Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Materials
2.2. Optimal Antibacterial Formulation of Honey and L. plantarum
2.3. In Vitro Antibacterial Activity of Honey–L. plantarum Formulation
2.4. Biofilm Formation Inhibition Assay
2.5. Live/Dead Bacterial Staining
2.6. Quantitative Real-Time PCR Analysis
2.7. Changes in the Growth of L. plantarum in the Formulation
2.8. Antibacterial Effect of Honey–L. plantarum Culture Supernatant
2.9. In Vivo Animal Experiment
2.9.1. Wound Infection Model
2.9.2. Evaluation of the Antibacterial Effect of Honey–L. plantarum Formulation on the Wounds
2.9.3. Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. Optimal Antibacterial Formulation of L. plantarum and Honey to Inhibit S. aureus
3.2. Honey–L. plantarum Formulation Inhibited the Growth of S. aureus, P. aeruginosa, and E. coli
3.3. Honey–L. plantarum Formulation Inhibited the Biofilm Formation of S. aureus and P. aeruginosa
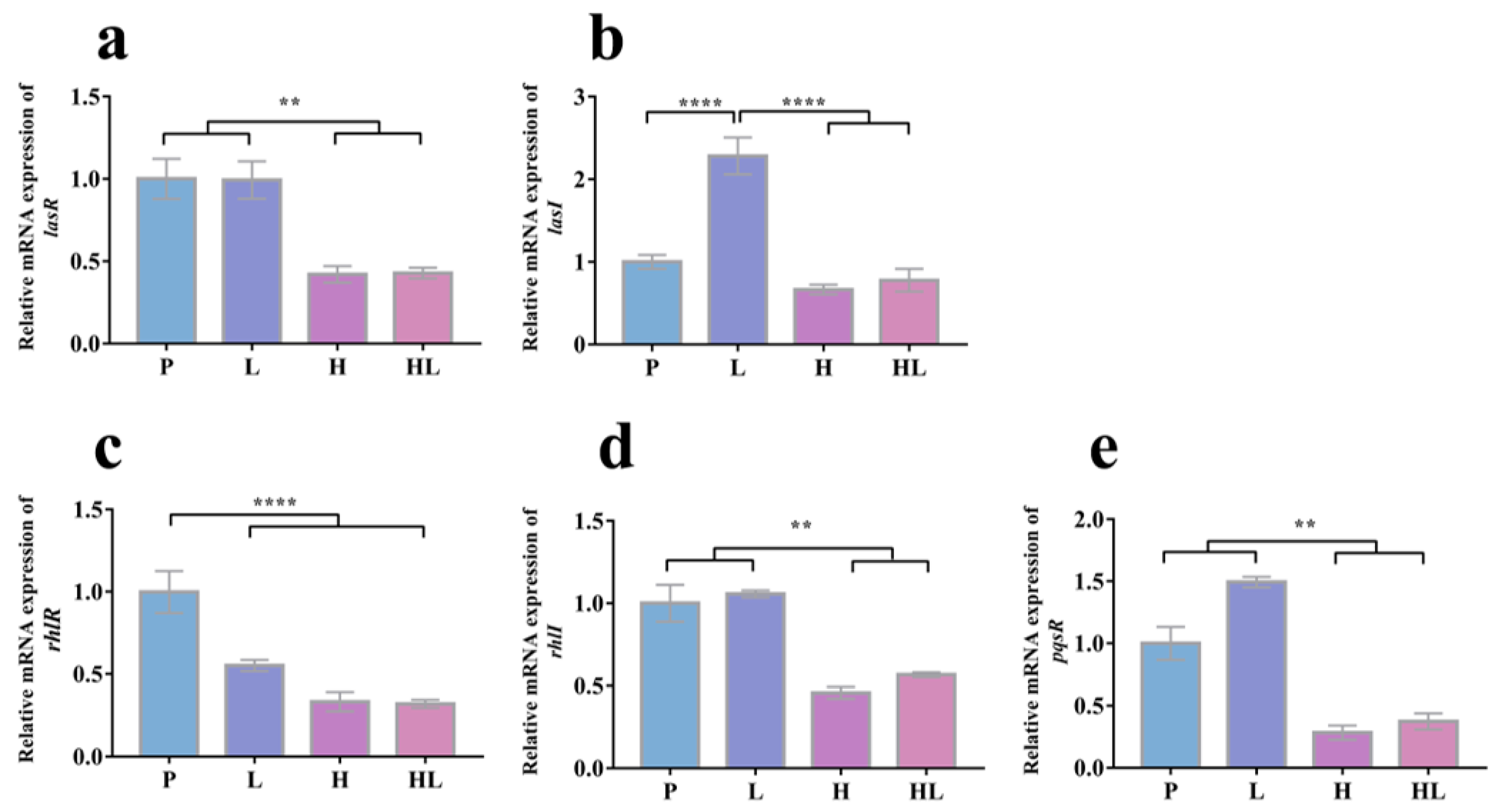
3.4. Honey–L. plantarum Formulation Increased the Transcription Level of icaA, icaR, sigB, sarA, and agrA, and Decreased the Transcription Level of lasI, lasR, rhlI, rhlR, and pqsR
3.5. The Synergistic Antibacterial Activity of Honey–L. plantarum Formulation May Depend on the Honey Promotion Growth of L. plantarum
3.6. Honey–L. plantarum Formulation Promoted Wound Healing in Rats with Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D. A study of biofilm-based wound management in subjects with critical limb ischaemia. J. Wound Care 2008, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Ahmad, I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J. Antimicrob. Chemother. 2012, 67, 618–621. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Imran, M.J.L. Antimicrobial and antibiofilm potential of bacteriocin loaded nano-vesicles functionalized with rhamnolipids against foodborne pathogens. LWT 2019, 116, 108583. [Google Scholar] [CrossRef]
- Furtado, D.N.; Favaro, L.; Nero, L.A.; de Melo Franco, B.D.G.; Todorov, S.D.J.P.; proteins, a. Nisin production by Enterococcus hirae DF105Mi isolated from Brazilian goat milk. Probiotics Antimicrob. Proteins 2019, 11, 1391–1402. [Google Scholar] [CrossRef]
- King, S.; Glanville, J.; Sanders, M.E.; Fitzgerald, A.; Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef]
- Fusieger, A.; Perin, L.M.; Teixeira, C.G.; de Carvalho, A.F.; Nero, L.A.J.A.v.L. The ability of Lactococcus lactis subsp. lactis bv. diacetylactis strains in producing nisin. Antonie Van Leeuwenhoek 2020, 113, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Rachid, M.M.; Gobbato, N.M.; Huaman Martinez, M.A.; Valdez, J.C. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010, 16, 281–286. [Google Scholar] [CrossRef]
- Argañaraz Aybar, J.N.; Ortiz Mayor, S.; Olea, L.; Garcia, J.J.; Nisoria, S.; Kolling, Y.; Melian, C.; Rachid, M.; Torres Dimani, R.; Werenitzky, C.; et al. Topical Administration of Accelerates the Healing of Chronic Diabetic Foot Ulcers through Modifications of Infection, Angiogenesis, Macrophage Phenotype and Neutrophil Response. Microorganisms 2022, 10, 634. [Google Scholar] [CrossRef]
