [Google Scholar] [CrossRef]
- Sun, T.; Rahman, S.U.; Cai, J.; Zeng, J.; Mi, R.; Zhang, Y.; Gong, H.; Ma, H.; Huang, Y.; Han, X. Seroprevalence and associated risk factors of Toxoplasma gondii infection in yaks (Bos grunniens) on the Qinghai–Tibetan Plateau of China. Parasite 2021, 28, 43. [Google Scholar] [CrossRef]
- Bahreh, M.; Hajimohammadi, B.; Eslami, G. Toxoplasma gondii in sheep and goats from central Iran. BMC Res. Notes 2021, 14, 46. [Google Scholar] [CrossRef]
- Aziz, M.N.; Iqbal, R.K.; Irfan, M.; Parveen, A.; Asif, M.; Ozubek, S.; Aktas, M.; Said, M.B.; Iqbal, F. First report on molecular epidemiology, seasonality and phylogeny of Toxoplasma gondii infecting goats from Khanewal district in Punjab, Pakistan. Acta Trop. 2022, 228, 106304. [Google Scholar] [CrossRef]
- Costa, J.M.; Cabaret, O.; Moukoury, S.; Bretagne, S. Genotyping of the protozoan pathogen Toxoplasma gondii using high-resolution melting analysis of the repeated B1 gene. J. Microbiol. Methods 2011, 86, 357–363. [Google Scholar] [CrossRef]
- Homan, W.L.; Limper, L.; Verlaan, M.; Borst, A.; Vercammen, M.; van Knapen, F. Comparison of the internal transcribed spacer, ITS 1, from Toxoplasma gondii isolates and Neospora caninum. Parasitol. Res. 1997, 83, 285–289. [Google Scholar] [CrossRef]
- Jones, C.D.; Okhravi, N.; Adamson, P.; Tasker, S.; Lightman, S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. Investig. Ophthalmol. Vis. Sci. 2000, 41, 634–644. [Google Scholar]
- Edvinsson, B.; Lappalainen, M.; Evengard, B.; Toxoplasmosis, E.S.G.f. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin. Microbiol. Infect. 2006, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; Sibley, L.D. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 1995, 172, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 2002, 185, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Mammari, N.; Halabi, M.A.; Yaacoub, S.; Chlala, H.; Darde, M.L.; Courtioux, B. Toxoplasma gondii modulates the host cell responses: An overview of apoptosis pathways. Biomed. Res. Int. 2019, 2019, 6152489. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernandez, E.; Ortega-Mora, L.M. Isolation, genotyping, and mouse virulence characterization of Toxoplasma gondii from free ranging Iberian pigs. Front. Vet. Sci. 2020, 7, 604782. [Google Scholar] [CrossRef]
- Grigg, M.E.; Boothroyd, J.C. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001, 39, 398–400. [Google Scholar] [CrossRef]
- Bossi, P.; Bricaire, F. Severe acute disseminated toxoplasmosis. Lancet 2004, 364, 579. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Ciampelli, A.; Sechi, P.; Veronesi, F.; Moretta, I.; Cambiotti, V.; Thompson, P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013, 9, 25. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Weiss, A.; Beer, O.; Tirosh-Levy, S.; Riklis, I.; Dveyrin, Z.; Rorman, E.; Cohen, N.Z.; Markovich, M.P.; Baneth, G. High infection rates of Toxoplasma gondii in cattle, sheep and pigs from Israel. Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101928. [Google Scholar] [CrossRef]
- Dessi, G.; Tamponi, C.; Pasini, C.; Porcu, F.; Meloni, L.; Cavallo, L.; Sini, M.F.; Knoll, S.; Scala, A.; Varcasia, A. A survey on Apicomplexa protozoa in sheep slaughtered for human consumption. Parasitol. Res. 2022, 121, 1437–1445. [Google Scholar] [CrossRef]
- Mancianti, F.; Nardoni, S.; Mugnaini, L.; Poli, A. Toxoplasma gondii in waterfowl: The first detection of this parasite in Anas crecca and Anas clypeata from Italy. J. Parasitol. 2013, 99, 561–563. [Google Scholar] [CrossRef]
- Sharif, M.; Sarvi, S.; Shokri, A.; Hosseini Teshnizi, S.; Rahimi, M.T.; Mizani, A.; Ahmadpour, E.; Daryani, A. Toxoplasma gondii infection among sheep and goats in Iran: A systematic review and meta-analysis. Parasitol. Res. 2015, 114, 1–16. [Google Scholar] [CrossRef]
- Alfonso, Y.; Fraga, J.; Jimenez, N.; Fonseca, C.; Dorta-Contreras, A.J.; Cox, R.; Capo, V.; Bandera, F.; Pomier, O.; Ginorio, D. Detection of Toxoplasma gondii in cerebrospinal fluid from AIDS patients by nested PCR and rapid identification of type I allele at B1 gene by RFLP analysis. Exp. Parasitol. 2009, 122, 203–207. [Google Scholar] [CrossRef]
- Ait Issad, N.; Abdelouahed, K.; Bekhouche, S.; Boubeuker, R.; Hamoudi Adjmi, H.; Ouchene-Khelifi, N.A.; Ouchene, N.; Ait Oudhia, K.; Khelef, D. Molecular detection of the B1 gene of Toxoplasma gondii in blood samples of female sheep and goats in Tebessa, northeastern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101530. [Google Scholar] [CrossRef]
