Cryptococcal Meningitis: Differences between Patients with and without HIV-Infection
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
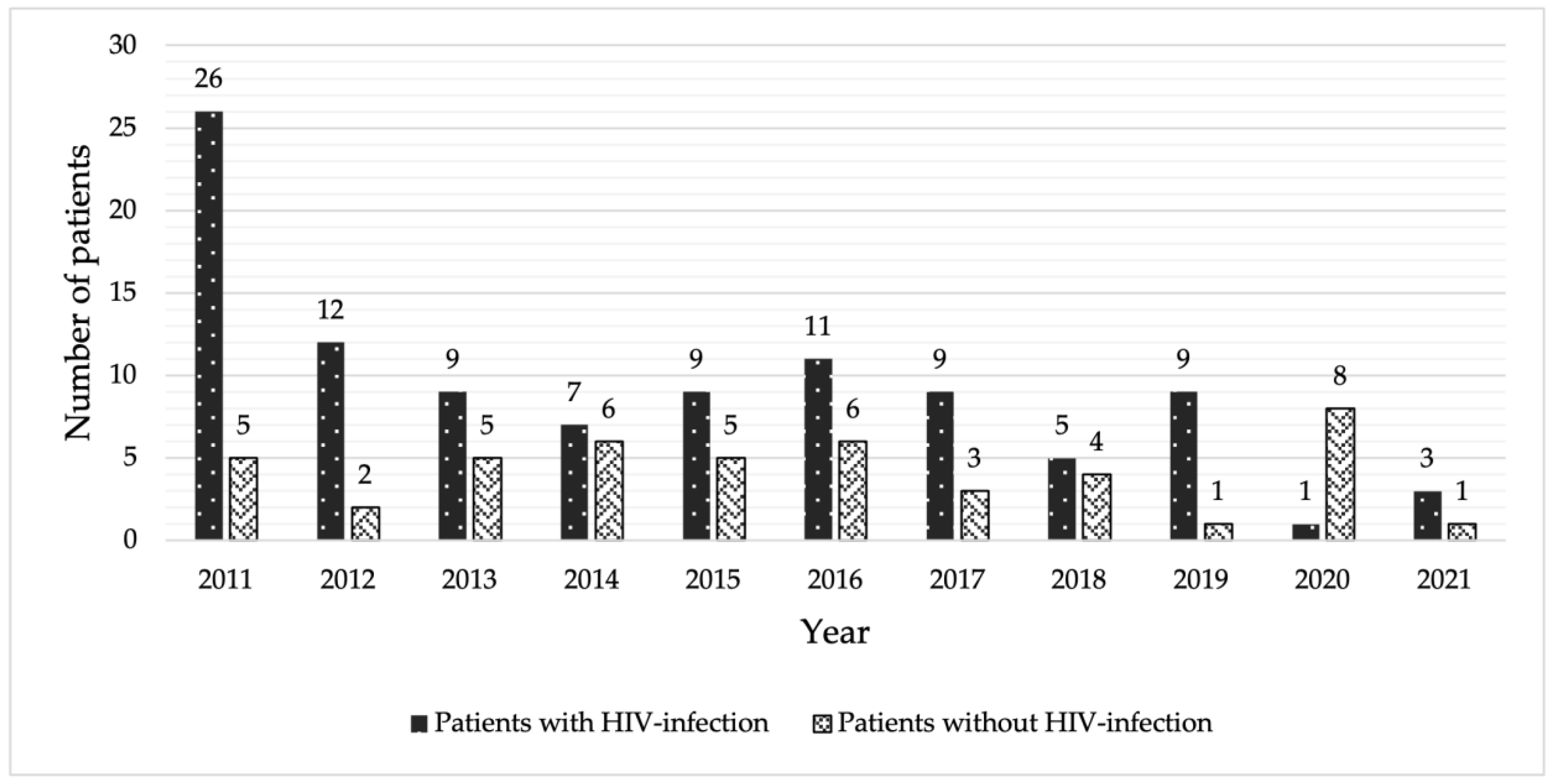
3.1. Demographic Clinical Characteristics
3.2. Laboratory Findings
3.3. Induction Therapy and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, P.R.; Jarvis, J.N.; Panackal, A.A.; Fisher, M.C.; Molloy, S.F.; Loyse, A.; Harrison, T.S. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat. Rev. Neurol. 2017, 13, 13–24. [Google Scholar] [CrossRef]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kongsiriwattanakul, S.; Suankratay, C. Central nervous system infections in HIV-infected patients hospitalized at King Chulalongkorn Memorial Hospital. J. Med. Assoc. Thai. 2011, 94, 551–558. [Google Scholar] [PubMed]
- O’Halloran, J.A.; Powderly, W.G.; Spec, A. Cryptococcosis today: It is not all about HIV infection. Curr. Clin. Microbiol. Rep. 2017, 4, 88–95. [Google Scholar] [CrossRef]
- Pappas, P.G.; Perfect, J.R.; Cloud, G.A.; Larsen, R.A.; Pankey, G.A.; Lancaster, D.J.; Henderson, H.; Kauffman, C.A.; Haas, D.W.; Saccente, M.; et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 2001, 33, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G. Cryptococcal infections in non-HIV-infected patients. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 61–79. [Google Scholar]
- Pinheiro, S.B.; Sousa, E.S.; Cortez, A.C.A.; da Silva Rocha, D.F.; Menescal, L.S.F.; Chagas, V.S.; Gomez, A.S.P.; Cruz, K.S.; Santos, L.O.; Alves, M.J.; et al. Cryptococcal meningitis in non-HIV patients in the State of Amazonas, Northern Brazil. Braz. J. Microbiol. 2021, 52, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bratton, E.W.; El Husseini, N.; Chastain, C.A.; Lee, M.S.; Poole, C.; Sturmer, T.; Juliano, J.J.; Weber, D.J.; Perfect, J.R. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS ONE 2012, 7, e43582. [Google Scholar] [CrossRef]
- Chetchotisakd, P.; Anunnatsiri, S.; Nithichanon, A.; Lertmemongkolchai, G. Cryptococcosis in Anti-Interferon-Gamma Autoantibody-Positive Patients: A Different Clinical Manifestation from HIV-Infected Patients. Jpn. J. Infect. Dis. 2017, 70, 69–74. [Google Scholar] [CrossRef]
- Rujirachun, P.; Sangwongwanich, J.; Chayakulkeeree, M. Triple infection with Cryptococcus, varicella-zoster virus, and Mycobacterium abscessus in a patient with anti-interferon-gamma autoantibodies: A case report. BMC Infect. Dis. 2020, 20, 232. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Mocroft, A.; Vella, S.; Benfield, T.L.; Chiesi, A.; Miller, V.; Gargalianos, P.; d’Arminio Monforte, A.; Yust, I.; Bruun, J.N.; Phillips, A.N.; et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet 1998, 352, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D.; HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef] [PubMed]
