Dermatological Manifestations in COVID-19: A Case Study of SARS-CoV-2 Infection in a Genetic Thrombophilic Patient with Mthfr Mutation
Abstract
1. Introduction
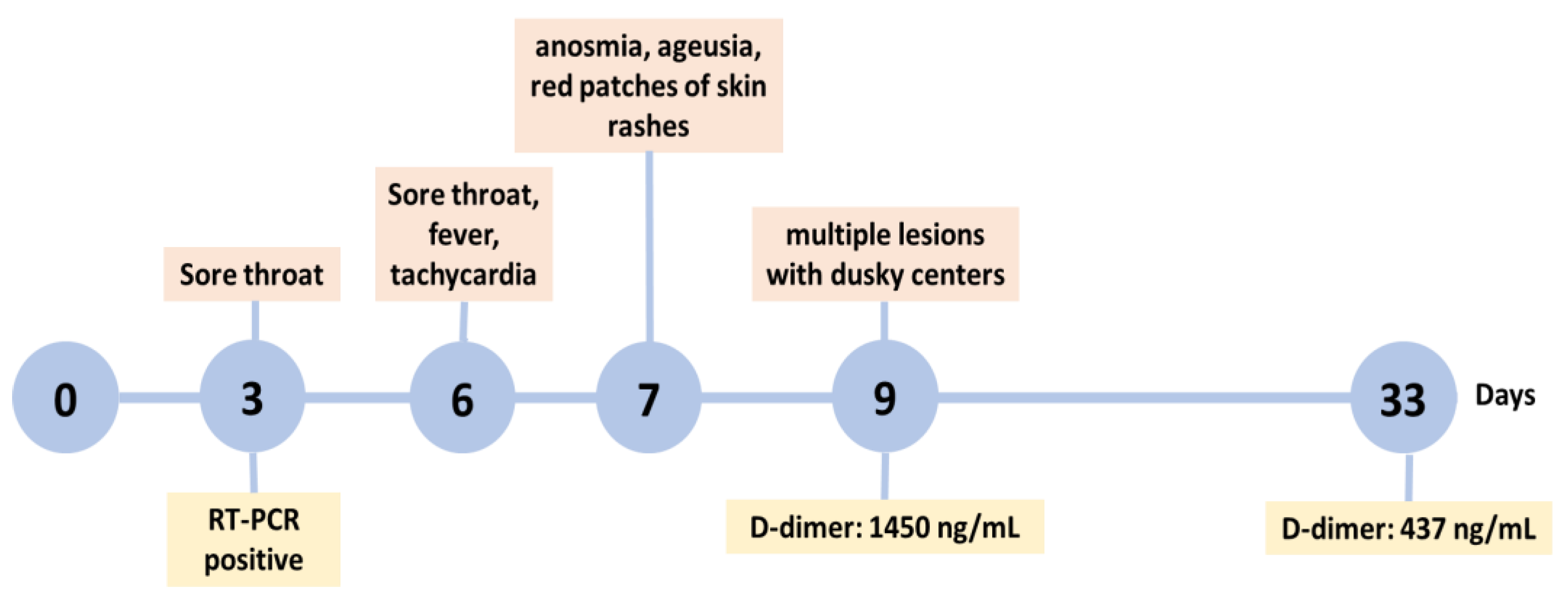
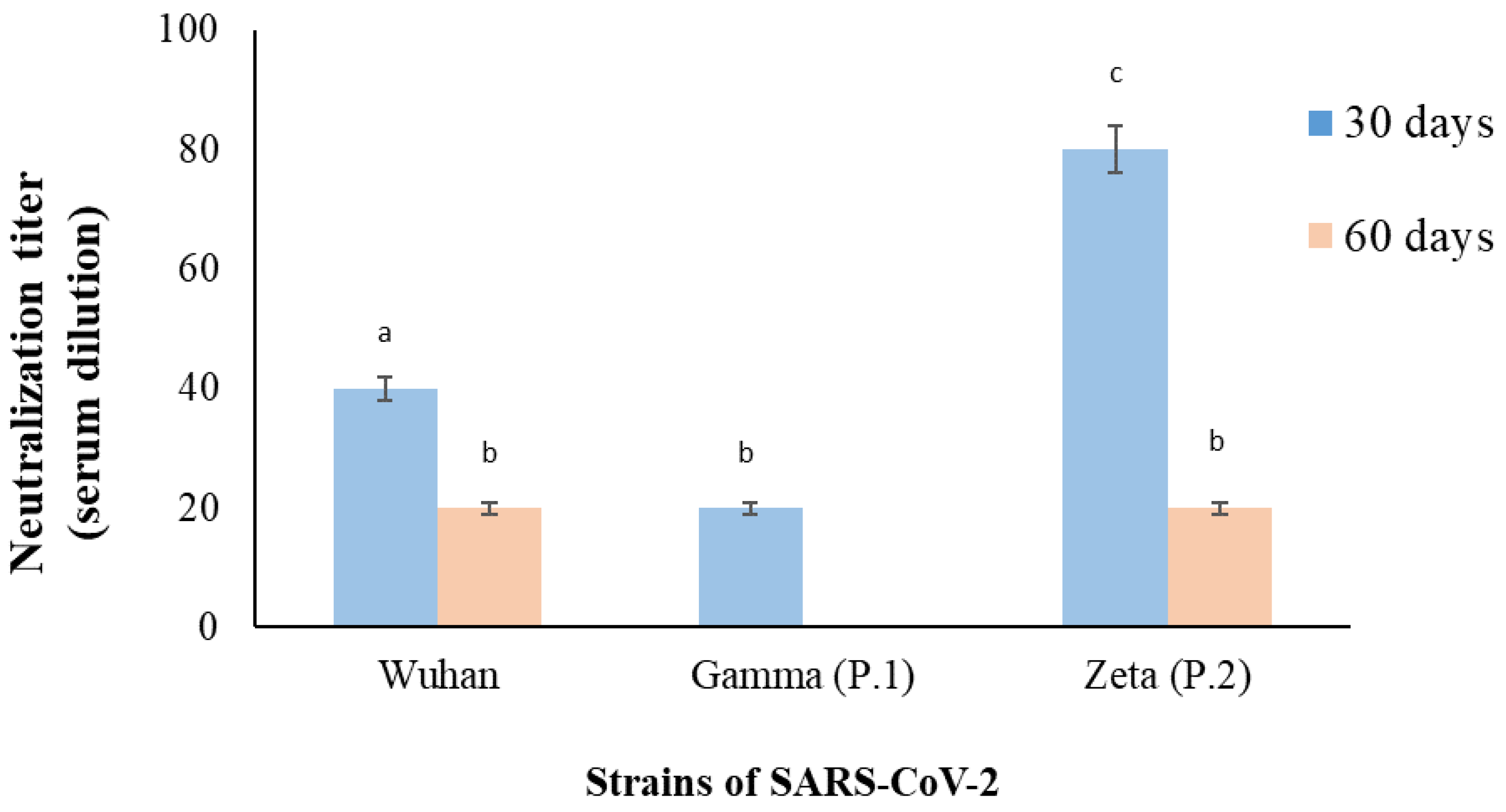
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, V.; Ratho, R.K.; Kumar, P.; Bhatia, S.K.; Bora, I.; Mohi, G.K.; Saxena, S.K.; Devi, M.; Yadav, D.; Mehariya, S. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. J. Clin. Med. 2021, 10, 446. [Google Scholar] [CrossRef]
- Kreutmair, S.; Kauffmann, M.; Unger, S.; Ingelfinger, F.; Núñez, N.G.; Alberti, C.; De Feo, D.; Krishnarajah, S.; Friebel, E.; Ulutekin, C.; et al. Preexisting comorbidities shape the immune response associated with severe COVID-19. J. Allergy Clin. Immunol. 2022, 150, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Seque, C.A.; Enokihara, M.M.S.e.S.; Porro, A.M.; Tomimori, J. Skin Manifestations Associated with COVID-19. An. Bras. Dermatol. 2022, 97, 75–88. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Leerunyakul, K.; Kositkuljorn, C. Cutaneous Manifestations In COVID-19: Lessons Learned From Current Evidence. J. Am. Acad. Dermatol. 2020, 83, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Tam, Y.C.; Oh, C.C. Skin Manifestations of COVID-19: A Worldwide Review. JAAD Int. 2021, 2, 119–133. [Google Scholar] [CrossRef]
- Genovese, G.; Moltrasio, C.; Berti, E.; Marzano, A.V. Skin Manifestations Associated with COVID-19: Current Knowledge and Future Perspectives. Dermatology 2021, 237, 1–12. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA J. Am. Med. Assoc. 2020, 324, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Greninger, A.L.; Naccache, S.N.; Federman, S.; Yu, G.; Mbala, P.; Bres, V.; Stryke, D.; Bouquet, J.; Somasekar, S.; Linnen, J.M.; et al. Rapid Metagenomic Identification of Viral Pathogens in Clinical Samples by Real-Time Nanopore Sequencing Analysis. Genome Med. 2015, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Wendel, S.; Kutner, J.M.; Machado, R.; Fontão-Wendel, R.; Bub, C.; Fachini, R.; Yokoyama, A.; Candelaria, G.; Sakashita, A.; Achkar, R.; et al. Screening for SARS-CoV-2 Antibodies in Convalescent Plasma in Brazil: Preliminary Lessons from a Voluntary Convalescent Donor Program. Transfusion 2020, 60, 2938–2951. [Google Scholar] [CrossRef]
- Agnihothri, R.; Fox, L.P. Clinical Patterns and Morphology of COVID-19 Dermatology. Dermatol. Clin. 2021, 39, 487–503. [Google Scholar] [CrossRef]
