In Vitro versus in Mice: Efficacy and Safety of Decoquinate and Quinoline-O-Carbamate Derivatives against Experimental Infection with Neospora caninum Tachyzoites
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Equipment and Media, Biochemicals and Compounds
2.2. Host Cells and Parasites
2.3. Cytotoxicity and Anti-N. caninum Efficacy Assessments In Vitro
2.4. Transmission Electron Microscopy
2.5. Long Term Treatment of N. caninum Infected HFF
2.6. Assessment of Susceptibility of Murine Splenocytes to DCQ, RMB054 and RMB060
2.7. Ethics Statement
2.8. Assessment of the Efficacy of DCQ and RMB060 in BALB/c Mice Infected with NcSp-7 Tachyzoites
2.9. Real-Time PCR-Based Determination of Cerebral Parasite Loads
2.10. Statistical Analysis
3. Results
3.1. In Vitro Efficacy and Safety Assessment of DCQ and Quinoline-O-Carbamate Derivatives
3.2. Structural Alterations Induced by In Vitro Drug Treatments of N. caninum Infected HFF
3.3. Treatments of Extended Drug Exposure to Reveal Parasitostatic versus Parasiticidal Effects
3.4. Efficacy of DCQ and RMB060 in BALB/c Mice Experimentally Infected with N. caninum Tachyzoites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-5254-1. [Google Scholar]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants: An Update. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef]
- Silva, R.C.; Machado, G.P. Canine Neosporosis: Perspectives on Pathogenesis and Management. Vet. Med. Res. Rep. 2016, 7, 59–70. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Vázquez, P.; Ferre, I.; Ortega-Mora, L.M. Treatment of Toxoplasmosis and Neosporosis in Farm Ruminants: State of Knowledge and Future Trends. Curr. Top. Med. Chem. 2018, 18, 1304–1323. [Google Scholar] [CrossRef]
- Quintero-de Leonardo, J.; Rosiles, R.; Bautista, J.; González-Monsón, N.; Sumano, H. Oral Pharmacokinetics and Milk Residues of Decoquinate in Milking Cows. J. Vet. Pharmacol. Ther. 2009, 32, 403–406. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; de Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of Deccox® (Decoquinate) for Chickens for Fattening. EFSA J. 2019, 17, e05541. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Butler, J.M.; Blagburn, B.L. Efficacy of Decoquinate against Neospora caninum Tachyzoites in Cell Cultures. Vet. Parasitol. 1997, 68, 35–40. [Google Scholar] [CrossRef]
- Buxton, D.; Brebner, J.; Wright, S.; Maley, S.W.; Thomson, K.M.; Millard, K. Decoquinate and the Control of Experimental Ovine Toxoplasmosis. Vet. Rec. 1996, 138, 434–436. [Google Scholar] [CrossRef]
- Pogany, S.; Tanol, M.; Baltezor, M.J. Decoquinate Prodrugs for Inhibiting or Treating a Parasitic Infection. WO Patent No. PCT/US2012/042265, 13 June 2012. Available online: https://patents.google.com/patent/WO2012174121A2/en (accessed on 15 April 2020).