- van Elden, L.J.; Walenkamp, A.M.; Lipovsky, M.M.; Reiss, P.; Meis, J.F.; de Marie, S.; Dankert, J.; Hoepelman, A.I. Declining number of patients with cryptococcosis in the Netherlands in the era of highly active antiretroviral therapy. AIDS 2000, 14, 2787–2788. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Sorrell, T.C.; Chen, S.C. Central Nervous System Cryptococcal Infections in Non-HIV Infected Patients. J. Fungi 2019, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chang, W.N.; Chang, H.W.; Chuang, Y.C. The prognostic factors of cryptococcal meningitis in HIV-negative patients. J. Hosp. Infect. 1999, 42, 313–320. [Google Scholar] [CrossRef]
- Lui, G.; Lee, N.; Ip, M.; Choi, K.W.; Tso, Y.K.; Lam, E.; Chau, S.; Lai, R.; Cockram, C.S. Cryptococcosis in apparently immunocompetent patients. QJM 2006, 99, 143–151. [Google Scholar] [CrossRef]
- Shih, C.C.; Chen, Y.C.; Chang, S.C.; Luh, K.T.; Hsieh, W.C. Cryptococcal meningitis in non-HIV-infected patients. QJM 2000, 93, 245–251. [Google Scholar] [CrossRef]
- Zhu, L.P.; Wu, J.Q.; Xu, B.; Ou, X.T.; Zhang, Q.Q.; Weng, X.H. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997–2007. Med. Mycol. 2010, 48, 570–579. [Google Scholar] [CrossRef]
- Jiang, Y.K.; Wu, J.Q.; Zhao, H.Z.; Wang, X.; Wang, R.Y.; Zhou, L.H.; Yip, C.W.; Huang, L.P.; Cheng, J.H.; Chen, Y.H.; et al. Genetic influence of Toll-like receptors on non-HIV cryptococcal meningitis: An observational cohort study. EBioMedicine 2018, 37, 401–409. [Google Scholar] [CrossRef]
- Chen, M.; Xu, N.; Xu, J. Cryptococcus neoformans Meningitis Cases Among China’s HIV-Infected Population may have been Severely Under-Reported. Mycopathologia 2020, 185, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Fa, Z.; Liao, W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet. Biol. 2015, 78, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.T.; Wu, J.Q.; Zhu, L.P.; Guan, M.; Xu, B.; Hu, X.P.; Wang, X.; Weng, X.H. Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J. Infect. Dis. 2011, 203, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Tjia, T.L.; Yeow, Y.K.; Tan, C.B. Cryptococcal meningitis. J. Neurol. Neurosurg. Psychiatry 1985, 48, 853–858. [Google Scholar] [CrossRef]
- Yao, Z.; Liao, W.; Chen, R. Management of cryptococcosis in non-HIV-related patients. Med. Mycol. 2005, 43, 245–251. [Google Scholar] [CrossRef]
- Emmons, C.W. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am. J. Hyg. 1955, 62, 227–232. [Google Scholar] [CrossRef]