- Burlando, M.; Russo, R.; Cozzani, E.; Parodi, A. COVID-19 “Second Wave” and Vaccines: The Dermatologists’ Perspective. Int. J. Dermatol. 2021, 60, 889–890. [Google Scholar] [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the Cutaneous Manifestations of COVID-19: A Rapid Prospective Nationwide Consensus Study in Spain with 375 Cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Galván-Casas, C.; Carretero-Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa, A.; Navarro-Fernández, Í.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas-Velasco, M.; et al. Maculopapular Eruptions Associated to COVID-19: A Subanalysis of the COVID-Piel Study. Dermatol. Ther. 2020, 33, e14170. [Google Scholar] [CrossRef]
- Sławińska, M.; Nowicki, R.J. Dermatological Manifestations of COVID-19: A Practical Summary of the Current State of Knowledge. Przegl. Dermatol. 2020, 107, 228–233. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Franco, M.M.; Gräf, T.; de Lorenzo Barcia, C.A.; de Ávila Mendonça, R.N.; de Sousa, K.A.F.; Costa Neiva, L.M.; Fosenca, V.; Mendes, A.V.A.; de Aguiar, R.S.; et al. Genomic Evidence of SARS-CoV-2 Reinfection Involving E484K Spike Mutation, Brazil. Emerg. Infect. Dis. 2021, 27, 1522–1524. [Google Scholar] [CrossRef]
- Yadav, P.; Mohandas, S.; Sarkale, P.; Nyayanit, D.; Shete, A.; Sahay, R.; Potdar, V.; Baradkar, S.; Gupta, N.; Sapkal, G.; et al. Isolation of SARS-CoV-2 B.1.1.28.2 (P2) Variant and Pathogenicity Comparison with D614G Variant in Hamster Model. J. Infect. Public Health 2022, 15, 164–171. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St. Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.M.; Amorim, M.R.; Sesti-Costa, R.; Coimbra, L.D.; Brunetti, N.S.; Toledo-Teixeira, D.A.; de Souza, G.F.; Muraro, S.P.; Parise, P.L.; Barbosa, P.P.; et al. Neutralisation of SARS-CoV-2 Lineage P.1 by Antibodies Elicited through Natural SARS-CoV-2 Infection or Vaccination with an Inactivated SARS-CoV-2 Vaccine: An Immunological Study. Lancet Microbe 2021, 2, e527–e535. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.W.; le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 Neutralising Antibody Responses and Duration of Immunity: A Longitudinal Study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Pang, N.Y.L.; Pang, A.S.R.; Chow, V.T.; Wang, D.Y. Understanding Neutralising Antibodies against SARS-CoV-2 and Their Implications in Clinical Practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.H.