- Watson, D.J.; Laing, L.; Beteck, R.M.; Gibhard, L.; Haynes, R.K.; Wiesner, L. The Evaluation of ADME and Pharmacokinetic Properties of Decoquinate Derivatives for the Treatment of Malaria. Front. Pharmacol. 2022, 13, 957690. [Google Scholar] [CrossRef]
- Beteck, R.M.; Seldon, R.; Coertzen, D.; van der Watt, M.E.; Reader, J.; Mackenzie, J.S.; Lamprecht, D.A.; Abraham, M.; Eribez, K.; Müller, J.; et al. Accessible and Distinct Decoquinate Derivatives Active against Mycobacterium tuberculosis and Apicomplexan Parasites. Commun. Chem. 2018, 1, 62. [Google Scholar] [CrossRef]
- Ramseier, J.; Imhof, D.; Anghel, N.; Hänggeli, K.; Beteck, R.M.; Balmer, V.; Ortega-Mora, L.-M.; Sanchez-Sanchez, R.; Ferre, I.; Haynes, R.K.; et al. Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts. Molecules 2021, 26, 6393. [Google Scholar] [CrossRef]
- Winzer, P.; Imhof, D.; Anghel, N.; Ritler, D.; Müller, J.; Boubaker, G.; Aguado-Martinez, A.; Ortega-Mora, L.-M.; Ojo, K.K.; VanVoorhis, W.C.; et al. The Impact of BKI-1294 Therapy in Mice Infected with the Apicomplexan Parasite Neospora caninum and Re-Infected during Pregnancy. Front. Vet. Sci. 2020, 7, 587570. [Google Scholar] [CrossRef] [PubMed]
- Imhof, D.; Anghel, N.; Winzer, P.; Balmer, V.; Ramseier, J.; Hänggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. In Vitro Activity, Safety and in Vivo Efficacy of the Novel Bumped Kinase Inhibitor BKI-1748 in Non-Pregnant and Pregnant Mice Experimentally Infected with Neospora caninum Tachyzoites and Toxoplasma gondii Oocysts. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado-Martínez, A.; Balmer, V.; Maly, D.J.; Fan, E.; Ortega-Mora, L.-M.; Ojo, K.K.; Van Voorhis, W.C.; Hemphill, A. Two Novel Calcium-Dependent Protein Kinase 1 Inhibitors Interfere with Vertical Transmission in Mice Infected with Neospora caninum Tachyzoites. Antimicrob. Agents Chemother. 2017, 61, e02324-16. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Anghel, N.; Imhof, D.; Balmer, V.; Ortega-Mora, L.-M.; Ojo, K.K.; Van Voorhis, W.C.; Müller, J.; Hemphill, A. Neospora Caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Müller, J.; Aguado-Martínez, A.; Rahman, M.; Balmer, V.; Manser, V.; Ortega-Mora, L.M.; Ojo, K.K.; Fan, E.; Maly, D.J.; et al. In Vitro and In Vivo Effects of the Bumped Kinase Inhibitor 1294 in the Related Cyst-Forming Apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob. Agents Chemother. 2015, 59, 6361–6374. [Google Scholar] [CrossRef]