- Diem, P.K.; Pimple, U.; Sitthi, A.; Varnakovida, P.; Kaewthongrach, R.; Chidthaisong, A. Responses of Tropical Deciduous Forest Phenology to Climate Variation in Northern Thailand. In Proceedings of the International Conference on Environmental Research and Technology (ICERT 2017), Georgetown, Penang, Malaysia, 23–25 August 2017. [Google Scholar]
- Lopez-Mondejar, R.; Brabcova, V.; Stursova, M.; Davidova, A.; Jansa, J.; Cajthaml, T.; Baldrian, P. Decomposer food web in a deciduous forest shows high share of generalist microorganisms and importance of microbial biomass recycling. ISME J. 2018, 12, 1768–1778. [Google Scholar] [CrossRef]
- Byrnes, E.J., 3rd; Li, W.; Ren, P.; Lewit, Y.; Voelz, K.; Fraser, J.A.; Dietrich, F.S.; May, R.C.; Chaturvedi, S.; Chaturvedi, V.; et al. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 2011, 7, e1002205. [Google Scholar] [CrossRef]
- Chen, S.C.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Zhang, L.; Liao, W.; Ling, L.; Li, X.; Lin, J.; Xu, B.; Pan, W.; Zhang, Q. Cerebrospinal fluid microscopy as an index for predicting the prognosis of cryptococcal meningitis patients with and without HIV. Future Microbiol. 2020, 15, 1645–1652. [Google Scholar] [CrossRef]
- Correa, K.; Craver, S.; Sandhu, A. An Uncommon Presentation of Cryptococcal Meningitis in an Immunocompetent Patient: A Case Report. Clin. Pract. Cases Emerg. Med. 2021, 5, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Poley, M.; Koubek, R.; Walsh, L.; McGillen, B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact. Case Rep. 2019, 7, 2324709619834578. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Almaeen, A.; Ahmed Wani, F.; Thirunavukkarasu, A. Hematologic derangements in HIV/AIDS patients and their relationship with the CD4 counts: A cross-sectional study. Int. J. Clin. Exp. Pathol. 2020, 13, 756–763. [Google Scholar]
- Xu, M.; Peng, Z.; Xu, C.; Chen, Y.; Cheng, J.; Chi, Y.; Wei, H.; Chen, W.; Hu, Z. Underlying Cryptococcal Diseases and the Correlation With Serum Cryptococcal Antigen Titers in Hospitalized HIV-Infected Patients Screened Positive for Cryptococcal Antigenemia. Front. Cell. Infect. Microbiol. 2020, 10, 170. [Google Scholar] [CrossRef]
- Chukwuanukwu, R.C.; Uchenna, N.; Mbagwu, S.I.; Chukwuanukwu, T.O.; Charles, O. Cryptococcus neoformans seropositivity and some haematological parameters in HIV seropositive subjects. J. Infect. Public Health 2020, 13, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Jongwutiwes, U.; Sungkanuparph, S.; Kiertiburanakul, S. Comparison of clinical features and survival between cryptococcosis in human immunodeficiency virus (HIV)-positive and HIV-negative patients. Jpn. J. Infect. Dis. 2008, 61, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Viriyavejakul, P.; Tangwanicharoen, T.; Punpoowong, B.; Chaisri, U.; Wilainam, P.; Nuntakomon, D.; Yimsamran, S.; Maneerat, Y.; Pongponratn, E.; Wilairatana, P.; et al. Cryptococcal meningitis in human immunodeficiency virus (HIV)-positive and HIV-negative patients. Southeast Asian J. Trop. Med. Public Health 2004, 35 (Suppl. 2), 33–38. [Google Scholar]
- Chen, S.C.; Slavin, M.A.; Heath, C.H.; Playford, E.G.; Byth, K.; Marriott, D.; Kidd, S.E.; Bak, N.; Currie, B.; Hajkowicz, K.; et al. Clinical manifestations of Cryptococcus gattii infection: Determinants of neurological sequelae and death. Clin. Infect. Dis. 2012, 55, 789–798. [Google Scholar] [CrossRef]
- Galanis, E.; Hoang, L.; Kibsey, P.; Morshed, M.; Phillips, P. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: Lessons learned from British Columbia. Can. J. Infect. Dis. Med. Microbiol. 2009, 20, 23–28. [Google Scholar] [CrossRef]
- Harris, J.R.; Lockhart, S.R.; Debess, E.; Marsden-Haug, N.; Goldoft, M.; Wohrle, R.; Lee, S.; Smelser, C.; Park, B.; Chiller, T. Cryptococcus gattii in the United States: Clinical aspects of infection with an emerging pathogen. Clin. Infect. Dis. 2011, 53, 1188–1195. [Google Scholar] [CrossRef]
- Tan, Z.R.; Long, X.Y.; Li, G.L.; Zhou, J.X.; Long, L. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China—A series of 18 cases. J. Neurol. Sci. 2016, 368, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhou, Z.; Fang, X.; Peng, F.; Zhang, W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1367–1372. [Google Scholar] [CrossRef]
- Abassi, M.; Boulware, D.R.; Rhein, J. Cryptococcal Meningitis: Diagnosis and Management Update. Curr. Trop. Med. Rep. 2015, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarith, R.; Vongsanim, S.; Supparatpinyo, K. Cryptococcal meningitis in HIV-infected patients at Chiang Mai University Hospital: A retrospective study. Southeast Asian J. Trop. Med. Public Health 2014, 45, 636–646. [Google Scholar] [PubMed]
- Martin, G.E.; Frater, J. Post-treatment and spontaneous HIV control. Curr. Opin. HIV AIDS 2018, 13, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, U.; Shrestha, U.; Guzman, N. Prevention of Opportunistic Infections In HIV/AIDS. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pasquier, E.; Kunda, J.; De Beaudrap, P.; Loyse, A.; Temfack, E.; Molloy, S.F.; Harrison, T.S.; Lortholary, O. Long-term Mortality and Disability in Cryptococcal Meningitis: A Systematic Literature Review. Clin. Infect. Dis. 2018, 66, 1122–1132. [Google Scholar] [CrossRef]