- Moraffah, F.; Kiani, M.; Abdollahi, M.; Yoosefi, S.; Vatanara, A.; Samadi, N. In Vitro-In Vivo Correlation for the Antibacterial Effect of Lactiplantibacillus plantarum as a Topical Healer for Infected Burn Wound. Probiotics Antimicrob. Proteins 2022, 14, 675–689. [Google Scholar] [CrossRef]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S.; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight Into the Beneficial Role of Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front. Pharm. 2021, 12, 728614. [Google Scholar] [CrossRef]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites. Probiotics Antimicrob. Proteins 2020, 12, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gulfraz, M.; Iftikhar, F.; Imran, M.; Zeenat, A.; Asif, S.; Shah, I. Compositional analysis and antimicrobial activity of various honey types of Pakistan. Int. J. Food Sci. Tech. 2011, 46, 263–267. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Vasquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Butler, E.; Alsterfjord, M.; Olofsson, T.C.; Karlsson, C.; Malmstrom, J.; Vasquez, A. Proteins of novel lactic acid bacteria from Apis mellifera mellifera: An insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol. 2013, 13, 235. [Google Scholar] [CrossRef]
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S.K. Myrtenol Attenuates MRSA Biofilm and Virulence by Suppressing sarA Expression Dynamism. Front. Microbiol. 2019, 10, 2027. [Google Scholar] [CrossRef] [PubMed]
- Ahmadrajabi, R.; Layegh-Khavidaki, S.; Kalantar-Neyestanaki, D.; Fasihi, Y. Molecular analysis of immune evasion cluster (IEC) genes and intercellular adhesion gene cluster (ICA) among methicillin-resistant and methicillin-sensitive isolates of Staphylococcus aureus. J. Prev. Med. Hyg. 2017, 58, E308–E314. [Google Scholar] [CrossRef] [PubMed]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell Infect. Microbiol. 2012, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.Y.; Parayno, A.; Hancock, R.E.W. Mucin promotes rapid surface motility in Pseudomonas aeruginosa. mBio 2012, 3, e00073-12. [Google Scholar] [CrossRef]
- Williams, P.; Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- de Kievit, T.R.; Iglewski, B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000, 68, 4839–4849. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Bharathidasan, V.; Manigandan, V.; Jeyapragash, D. Quebrachitol inhibits biofilm formation and virulence production against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2020, 149, 104286. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, P.W.; Wan, S.; Yao, Y.; Song, C.R.; Song, P.P.; Xu, G.B.; Hu, Z.Q.; Zeng, Z.; Wang, C.; et al. Ginkgo biloba exocarp extracts inhibit S. aureus and MRSA by disrupting biofilms and affecting gene expression. J. Ethnopharmacol. 2021, 271, 113895. [Google Scholar] [CrossRef]