- Müller, J.; Boubaker, G.; Imhof, D.; Hänggeli, K.; Haudenschild, N.; Uldry, A.-C.; Braga-Lagache, S.; Heller, M.; Ortega-Mora, L.-M.; Hemphill, A. Differential Affinity Chromatography Coupled to Mass Spectrometry: A Suitable Tool to Identify Common Binding Proteins of a Broad-Range Antimicrobial Peptide Derived from Leucinostatin. Biomedicines 2022, 10, 2675. [Google Scholar] [CrossRef]
- Aguado-Martínez, A.; Basto, A.P.; Tanaka, S.; Ryser, L.T.; Nunes, T.P.; Ortega-Mora, L.-M.; Arranz-Solís, D.; Leitão, A.; Hemphill, A. Immunization with a Cocktail of Antigens Fused with OprI Reduces Neospora caninum Vertical Transmission and Postnatal Mortality in Mice. Vaccine 2018, 37, 473–483. [Google Scholar] [CrossRef]
- Aguado-Martínez, A.; Basto, A.P.; Müller, J.; Balmer, V.; Manser, V.; Leitão, A.; Hemphill, A. N-Terminal Fusion of a Toll-like Receptor 2-Ligand to a Neospora caninum Chimeric Antigen Efficiently Modifies the Properties of the Specific Immune Response. Parasitology 2016, 143, 606–616. [Google Scholar] [CrossRef]
- Müller, N.; Zimmermann, V.; Hentrich, B.; Gottstein, B. Diagnosis of Neospora caninum and Toxoplasma gondii Infection by PCR and DNA Hybridization Immunoassay. J. Clin. Microbiol. 1996, 34, 2850–2852. [Google Scholar] [CrossRef]
- Müller, N.; Vonlaufen, N.; Gianinazzi, C.; Leib, S.L.; Hemphill, A. Application of Real-Time Fluorescent PCR for Quantitative Assessment of Neospora caninum Infections in Organotypic Slice Cultures of Rat Central Nervous System Tissue. J. Clin. Microbiol. 2002, 40, 252–255. [Google Scholar] [CrossRef]
- Longo, M.C.; Berninger, M.S.; Hartley, J.L. Use of Uracil DNA Glycosylase to Control Carry-over Contamination in Polymerase Chain Reactions. Gene 1990, 93, 125–128. [Google Scholar] [CrossRef]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent Progress in the Development of Anti-Malarial Quinolones. Malar. J. 2014, 13, 339. [Google Scholar] [CrossRef]
- Jiménez-Meléndez, A.; Rico-San Román, L.; Hemphill, A.; Balmer, V.; Ortega-Mora, L.M.; Álvarez-García, G. Repurposing of Commercially Available Anti-Coccidials Identifies Diclazuril and Decoquinate as Potential Therapeutic Candidates against Besnoitia besnoiti Infection. Vet. Parasitol. 2018, 261, 77–85. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Nazir, M.M.; Maqbool, A.; Ellison, S.P.; Strobl, J.S. Efficacy of Decoquinate against Sarcocystis neurona in Cell Cultures. Vet. Parasitol. 2013, 196, 21–23. [Google Scholar] [CrossRef]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; Nilsen, A.; Riscoe, M.; Francisco, S.; Leitao, A.; et al. Activities of Endochin-Like Quinolones Against In Vitro Cultured Besnoitia besnoiti Tachyzoites. Front. Vet. Sci. 2020, 7, 96. [Google Scholar] [CrossRef]