- Amdouni, Y.; Rjeibi, M.R.; Rouatbi, M.; Amairia, S.; Awadi, S.; Gharbi, M. Molecular detection of Toxoplasma gondii infection in slaughtered ruminants (sheep, goats and cattle) in northwest Tunisia. Meat Sci. 2017, 133, 180–184. [Google Scholar] [CrossRef]
- Sroka, J.; Karamon, J.; Wojcik-Fatla, A.; Piotrowska, W.; Dutkiewicz, J.; Bilska-Zajac, E.; Zajac, V.; Kochanowski, M.; Dabrowska, J.; Cencek, T. Toxoplasma gondii infection in slaughtered pigs and cattle in Poland: Seroprevalence, molecular detection and characterization of parasites in meat. Parasit. Vectors 2020, 13, 223. [Google Scholar] [CrossRef]
- Tavassoli, M.; Ghorbanzadehghan, M.; Esmaeilnejad, B. Detection of Toxoplasma gondii in sheep and goats blood samples by PCR-RFLP in Urmia. Vet. Res. Forum 2013, 4, 43–47. [Google Scholar]
- Fajardo, H.V.; D’ávila, S.; Bastos, R.R.; Cyrino, C.D.; de Lima Detoni, M.; Garcia, J.L.; das Neves, L.B.; Nicolau, J.L.; Amendoeira, M.R. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, southern Brazil. Parasit. Vectors 2013, 6, 191. [Google Scholar] [CrossRef]
- Magalhaes, F.J.; Ribeiro-Andrade, M.; Alcantara, A.M.; Pinheiro, J.W.J.; Sena, M.J.; Porto, W.J.; Vieira, R.F.; Mota, R.A. Risk factors for Toxoplasma gondii infection in sheep and cattle from Fernando de Noronha island, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 511–515. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of tick-borne pathogens in ticks parasitizing native Korean goats (Capra hircus coreanae) in South Korea. Pathogens 2020, 9, 71. [Google Scholar] [CrossRef]
- Su, Y.J.; Ma, Z.D.; Qiao, X.; Wang, P.T.; Kang, Y.T.; Yang, N.A.; Jia, W.; Zhao, Z.J. Geospatial epidemiology of Toxoplasma gondii infection in livestock, pets, and humans in China, 1984–2020. Parasitol. Res. 2022, 121, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cezar, C.K.; Kwok, O.C.H. Public health and economic importance of Toxoplasma gondii infections in goats: The last decade. Res. Vet. Sci. 2020, 132, 292–307. [Google Scholar] [CrossRef]
- Silva, B.M.; Queiroz, W.C.C.; Maia, M.O.; Pacheco, R.C.; Aguiar, D.M.; Campos, M.S.; Bresciani, K.D.S.; Costa, A.J.; Gomes, A.A.D.; Santos-Doni, T.R. Seroprevalence and risk factors of Toxoplasma gondii in cattle from Unai, Minas Gerais state, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100610. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodriguez, L.C.; Tafur-Gómez, G.A.; Guzman-Barragan, B.L. Toxoplasma gondii in small ruminants in northeastern areas of Colombia: Seroprevalence and risk factors. Parasite Epidemiol. Control. 2020, 10, e00147. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, N.; Maksimov, P.; Conraths, F.J.; Kiossis, E.; Brozos, C.; Sotiraki, S.; Schares, G. Toxoplasma gondii in sheep and goats: Seroprevalence and potential risk factors under dairy husbandry practices. Vet. Parasitol. 2012, 190, 340–348. [Google Scholar] [CrossRef]
- Switaj, K.; Master, A.; Skrzypczak, M.; Zaborowski, P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin. Microbiol. Infect. 2005, 11, 170–176. [Google Scholar] [CrossRef]

| Species (No. of Samples Sequenced) | No. of T. gondii Positive Samples | χ2 (p-Value) | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Beef cattle (3) | 3/105 (2.9%) | 14.348 (0.002) | 1.000 | - | - |
| Dairy cattle (1) | 2/61 (3.3%) | 1.153 | 0.187–7.097 | 0.878 | |
| Boer goats (3) | 11/78 (14.1%) | 5.582 | 1.501–20.756 | 0.005 | |
| Korean native goats (11) | 14/91 (15.4%) | 6.182 | 1.716–22.269 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.-J.; Cho, H.-C.; Park, Y.-J.; Jang, D.-H.; Park, J.; Choi, K.-S. Molecular Detection of Toxoplasma gondii in Blood Samples of Domestic Livestock in the Republic of Korea. Pathogens 2023, 12, 547. https://doi.org/10.3390/pathogens12040547
Ji M-J, Cho H-C, Park Y-J, Jang D-H, Park J, Choi K-S. Molecular Detection of Toxoplasma gondii in Blood Samples of Domestic Livestock in the Republic of Korea. Pathogens. 2023; 12(4):547. https://doi.org/10.3390/pathogens12040547
Chicago/Turabian StyleJi, Min-Jeong, Hyung-Chul Cho, Yu-Jin Park, Dong-Hun Jang, Jinho Park, and Kyoung-Seong Choi. 2023. "Molecular Detection of Toxoplasma gondii in Blood Samples of Domestic Livestock in the Republic of Korea" Pathogens 12, no. 4: 547. https://doi.org/10.3390/pathogens12040547
APA StyleJi, M.-J., Cho, H.-C., Park, Y.-J., Jang, D.-H., Park, J., & Choi, K.-S. (2023). Molecular Detection of Toxoplasma gondii in Blood Samples of Domestic Livestock in the Republic of Korea. Pathogens, 12(4), 547. https://doi.org/10.3390/pathogens12040547