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Voloch, C.M.; da Silva Francisco, R.; de Almeida, L.G.P.; Cardoso, C.C.; Brustolini, O.J.; Gerber, A.L.; Guimarães, A.P.d.C.; Mariani, D.; da Costa, R.M.; Ferreira, O.C.; et al. Genomic Characterization of a Novel SARS-CoV-2 Lineage from Rio de Janeiro, Brazil. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Demoliner, M.; da Silva, M.S.; Gularte, J.S.; Hansen, A.W.; de Almeida, P.R.; Weber, M.N.; Heldt, F.H.; Silveira, F.; Filippi, M.; de Abreu Góes Pereira, V.M.; et al. Predominance of SARS-CoV-2 P.1 (Gamma) Lineage Inducing the Recent COVID-19 Wave in Southern Brazil and the Finding of an Additional S: D614A Mutation. Infect. Genet. Evol. 2021, 96, 105134. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence That D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-Analysis of Studies from South Asia. ACS Chem. Neurosci. 2021, 12, 3535–3549. [Google Scholar] [CrossRef]
- Majumdar, P.; Niyogi, S. SARS-CoV-2 Mutations: The Biological Trackway towards Viral Fitness. Epidemiol. Infect. 2021, 149, e110. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Delaunay-Moisan, A.; Timmis, K.; Danchin, A. SARS-CoV-2 Biology and Variants: Anticipation of Viral Evolution and What Needs to Be Done. Environ. Microbiol. 2021, 23, 2339–2363. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Murray, B.; Rossi, N.; Wolf, J.; Ourselin, S.; Spector, T.D.; Freeman, E.E.; Bataille, V.; Falchi, M. Cutaneous Manifestations of SARS-CoV-2 Infection during the Delta and Omicron Waves in 348,691 UK Users of the UK ZOE COVID Study App. Br. J. Dermatol. 2022, 187, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Ko, W.C.; Lee, P.I.; Jean, S.S.; Hsueh, P.R. Extra-Respiratory Manifestations of COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106024. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, H.; Singh, K.; Sen, C.K. Cutaneous Manifestations of COVID-19: A Systematic Review. Adv. Wound Care 2021, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.; Chauhan, P. Cutaneous Manifestations of Coronavirus Disease 2019 in 458 Confirmed Cases: A Systematic Review. J. Fam. Med. Prim. Care 2020, 9, 4563. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Cuenca-Barrales, C.; Montero-Vilchez, T.; Molina-Leyva, A.; Arias-Santiago, S. Review of Adverse Cutaneous Reactions of Pharmacologic Interventions for COVID-19: A Guide for the Dermatologist. J. Am. Acad. Dermatol. 2020, 83, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Florêncio, F.K.Z.; de Oliveira Tenório, M.; Andrade, A.R.M.; de Lima, S.G. Angioedema, Endothelium, ACE2, and Bradykinin—Interrelationships in COVID-19: A Case Report. Medicina 2020, 53, 309–312. [Google Scholar] [CrossRef]