- Silva, M.G.; Bastos, R.G.; Stone Doggett, J.; Riscoe, M.K.; Pou, S.; Winter, R.; Dodean, R.A.; Nilsen, A.; Suarez, C.E. Endochin-like Quinolone-300 and ELQ-316 Inhibit Babesia bovis, B. bigemina, B. caballi and Theileria equi. Parasites Vectors 2020, 13, 606. [Google Scholar] [CrossRef]
- Doggett, J.S.; Schultz, T.; Miller, A.J.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.; Zakharov, L.N.; Nilsen, A.; Riscoe, M.K.; et al. Orally Bioavailable Endochin-Like Quinolone Carbonate Ester Prodrug Reduces Toxoplasma gondii Brain Cysts. Antimicrob. Agents Chemother. 2020, 64, e00535-20. [Google Scholar] [CrossRef]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like Quinolones Are Highly Efficacious against Acute and Latent Experimental Toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef]
- Martynowicz, J.; Doggett, J.S.; Sullivan, W.J., Jr. Efficacy of Guanabenz Combination Therapy against Chronic Toxoplasmosis across Multiple Mouse Strains. Antimicrob. Agents Chemother. 2020, 64, 11. [Google Scholar] [CrossRef]
- Anghel, N.; Balmer, V.; Müller, J.; Winzer, P.; Aguado-Martínez, A.; Roozbehani, M.; Pou, S.; Nilsen, A.; Riscoe, M.K.; Doggett, J.S.; et al. Endochin-Like Quinolones Exhibit Promising Efficacy Against Neospora caninum In Vitro and in Experimentally Infected Pregnant Mice. Front. Vet. Sci. 2018, 5, 15. [Google Scholar] [CrossRef]
- Anghel, N.; Imhof, D.; Winzer, P.; Balmer, V.; Ramseier, J.; Haenggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. Endochin-like Quinolones (ELQs) and Bumped Kinase Inhibitors (BKIs): Synergistic and Additive Effects of Combined Treatments against Neospora caninum Infection in Vitro and In Vivo. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Protonmotive Redox Mechanism of the Cytochrome b-c1 Complex in the Respiratory Chain: Protonmotive Ubiquinone Cycle. FEBS Lett. 1975, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, F.P.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sönnichsen, B.; Moreira, R.; Gamo, F.-J.; Marti, M.; Mota, M.M.; et al. Drug Screen Targeted at Plasmodium Liver Stages Identifies a Potent Multistage Antimalarial Drug. J. Infect. Dis. 2012, 205, 1278–1286. [Google Scholar] [CrossRef]
- Nam, T.; McNamara, C.W.; Bopp, S.; Dharia, N.V.; Meister, S.; Bonamy, G.M.C.; Plouffe, D.M.; Kato, N.; McCormack, S.; Bursulaya, B.; et al. A Chemical Genomic Analysis of Decoquinate, a Plasmodium falciparum Cytochrome b Inhibitor. ACS Chem. Biol. 2011, 6, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Forquer, I.; Boitz, J.; Soysa, R.; Elya, C.; Fulwiler, A.; Nilsen, A.; Polley, T.; Riscoe, M.K.; Ullman, B.; et al. Targeting the Cytochrome Bc1 Complex of Leishmania Parasites for Discovery of Novel Drugs. Antimicrob. Agents Chemother. 