- Baddley, J.W.; Chen, S.C.; Huisingh, C.; Benedict, K.; DeBess, E.E.; Galanis, E.; Jackson, B.R.; MacDougall, L.; Marsden-Haug, N.; Oltean, H.; et al. MSG07: An International Cohort Study Comparing Epidemiology and Outcomes of Patients With Cryptococcus neoformans or Cryptococcus gattii Infections. Clin. Infect. Dis. 2021, 73, 1133–1141. [Google Scholar] [CrossRef]
- Phillips, P.; Galanis, E.; MacDougall, L.; Chong, M.Y.; Balshaw, R.; Cook, V.J.; Bowie, W.; Steiner, T.; Hoang, L.; Morshed, M.; et al. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin. Infect. Dis. 2015, 60, 1368–1376. [Google Scholar] [CrossRef]
- Sim, B.Z.; Conway, L.; Smith, L.K.; Fairhead, L.; Der, Y.S.; Payne, L.; Binotto, E.; Smith, S.; Hanson, J. The aetiology and clinical characteristics of cryptococcal infections in Far North Queensland, tropical Australia. PLoS ONE 2022, 17, e0265739. [Google Scholar] [CrossRef]
- Smith, R.M.; Mba-Jonas, A.; Tourdjman, M.; Schimek, T.; DeBess, E.; Marsden-Haug, N.; Harris, J.R. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS ONE 2014, 9, e88875. [Google Scholar] [CrossRef]
- Chen, S.C.; Korman, T.M.; Slavin, M.A.; Marriott, D.; Byth, K.; Bak, N.; Currie, B.J.; Hajkowicz, K.; Heath, C.H.; Kidd, S.; et al. Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin. Infect. Dis. 2013, 57, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; He, J.; Cheng, Z.; Wu, S.; Wang, M.; Niu, T. Clinical Characteristics and Risk Factors for Mortality in Cryptococcal Meningitis: Evidence From a Cohort Study. Front. Neurol. 2022, 13, 779435. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Tansuphasawadikul, S.; Simpson, A.J.; Howe, P.A.; White, N.J. A prospective study of AIDS-associated cryptococcal meningitis in Thailand treated with high-dose amphotericin B. J. Infect. 2001, 43, 226–233. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | HIV-Infected Patients (n = 101) | HIV-Uninfected Patients (n = 46) | p-Value |
|---|---|---|---|
| Male—n (%) | 66 (65.4) | 29 (63.0) | 0.787 |
| Age at diagnosis (years)—mean (SD) | 45.0 (12.4) | 61.9 (16.3) | <0.001 |
| Opportunistic infections—n (%) | 19 (18.8) | 3 (6.5) | 0.053 |
| ▪ Candidiasis | 7 (6.9) | 0 (0) | 0.099 |
| ▪ Tuberculosis | 6 (5.9) | 2 (4.3) | 1.000 |
| ▪ Pneumocystis jiroveci pneumonia | 4 (4.0) | 1 (2.2) | 1.000 |
| ▪ Cytomegalovirus infection | 4 (4.0) | 0 (0) | 0.310 |
| ▪ Herpes simplex virus infection | 4 (4.0) | 0 (0) | 0.310 |
| ▪ Progressive multifocal leukoencephalopathy | 0 (0) | 1 (2.2) | 0.313 |
| Co-infections—n (%) | |||
| Hepatitis B virus infection | 6 (5.9) | 0 (0) | 0.177 |
| Hepatitis C virus infection | 0 (0) | 1 (2.2) | 0.313 |