- Shi, N.; Gao, Y.; Yin, D.; Song, Y.; Kang, J.; Li, X.; Zhang, Z.; Feng, X.; Duan, J. The effect of the sub-minimal inhibitory concentration and the concentrations within resistant mutation window of ciprofloxacin on MIC, swimming motility and biofilm formation of Pseudomonas aeruginosa. Microb. Pathog. 2019, 137, 103765. [Google Scholar] [CrossRef]
- Kot, B.; Sytykiewicz, H.; Sprawka, I. Expression of the Biofilm-Associated Genes in Methicillin-Resistant Staphylococcus aureus in Biofilm and Planktonic Conditions. Int. J. Mol. Sci. 2018, 19, 3487. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Bright, R.; Strudwick, X.L.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds. Acta Biomater. 2021, 128, 420–434. [Google Scholar] [CrossRef]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed Biotechnol. 2019, 47, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ou-Yang, W.; Zhang, C.; Wang, Q.; Pan, X.; Huang, P.; Zhang, C.; Li, Y.; Kong, D.; Wang, W. Synthetic Polymeric Antibacterial Hydrogel for Methicillin-Resistant Infected Wound Healing: Nanoantimicrobial Self-Assembly, Drug- and Cytokine-Free Strategy. ACS Nano 2020, 14, 12905–12917. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fang, W.W.; Xue, J.; Sun, T.C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol-Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef] [PubMed]
- Squarzanti, D.F.; Zanetta, P.; Ormelli, M.; Manfredi, M.; Barberis, E.; Vanella, V.V.; Amoruso, A.; Pane, M.; Azzimonti, B. An animal derivative-free medium enhances Lactobacillus johnsonii LJO02 supernatant selective efficacy against the methicillin (oxacillin)-resistant Staphylococcus aureus virulence through key-metabolites. Sci. Rep. 2022, 12, 8666. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, M.; Gaisford, S. Assessing inhibitory activity of probiotic culture supernatants against Pseudomonas aeruginosa: A comparative methodology between agar diffusion, broth culture and microcalorimetry. World J. Microbiol. Biotechnol. 2019, 35, 49. [Google Scholar] [CrossRef] [PubMed]
- Mariam, S.H.; Zegeye, N.; Tariku, T.; Andargie, E.; Endalafer, N.; Aseffa, A. Potential of cell-free supernatants from cultures of selected lactic acid bacteria and yeast obtained from local fermented foods as inhibitors of Listeria monocytogenes, Salmonella spp. and Staphylococcus aureus. BMC Res. Notes 2014, 7, 606. [Google Scholar] [CrossRef]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef]
- Danilova, T.A.; Adzhieva, A.A.; Danilina, G.A.; Polyakov, N.B.; Soloviev, A.I.; Zhukhovitsky, V.G. Antimicrobial Activity of Supernatant of Lactobacillus plantarum against Pathogenic Microorganisms. Bull Exp. Biol. Med. 2019, 167, 751–754. [Google Scholar] [CrossRef]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The Battle of Probiotics and Their Derivatives Against Biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef]