2016, 60, 4972–4982. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Manser, V.; Hemphill, A. In Vitro Treatment of Besnoitia besnoiti with the Naphto-Quinone Buparvaquone Results in Marked Inhibition of Tachyzoite Proliferation, Mitochondrial Alterations and Rapid Adaptation of Tachyzoites to Increased Drug Concentrations. Parasitology 2019, 146, 112–120. [Google Scholar] [CrossRef]
- Müller, J.; Aguado, A.; Laleu, B.; Balmer, V.; Ritler, D.; Hemphill, A. In Vitro Screening of the Open Source Pathogen Box Identifies Novel Compounds with Profound Activities against Neospora caninum. Int. J. Parasitol. 2017, 47, 801–809. [Google Scholar] [CrossRef]
- Winzer, P.; Müller, J.; Imhof, D.; Ritler, D.; Uldry, A.-C.; Braga-Lagache, S.; Heller, M.; Ojo, K.K.; Van Voorhis, W.C.; Ortega-Mora, L.-M.; et al. Neospora caninum: Differential Proteome of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Microorganisms 2020, 8, 801. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Barenco, P.V.C.; Lourenço, E.V.; Cunha-Júnior, J.P.; Almeida, K.C.; Roque-Barreira, M.C.; Silva, D.A.O.; Araújo, E.C.B.; Coutinho, L.B.; Oliveira, M.C.; Mineo, T.W.P.; et al. Toxoplasma gondii 70 KDa Heat Shock Protein: Systemic Detection Is Associated with the Death of the Parasites by the Immune Response and Its Increased Expression in the Brain Is Associated with Parasite Replication. PLoS ONE 2014, 9, e96527. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, H.; Sun, Z.; Ying, Z.; Wu, Y.; Xu, J.; Liu, Q. Toxoplasma gondii Glutathione S-transferase 2 Plays an Important Role in Partial Secretory Protein Transport. FASEB J. 2021, 35, e21352. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.K.; Alam, M.N.; Roy Chowdhury, D.; Chakraborti, T. Drug Resistance in Protozoan Parasites: An Incessant Wrestle for Survival. J. Glob. Antimicrob. Resist. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharifiyazdi, H.; Namazi, F.; Oryan, A.; Shahriari, R.; Razavi, M. Point Mutations in the Theileria annulata Cytochrome b Gene Is Associated with Buparvaquone Treatment Failure. Vet. Parasitol. 2012, 187, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Journel, C.; Chatagnon, G.; Martin, D.; Richard, A.; Tainturier, D. Prevention of Abortions and Fetal Infections due to Neospora caninum in Heifers: Trials of Decoquinate Treatments during Pregnancy at the Rate of 2 mg/kg/d; Institut de l’Elevage: Paris, France, 2002. [Google Scholar]