- Kroumpouzos, G. Cutaneous Manifestations of COVID-19: An Unusual Presentation with Edematous Plaques and Pruritic, Erythematous Papules, and Comment on the Role of Bradykinin Storm and Its Therapeutic Implications. Dermatol. Ther. 2021, 34, e14753. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, P.; Millan, C.; Matus, C.E.; Figueroa, J.E.; Burgos, R.A.; Nualart, F.; Bhoola, K.D.; Figueroa, C.D. Activation of Kinin B1 Receptors Induces Chemotaxis of Human Neutrophils. J. Leukoc. Biol. 2006, 80, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kenne, E.; Rasmuson, J.; Renné, T.; Vieira, M.L.; Müller-Esterl, W.; Herwald, H.; Lindbom, L. Neutrophils Engage the Kallikrein-Kinin System to Open up the Endothelial Barrier in Acute Inflammation. FASEB J. 2019, 33, 2599–2609. [Google Scholar] [CrossRef]
- Kaya, G.; Kaya, A.; Saurat, J.-H. Clinical and Histopathological Features and Potential Pathological Mechanisms of Skin Lesions in COVID-19: Review of the Literature. Dermatopathology 2020, 7, 2. [Google Scholar] [CrossRef]
- Salimi-Jeda, A.; Abbassi, S.; Mousavizadeh, A.; Esghaie, M.; Bokharaei-Salim, F.; Jeddi, F.; Shafaati, M.; Abdoli, A. SARS-CoV-2: Current Trends in Emerging Variants, Pathogenesis, Immune Responses, Potential Therapeutic, and Vaccine Development Strategies. Int. Immunopharmacol. 2021, 101, 108232. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Miesbach, W.; Makris, M. COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620938149. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Elevated Level of C-Reactive Protein May Be an Early Marker to Predict Risk for Severity of COVID-19. J. Med. Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef]
- Capoferri, G.; Daikeler, T.; Mühleisen, B.; Trendelenburg, M.; Müller, S. Cutaneous Leukocytoclastic Vasculitis Secondary to COVID-19 Infection Leading to Extensive Skin Necrosis. Clin. Dermatol. 2022, 40, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Hollenhorst, J.; Achenbach, J. Life-Threatening Course in Coronavirus Disease 2019 (COVID-19): Is There a Link to Methylenetetrahydrofolic Acid Reductase (MTHFR) Polymorphism and Hyperhomocysteinemia? Med. Hypotheses 2020, 144, 110234. [Google Scholar] [CrossRef]