| Syphilis | 1 (1.0) | 0 (0) | 1.000 |
| Charlson Comorbidity Index—median (IQR) | 6 (6, 7) | 3 (2, 4) | <0.001 |
| Duration of symptoms before diagnosis (days)—median (IQR) | 14 (4, 30) | 14 (7, 30) | 0.921 |
| Presenting symptoms—n (%) | |||
| ▪ Fever | 64 (63.4) | 27 (58.7) | 0.589 |
| ▪ Headache | 73 (72.3) | 29 (63.0) | 0.260 |
| ▪ Alteration of consciousness | 14 (13.9) | 18 (39.1) | 0.001 |
| ▪ Nausea/vomiting | 29 (28.7) | 5 (10.9) | 0.017 |
| ▪ Seizure | 15 (14.9) | 7 (15.2) | 0.954 |
| ▪ Diplopia | 14 (13.9) | 6 (13.0) | 0.893 |
| ▪ Gait disturbance | 1 (1.0) | 3 (6.5) | 0.091 |
| ▪ Speech problems | 2 (2.0) | 2 (4.3) | 0.589 |
| ▪ Weakness | 9 (8.9) | 3 (6.5) | 0.754 |
| ▪ Sensory deficit | 2 (2.0) | 3 (6.5) | 0.177 |
| Characteristics | HIV-Infected Patients (n = 101) | HIV-Uninfected Patients (n = 46) | p-Value |
|---|---|---|---|
| Blood tests | |||
| Hemoglobin—g/dL—mean (SD) | 10.9 (2.2) | 11.8 (1.9) | 0.027 |
| Hemoglobin ≤ 10 g/dL | 39 (84.8) | 64 (63.4) | 0.009 |
| White blood cells—cells/cu.mm.—median (IQR) | 5100 (3800–6900) | 8985 (6290–14830) | <0.001 |
| White blood cells >15,000—cells/cu.mm.—n (%) | 2 (2.0) | 11 (23.9) | <0.001 |
| White blood cells <5000—cells/cu.mm.—n (%) | 49 (48.5) | 6 (13.0) | <0.001 |
| Platelets ×1000/cu.mm—mean (SD) | 240.3 (120.2) | 309.6 (138.1) | 0.002 |
| CD4 cell count—median (IQR) | 23 (10, 68) (n = 96) | 226 (150, 449) (n = 6) | <0.001 |
| Serum cryptococcal antigen titer—n (%) | <0.001 | ||
| Undetectable | 0/97 (0.0) | 3/40 (7.5) | |
| 1:10 | 9/97 (9.3) | 4/40 (10.0) | |
| 1:100 | 17/97 (17.5) | 19/40 (47.5) | |
| 1:1000 | 32/97 (33.0) | 9/40 (22.5) | |
| 1:10,000 | 38/97 (39.2) | 5/40 (12.50) | |
| >1:10,000 | 1/97 (1.0) | 0/40 (0.00) | |
| Serum cryptococcal antigen titer ≥1:10,000—n (%) | 39/97 (40.2) | 5/40 (12.5) | 0.002 |
| Hemoculture grew Cryptococcus species—n (%) | 44 (43.6) | 10 (21.7) | 0.011 |
| Cerebrospinal fluid (CSF) analysis | |||
| Opening pressure > 20 cm.H2O- n (%) | 69 (72.6) | 17 (51.5) | 0.026 |
| White blood cells –cells/cu.mm.—median (IQR) | 49 (10, 220) | 90 (20, 183) | 0.130 |
| Mononuclear—%—median (IQR) | 100.0 (86.0, 100.0) | 100.0 (66.0, 100.0) | 0.481 |
| Protein—g/dL—median (IQR) | 65.5 (45.0, 108.5) | 117.5 (71.0, 224.0) | <0.001 |
| CSF: serum sugar ratio –% | 36.5 (16.3) | 24.9 (16.3) | <0.001 |
| India ink positive—n (%) | 66/100 (66.0) | 23/44 (52.3) | 0.118 |
| Culture grew Cryptococcus species—n (%) | 75/100 (75.0) | 30/44 (68.2) | 0.396 |
| ▪ Cryptococcus neoformans species complex | 101 (100.0) | 38 (82.6) | <0.001 |
| ▪ Cryptococcus gattii species complex | 0 (0.0) | 8 (17.4) | <0.001 |