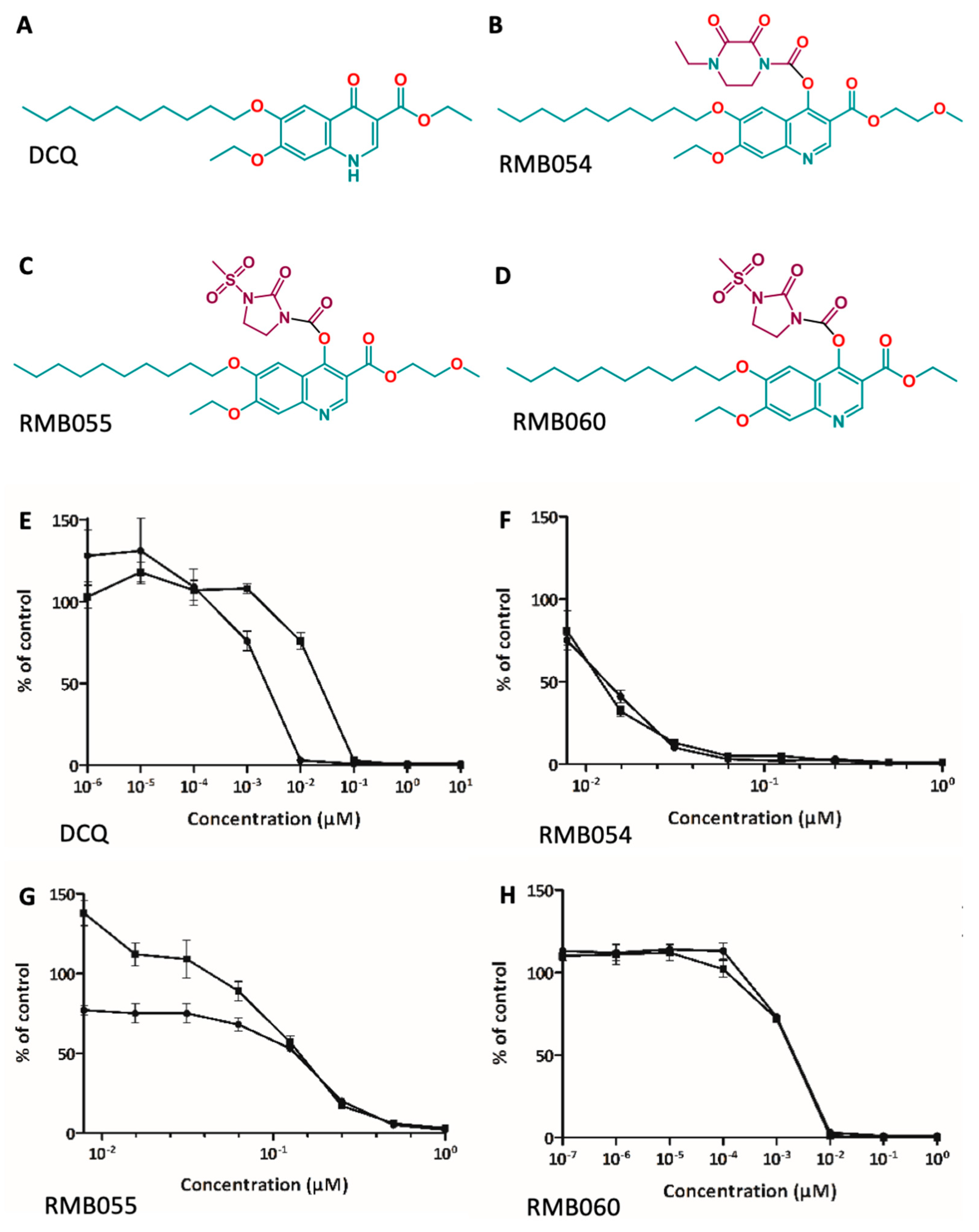



| Compounds | IC50 N. caninum (nM) [LS; LI] a Compound Added Prior to Infection | IC50 N. caninum (nM) [LS; LI] a Compound Added after Infection | IC50 HFF (µM) |
|---|---|---|---|
| DCQ | 2.4 [18.5; 0.3] | 16.6 [52.4; 5.3] | >5 b |
| RMB054 | 11.7 [18.7; 7.4] | 12.7 [18.8; 8.6] | >5 b |
| RMB055 | 60 [77.1; 46.7] | 146.4 [161.7; 132.6] | >10 b |
| RMB060 | 1.7 [6.6; 0.5] | 1.2 [3.6; 0.4] | >10 b |
| Treatment | Challenge | N. caninum Seropositive | N. caninum Brain Positive Non-Pregnant | N. caninum Brain Positive Dams | Number of Pups/Dams | Neonatal Mortality | Postnatal Mortality | N. caninum Brain Positive Pups a |
|---|---|---|---|---|---|---|---|---|
| 10 mg/kg DCQ | 105 NcSp7 tachyzoites | 12/12 | 4/7 | 5/5 | 29/5 | 1/29 | 28/28 | - |
| 10 mg/kg RMB060 | 105 NcSp7 tachyzoites | 12/12 | 4/4 | 7/8 | 44/8 | 4/44 | 40/40 | - |
| Corn oil | 105 NcSp7 tachyzoites | 12/12 | 4/5 | 7/7 | 43/7 | 6/43 | 37/37 | - |
| Corn oil | PBS | 0/6 | 0/1 | 0/5 | 27/6 | 1/27 | 0/26 | 0/26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramseier, J.; Imhof, D.; Hänggeli, K.P.A.; Anghel, N.; Boubaker, G.; Beteck, R.M.; Ortega-Mora, L.-M.; Haynes, R.K.; Hemphill, A. In Vitro versus in Mice: Efficacy and Safety of Decoquinate and Quinoline-O-Carbamate Derivatives against Experimental Infection with Neospora caninum Tachyzoites. Pathogens 2023, 12, 447. https://doi.org/10.3390/pathogens12030447
Ramseier J, Imhof D, Hänggeli KPA, Anghel N, Boubaker G, Beteck RM, Ortega-Mora L-M, Haynes RK, Hemphill A. In Vitro versus in Mice: Efficacy and Safety of Decoquinate and Quinoline-O-Carbamate Derivatives against Experimental Infection with Neospora caninum Tachyzoites. Pathogens. 2023; 12(3):447. https://doi.org/10.3390/pathogens12030447
Chicago/Turabian StyleRamseier, Jessica, Dennis Imhof, Kai Pascal Alexander Hänggeli, Nicoleta Anghel, Ghalia Boubaker, Richard M. Beteck, Luis-Miguel Ortega-Mora, Richard K. Haynes, and Andrew Hemphill. 2023. "In Vitro versus in Mice: Efficacy and Safety of Decoquinate and Quinoline-O-Carbamate Derivatives against Experimental Infection with Neospora caninum Tachyzoites" Pathogens 12, no. 3: 447. https://doi.org/10.3390/pathogens12030447
APA StyleRamseier, J., Imhof, D., Hänggeli, K. P. A., Anghel, N., Boubaker, G., Beteck, R. M., Ortega-Mora, L.-M., Haynes, R. K., & Hemphill, A. (2023). In Vitro versus in Mice: Efficacy and Safety of Decoquinate and Quinoline-O-Carbamate Derivatives against Experimental Infection with Neospora caninum Tachyzoites. Pathogens, 12(3), 447. https://doi.org/10.3390/pathogens12030447