- Annunziata, A.; Coppola, A.; di Spirito, V.; Cauteruccio, R.; Marotta, A.; di Micco, P.; Fiorentino, G. The Angiotensin Converting Enzyme Deletion/Deletion Genotype Is a Risk Factor for Severe COVID-19: Implication and Utility for Patients Admitted to Emergency Department. Medicina 2021, 57, 844. [Google Scholar] [CrossRef] [PubMed]
- Cantanhede, M.H.D.; Sarges, K.M.d.L.; Leite, M.d.M.; Miyajima, F.; dos Santos, E.J.M. Suscetibilidade de polimorfismos genéticos a trombofilia e seu papel na COVID-19. Braz. J. Infect. Dis. 2022, 26, 47. [Google Scholar] [CrossRef]
- Yafei, W.; Lijun, P.; Jinfeng, W.; Xiaoying, Z. Is the Prevalence of MTHFR C677T Polymorphism Associated with Ultraviolet Radiation in Eurasia? J. Hum. Genet. 2012, 57, 780–786. [Google Scholar] [CrossRef]
- Yadav, U.; Kumar, P.; Gupta, S.; Rai, V. Distribution of MTHFR C677T Gene Polymorphism in Healthy North Indian Population and an Updated Meta-Analysis. Indian J. Clin. Biochem. 2017, 32, 399–410. [Google Scholar] [CrossRef]
- Wilcken, B.; Bamforth, F.; Li, Z.; Zhu, H.; Ritvanen, A.; Redlund, M.; Stoll, C.; Alembik, Y.; Dott, B.; Czeizel, A.E.; et al. Geographical and Ethnic Variation of the 677C>T Alleleof 5,10 Methylenetetrahydrofolate Reductase (MTHFR): Findings from over 7000 Newborns from 16 Areas Worldwide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef]
- Russo, G.T.; Friso, S.; Jacques, P.F.; Rogers, G.; Cucinotta, D.; Wilson, P.W.F.; Ordovas, J.M.; Rosenberg, I.H.; Selhub, J. Age and Gender Affect the Relation between Methylenetetrahydrofolate Reductase C677T Genotype and Fasting Plasma Homocysteine Concentrations in the Framingham Offspring Study Cohort. J. Nutr. 2003, 133, 3416–3421. [Google Scholar] [CrossRef]
- Smits, S.L.; de Lang, A.; van den Brand, J.M.A.; Leijten, L.M.; van Ijcken, W.F.; Eijkemans, M.J.C.; van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.D.M.E.; et al. Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef] [PubMed]
- Berbert, A. Further comment on articles pertaining to: “Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19”. Med. Hypotheses 2021, 155, 110676. [Google Scholar] [CrossRef] [PubMed]
- Lord, N.; Ruwart, M.J. Homocysteine and the SARS-CoV-2 Coronavirus—The X factor of severe disease and death. SSRN 2020, 1–6. [Google Scholar] [CrossRef]