| CSF cryptococcal antigen titer—n (%) | 0.212 | ||
| Undetectable | 4/99 (4.0) | 0 (0.0) | |
| 1:10 | 9/99 (9.1) | 8/44 (18.2) | |
| 1:100 | 26/99 (26.3) | 14/44 (31.8) | |
| 1:1000 | 30/99 (30.3) | 14/44 (31.8) | |
| 1:10,000 | 29/99 (29.3) | 7/44 (15.9) | |
| >1:10,000 | 1/99 (1.0) | 1/44 (2.3) | |
| CSF cryptococcal antigen titer ≥1:10,000—n (%) | 30/99 (30.3) | 8/44 (18.2) | 0.130 |
| Imaging study—n (%) | |||
| Chest radiograph abnormality | 27 (26.7) | 16 (34.8) | 0.320 |
| Computerized tomography abnormality | 61 (60.4) | 37 (80.4) | 0.017 |
| ▪ Hypodensity lesion | 15 (14.9) | 10 (21.7) | 0.303 |
| ▪ Gelatinous pseudocyst | 10 (9.9) | 5 (10.9) | 1.000 |
| ▪ Hydrocephalus | 16 (15.8) | 24 (52.2) | <0.001 |
| ▪ Abscess | 1 (1.0) | 2 (4.3) | 0.230 |
| ▪ Leptomeningeal enhancement | 20 (19.8) | 10 (21.7) | 0.787 |
| ▪ Infarction | 11 (10.9) | 3 (6.5) | 0.550 |
| Characteristics | HIV-Infected Patients (n = 101) | HIV-Uninfected Patients (n = 46) | p-Value |
|---|---|---|---|
| Induction therapy—n (%) | 0.001 | ||
| Amphotericin B+ flucytosine | 0 (0.0) | 5 (10.9) | |
| Amphotericin B + fluconazole | 65 (64.4) | 35 (76.1) | |
| Amphotericin + flucytosine + fluconazole | 1 (1.0) | 0 (0.0) | |
| Amphotericin B | 30 (29.7) | 6 (13.0) | |
| Fluconazole | 5 (5.0) | 0 (0.0) | |
| Dose –mg/kg/day—n (%) | 0.183 | ||
| 0.7 | 40 (41.7) | 15 (32.6) | |
| 0.8 | 0 (0.0) | 1 (2.2) | |
| 1.0 | 56 (58.3) | 30 (65.2) | |
| Duration of induction—days—median (IQR) | 14 (13, 16) | 14 (13, 27) | 0.088 |
| Mortality—n (%) | 18 (17.8) | 17 (37.0) | 0.020 |
| Median time to death—days—median (IQR) | 38 (25, 84) (n = 18) | 66 (29, 124) (n = 17) | 0.030 |
| In-hospital—n (%) | 1 (1.0) | 2 (4.3) | 0.231 |
| 30-day—n (%) | 6 (5.9) | 5 (10.9) | 0.320 |
| 90-day—n (%) | 14 (13.9) | 12 (26.1) | 0.101 |
| 1-year—n (%) | 18 (17.8) | 17 (37.0) | 0.020 |
| Other outcomes | |||
| Amphotericin-induced acute kidney injury—n (%) | 24 (23.8) | 16 (34.8) | 0.169 |
| Length of hospital stay—days—median (IQR) | 15 (8, 23) | 19 (14, 36) | 0.002 |
| Characteristics | Patients Who Survived (n = 112) | Patients Who Died (n = 35) | p-Value |
|---|---|---|---|
| Male—n (%) | 73 (65.2) | 22 (62.9) | 0.841 |
| Age at diagnosis (years)—mean (SD) | 48.9 (15.3) | 54.8 (16.6) | 0.045 |
| HIV infection | 83 (74.1) | 18 (51.4) | 0.020 |
| Opportunistic infections—n (%) | 15 (13.4) | 7 (20) | 0.415 |
| ▪ Candidiasis | 5 (4.5) | 2 (5.7) | 0.671 |
| ▪ Tuberculosis | 6 (5.4) | 2 (5.7) | 1.000 |
| ▪ Pneumocystis jiroveci pneumonia | 2 (1.8) | 3 (8.6) | 0.088 |
| ▪ Cytomegalovirus infection | 3 (2.7) | 1 (2.9) | 1.000 |
| ▪ Herpes simplex virus infection | 3 (2.7) | 1 (2.9) | 1.000 |