- Mekky, A.F.; Hassanein, W.A.; Reda, F.M.; Elsayed, H.M. Anti-biofilm potential of Lactobacillus plantarum Y3 culture and its cell-free supernatant against multidrug-resistant uropathogen Escherichia coli U12. Saudi J. Biol. Sci. 2022, 29, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yuan, L.; Sadiq, F.A.; He, G. Inhibitory effect of Lactobacillus plantarum metabolites against biofilm formation by Bacillus licheniformis isolated from milk powder products. Food Control 2019, 106, 106721. [Google Scholar] [CrossRef]
- Kong, K.F.; Vuong, C.; Otto, M. Staphylococcus quorum sensing in biofilm formation and infection. Int. J. Med. Microbiol. IJMM 2006, 296, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Mrak, L.N.; Griffin, L.M.; Zielinska, A.K.; Shaw, L.N.; Rice, K.C.; Horswill, A.R.; Bayles, K.W.; Smeltzer, M.S. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS ONE 2010, 5, e10790. [Google Scholar] [CrossRef]
- Karuppiah, V.; Seralathan, M. Quorum sensing inhibitory potential of vaccenic acid against Chromobacterium violaceum and methicillin-resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2022, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Hnamte, S.; Parasuraman, P.; Ranganathan, S.; Ampasala, D.R.; Reddy, D.; Kumavath, R.N.; Suchiang, K.; Mohanty, S.K.; Busi, S. Mosloflavone attenuates the quorum sensing controlled virulence phenotypes and biofilm formation in Pseudomonas aeruginosa PAO1: In vitro, in vivo and in silico approach. Microb. Pathog. 2019, 131, 128–134. [Google Scholar] [CrossRef] [PubMed]
- das Neves Selis, N.; de Oliveira, H.B.M.; Leão, H.F.; Dos Anjos, Y.B.; Sampaio, B.A.; Correia, T.M.L.; Almeida, C.F.; Pena, L.S.C.; Reis, M.M.; Brito, T.L.S.; et al. Lactiplantibacillus plantarum strains isolated from spontaneously fermented cocoa exhibit potential probiotic properties against Gardnerella vaginalis and Neisseria gonorrhoeae. BMC Microbiol. 2021, 21, 198. [Google Scholar] [CrossRef]
- Rather, I.A.; Wani, M.Y.; Kamli, M.R.; Sabir, J.S.M.; Hakeem, K.R.; Firoz, A.; Park, Y.H.; Hor, Y.Y. Lactiplantibacillus plantarum KAU007 Extract Modulates Critical Virulence Attributes and Biofilm Formation in Sinusitis Causing Streptococcus pyogenes. Pharmaceutics 2022, 14, 2702. [Google Scholar] [CrossRef]
- Ray, S.; Jin, J.O.; Choi, I.; Kim, M. Cell-Free Supernatant of Bacillus thuringiensis Displays Anti-Biofilm Activity Against Staphylococcus aureus. Appl. Biochem. Biotechnol. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pancheniak, E.D.E.F.R.; Soccol, C.R. Biochemical characterization and identification of probiotic lactobacillus for swine. Bol. Do Cent. De Pesqui. De Process. De Aliment. 2005, 23, 299–310. [Google Scholar] [CrossRef]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic Properties of Lactobacilli and Their Ability to Inhibit the Adhesion of Enteropathogenic Bacteria to Caco-2 and HT-29 Cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Argenta, A.; Satish, L.; Gallo, P.; Liu, F.; Kathju, S.J.P.o. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS ONE 2016, 11, e0165294. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Derm. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement; [... Provides Updated Tables for... M02-A11, M07-A9, and M11-A8]; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2013. [Google Scholar]







| Factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| X1 (Honey ratio, %, v/v) | 10 | 20 | 30 |
| X2 (L. plantarum, CFU/mL) | 107 | 108 | 109 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| rpoB | CAGCTGACGAAGAAGATAGCTATGT | ACTTCATCATCCATGAAACGACCAT |
| icaA | CTGGCGCAGTCAATACTATTTCGGGTGTCT | GACCTCCCAATGTTTCTGGAACCAACTCC |
| icaR | TGCTTTCAAATACCAACTTTCAAGA | ACGTTCAATTATCTAATACGCCTGA |
| sigB | AAGTGATTCGTAAGGACGTCT | TCGATAACTATAACCAAAGCCT |
| agrA | TGATAATCCTTATGAGGTGCTT | CACTGTGACTCGTAACGAAAA |
| sarA | CAAACAACCACAAGTTGTTAAAGC | TGTTTGCTTCAGTGATTCGTTT |
| lasI | CGATACCACTGGCCCCTACA | GGCTGAGTTCCCAGATGTGC |
| lasR | AGGAAGTGTTGCAGTGGTGC | GGAGGTCACACCGAACTTCC |
| rhlI | GTCTCGCCCTTGACCTTCTG | ATTCTGGTCCAGCCTGCAAT |
| rhlR | CGGGTGAAGGGAATCGTGTG | ACGGTTTGCGTAGCGAGATG |
| pqsR | CTGCTCACCGTATCGCAGAA | CGCCTGATCCCTTACATGCG |
| GAPDH | CACTCCAGCCGTTTCGAACT | CGGCTTGAACACCACCGTAT |
| Source of Variation | Degrees of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 8 | 48.052 | 6.007 | 5115.985 | <0.001 |
| H (X1) | 2 | 32.202 | 16.101 | 13,713.656 | <0.001 |
| L (X2) | 2 | 5.095 | 2.548 | 2169.997 | <0.001 |
| H × L (X12) | 4 | 10.755 | 2.689 | 2290.144 | <0.001 |
| Residual | 18 | 0.021 | 0.001 | ||
| Cor Total | 26 | 48.073 |
| Formulations | Honey (%) | L. plantarum (CFU/mL) | S. aureus (Log10CFU/mL) | Inhibition (%) |
|---|---|---|---|---|
| F0 | 0 | 0 | 9.60 ± 0.04 | - |
| F1 | 10 | 107 | 9.39 ± 0.01 | 2.20 ± 0.09 |
| F2 | 10 | 108 | 8.54 ± 0.04 | 11.04 ± 0.37 |
| F3 | 10 | 109 | 4.32 ± 0.12 | 55.05 ± 1.23 |
| F4 | 20 | 107 | 9.08 ± 0.02 | 5.40 ± 0.16 |
| F5 | 20 | 108 | 8.64 ± 0.03 | 10.01 ± 0.32 |
| F6 | 20 | 109 | 6.70 ± 0.10 | 30.26 ± 0.1.06 |
| F7 | 30 | 107 | 9.16 ± 0.01 | 4.62 ± 0.13 |
| F8 | 30 | 108 | 8.81 ± 0.02 | 8.25 ± 0.25 |
| F9 | 30 | 109 | 7.95 ± 0.05 | 17.23 ± 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xiao, H.; Su, Y.; Cheng, D.; Jia, Y.; Li, Y.; Yin, Q.; Gao, J.; Tang, Y.; Bai, Q. Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds. Pathogens 2023, 12, 501. https://doi.org/10.3390/pathogens12030501
Li M, Xiao H, Su Y, Cheng D, Jia Y, Li Y, Yin Q, Gao J, Tang Y, Bai Q. Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds. Pathogens. 2023; 12(3):501. https://doi.org/10.3390/pathogens12030501
Chicago/Turabian StyleLi, Mei, Hong Xiao, Yongmei Su, Danlin Cheng, Yan Jia, Yingli Li, Qi Yin, Jieying Gao, Yong Tang, and Qunhua Bai. 2023. "Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds" Pathogens 12, no. 3: 501. https://doi.org/10.3390/pathogens12030501
APA StyleLi, M., Xiao, H., Su, Y., Cheng, D., Jia, Y., Li, Y., Yin, Q., Gao, J., Tang, Y., & Bai, Q. (2023). Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds. Pathogens, 12(3), 501. https://doi.org/10.3390/pathogens12030501