- Ponti, G.; Roli, L.; Oliva, G.; Manfredini, M.; Trenti, T.; Kaleci, S.; Iannella, R.; Balzano, B.; Coppola, A.; Fiorentino, G.; et al. Homocysteine (Hcy) assessment to predict outcomes of hospitalized COVID-19 patients: A multicenter study on 313 COVID-19 patients. Clin. Chem. Lab. Med. 2021, 59, e354–e357. [Google Scholar] [CrossRef]
- Tavares, L.S.; Ortiz, J.V. Development of thrombosis in patients with and without SARS-Cov-2 infection—Literature review. Res. Soc. Dev. 2021, 10, e410101522959. [Google Scholar] [CrossRef]
- de la Morena-Barrio, M.E.; Bravo-Pérez, C.; de la Morena-Barrio, B.; Orlando, C.; Cifuentes, R.; Padilla, J.; Miñano, A.; Herrero, S.; Marcellini, S.; Revilla, N.; et al. A pilot study on the impact of congenital thrombophilia in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13546. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential Interventions for Novel Coronavirus in China: A Systematic Review. J. Med Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, M. Identification, Mechanism, and Treatment of Skin Lesions in COVID-19: A Review. Viruses 2021, 13, 1916. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef]
- Chan, N.C.; Weitz, J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020, 136, 381–833. [Google Scholar] [CrossRef]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Lannella, R.; Tomasi, A. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [Google Scholar] [CrossRef] [PubMed]
- Dusse, L.M.; Carvalho, M.; Braganca, W.F.; Paiva, S.G.; Godoi, L.C.; Guimaraes, D.A.; Godoi, L.C.; Guimarães, D.A.M.; Fernandes, A.P. Inherited thrombophilias and pre-eclampsia in Brazilian women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Filho, I.L.; Leite, A.C.; Moura, P.G.; Ribeiro, G.S.; Cavalcante, A.C.; Azevedo, F.C.; Andrada-Serpa, M.J. Genetic polymorphisms and cerebrovascular disease in children with sickle cell anemia from Rio de Janeiro, Brazil. Arq. Neuropsiquiatr. 2011, 69, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Sabino, A.P.; Guimarães, D.A.M.; Ribeiro, D.D.; Paiva, S.G.; Dusse, L.M.; Carvalho, M.G.; Fernandes, A.P. Increased Factor V Leiden frequency is associated with venous thrombotic events among young Brazilian patients. J. Thromb. Thrombolysis 2007, 24, 261–266. [Google Scholar] [CrossRef]
- Dick-Guareschi, J.; Fontana, J.C.; Sanseverino, M.T.V.; Kubaski, F.; Sekine, L.; Mesquita, N.F.; Onsten, T.G.H.; Leistner-Segala, S. Prevalence of thrombophilia-associated genetic risk factors in blood donors of a regional hospital in southern Brazil. Hematol. Transfus. Cell Ther. 2022, 44, 379–385. [Google Scholar] [CrossRef]