| ▪ Progressive multifocal leukoencephalopathy | 1 (2.2) | 0 (0) | 1.000 |
| Charlson Comorbidity Index—median (IQR) | 6 (6, 7) | 6 (3, 7) | 0.182 |
| Median (IQR) duration of symptoms before diagnosis (days) | 14 (5.5, 30) | 14 (3, 30) | 0.411 |
| Presenting symptoms—n (%) | |||
| ▪ Fever | 69 (61.6) | 22 (62.9) | 0.894 |
| ▪ Alteration of consciousness | 16 (14.3) | 16 (45.7) | <0.001 |
| ▪ Seizure | 14 (12.5) | 8 (22.9) | 0.134 |
| Laboratory findings | |||
| Hemoglobin ≤ 10 g/dL | 73 (65.2) | 30 (85.7) | 0.021 |
| White blood cell count >20,000 cells/cu.mm.—n (%) | 4 (3.6) | 4 (11.4) | 0.092 |
| Platelets ×1000/cu.mm—mean (SD) | 260.1 (128.8) | 267.9 (134.2) | 0.757 |
| CD4 cell count—median (IQR) | 26.5 (11, 78) | 31 (11, 159.5) | 0.519 |
| Serum cryptococcal antigen titer >=1:1000—n (%) | 66 (62.3) | 19 (61.3) | 0.992 |
| Hemoculture grew Cryptococcus species—n (%) | 43 (38.4) | 11 (31.4) | 0.456 |
| Cerebrospinal fluid (CSF) analysis | |||
| Opening pressure > 20 cm.H2O- n (%) | 69 (61.6) | 17 (48.6) | 0.826 |
| White blood cells –cells/cu.mm.—median (IQR) | 66 (10, 229) | 50 (10, 147) | 0.323 |
| Protein—g/dL—median (IQR) | 77.5 (50, 125.5) | 95.5 (50, 194) | 0.375 |
| CSF: serum sugar ratio –% | 32.8 (15.9) | 33.1 (21.1) | 0.950 |
| Culture grew Cryptococcus species—n (%) | 82 (73.2) | 23 (65.7) | 0.880 |
| ▪ Cryptococcus neoformans species complex | 110 (98.2) | 29 (82.9) | 0.002 |
| ▪ Cryptococcus gattii species complex | 2 (1.8) | 6 (17.1) | |
| CSF cryptococcal antigen titer ≥1:1000—n (%) | 62 (55.9) | 20 (62.5) | 0.503 |
| Induction therapy—n (%) | 0.039 | ||
| Amphotericin B combination with fluconazole or flucytosine | 75 (67.0) | 31 (88.6) | |
| Amphotericin B monotherapy | 32 (28.6) | 4 (11.4) | |
| Fluconazole | 5 (4.5) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teekaput, C.; Yasri, S.; Chaiwarith, R. Cryptococcal Meningitis: Differences between Patients with and without HIV-Infection. Pathogens 2023, 12, 427. https://doi.org/10.3390/pathogens12030427
Teekaput C, Yasri S, Chaiwarith R. Cryptococcal Meningitis: Differences between Patients with and without HIV-Infection. Pathogens. 2023; 12(3):427. https://doi.org/10.3390/pathogens12030427
Chicago/Turabian StyleTeekaput, Chutithep, Saowaluck Yasri, and Romanee Chaiwarith. 2023. "Cryptococcal Meningitis: Differences between Patients with and without HIV-Infection" Pathogens 12, no. 3: 427. https://doi.org/10.3390/pathogens12030427
APA StyleTeekaput, C., Yasri, S., & Chaiwarith, R. (2023). Cryptococcal Meningitis: Differences between Patients with and without HIV-Infection. Pathogens, 12(3), 427. https://doi.org/10.3390/pathogens12030427