- Soligo, A.G.; Barini, R.; Annichino-Bizzacchi, J.M. Prevalence of the MTHFR C677T mutation in fertile and infertile women. Rev. Bras. Ginecol. Obstet. 2017, 39, 659–662. [Google Scholar] [CrossRef]
- Dupont, L.; Duquia, R.P.; Pizutti, G.W.; Nunes, F.B.; Branchini, G.; Mosquera, E.S.B.; Bonamigo, R.R. Cutaneous manifestations in patients with COVID-19 treated at an university hospital in southern Brazil. Cureus 2022, 14, e31566. [Google Scholar] [CrossRef]
- Passarelli-Araujo, H.; Pott-Junior, H.; Susuki, A.M.; Olak, A.S.; Pescim, R.R.; Tomimatsu, M.F.A.I.; Volce, C.J.; Neves, M.A.Z.; Silva, F.F.; Narciso, S.G.; et al. The impact of COVID-19 vaccination on case fatality rates in a city in Southern Brazil. Am. J. Infect. Control. 2022, 50, 491–496. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celestino, G.G.; Amarante, M.K.; Vespero, E.C.; Tavares, E.R.; Yamauchi, L.M.; Candido, É.D.; de Oliveira, D.B.L.; Durigon, E.L.; Yamada-Ogatta, S.F.; Faccin-Galhardi, L.C. Dermatological Manifestations in COVID-19: A Case Study of SARS-CoV-2 Infection in a Genetic Thrombophilic Patient with Mthfr Mutation. Pathogens 2023, 12, 438. https://doi.org/10.3390/pathogens12030438
Celestino GG, Amarante MK, Vespero EC, Tavares ER, Yamauchi LM, Candido ÉD, de Oliveira DBL, Durigon EL, Yamada-Ogatta SF, Faccin-Galhardi LC. Dermatological Manifestations in COVID-19: A Case Study of SARS-CoV-2 Infection in a Genetic Thrombophilic Patient with Mthfr Mutation. Pathogens. 2023; 12(3):438. https://doi.org/10.3390/pathogens12030438
Chicago/Turabian StyleCelestino, Gabriela Gomes, Marla Karine Amarante, Eliana Carolina Vespero, Eliandro Reis Tavares, Lucy Megumi Yamauchi, Érika Donizetti Candido, Danielle Bruna Leal de Oliveira, Edison Luiz Durigon, Sueli Fumie Yamada-Ogatta, and Ligia Carla Faccin-Galhardi. 2023. "Dermatological Manifestations in COVID-19: A Case Study of SARS-CoV-2 Infection in a Genetic Thrombophilic Patient with Mthfr Mutation" Pathogens 12, no. 3: 438. https://doi.org/10.3390/pathogens12030438
APA StyleCelestino, G. G., Amarante, M. K., Vespero, E. C., Tavares, E. R., Yamauchi, L. M., Candido, É. D., de Oliveira, D. B. L., Durigon, E. L., Yamada-Ogatta, S. F., & Faccin-Galhardi, L. C. (2023). Dermatological Manifestations in COVID-19: A Case Study of SARS-CoV-2 Infection in a Genetic Thrombophilic Patient with Mthfr Mutation. Pathogens, 12(3), 438. https://doi.org/10.3390/pathogens12030438







