Increased HIV Incidence in Wuchereria bancrofti Microfilaria Positive Individuals in Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HIV and Filarial Antigen Testing
2.3. DNA Isolation and Purification
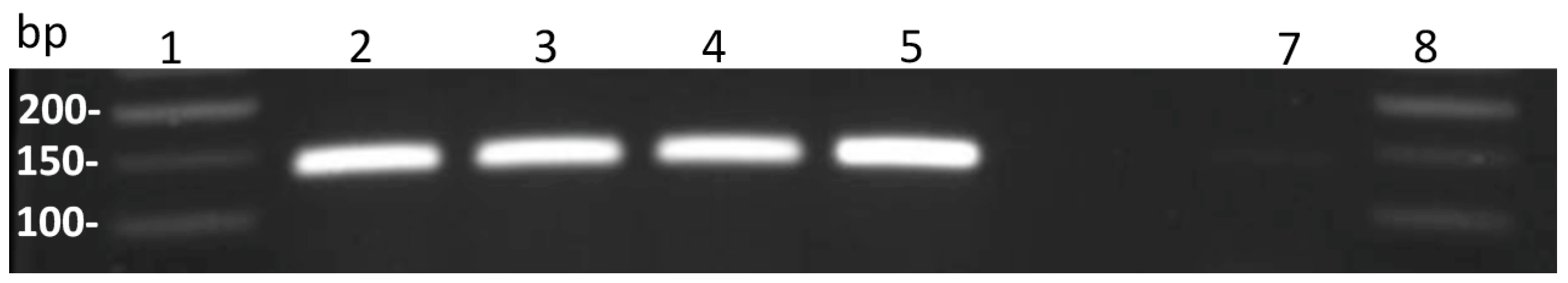
2.4. W. bancrofti Microfilaria Chitinase qPCR
2.5. Validation and Optimization of the Established Chitinase PCR Protocol
2.6. Statistical Analysis
3. Results
3.1. Description of Study Participants
3.2. Quality Control, Optimization and Validation of W. bancrofti Microfilariae Chitinase PCR
3.3. HIV Incidence in Wuchereria bancrofti Microfilariae Positive Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cromwell, A.E.; Schmidt, A.C.; Kwong, K.T.; Pigott, D.M.; Mupfasoni, D.; Biswas, G.; Shirude, S.; Hill, E.; Donkers, K.M.; Abdoli, A.; et al. The global distribution of lymphatic filariasis, 2000–2018: A geospatial analysis. Lancet Glob. Health 2020, 8, e1186–e1194. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, K.D.; Ottesen, E.A. Progress and Impact of 13 Years of the Global Programme to Eliminate Lymphatic Filariasis on Reducing the Burden of Filarial Disease. PLoS Negl. Trop. Dis. 2014, 8, e3319. [Google Scholar] [CrossRef]
- Dolo, H.; Coulibaly, Y.I.; Kelly-Hope, L.; Konate, S.; Dembele, B.; Coulibaly, S.Y.; Sanogo, D.; Soumaoro, L.; Coulibaly, M.E.; Doumbia, S.S.; et al. Factors Associated with Wuchereria bancrofti Microfilaremia in an Endemic Area of Mali. Am. J. Trop. Med. Hyg. 2018, 98, 1782–1787. [Google Scholar] [CrossRef]
- Simonsen, P.E. The Filariases. In Manson’s Tropical Diseases, 23rd ed.; Cook, G.C., Zumla, A.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 737–765. [Google Scholar]
- Kroidl, I.; Saathof, E.; Maganga, L.; Clowes, P.; Maboko, L.; Hoerauf, A.; Makunde, W.H.; Haule, A.; Mviombo, P.; Pitter, B.; et al. Prevalence of Lymphatic Filariasis and Treatment Effectiveness of Albendazole/ Ivermectin in Individuals with HIV Co-infection in Southwest-Tanzania. PLoS Negl. Trop. Dis. 2016, 10, e0004618. [Google Scholar] [CrossRef]
- Mnkai, J.; Marandu, T.F.; Mhidze, J.; Urio, A.; Maganga, L.; Haule, A.; Kavishe, G.; Ntapara, E.; Chiwerengo, N.; Clowes, P.; et al. Step towards elimination of Wuchereria bancrofti in Southwest Tanzania 10 years after mass drug administration with Albendazole and Ivermectin. PLoS Negl. Trop. Dis. 2022, 16, e0010044. [Google Scholar] [CrossRef]
- Arndts, K.; Deininger, S.; Specht, S.; Klarmann, U.; Mand, S.; Adjobimey, T.; Debrah, A.; Batsa, L.; Kwarteng, A.; Epp, C.; et al. Elevated Adaptive Immune Responses Are Associated with Latent Infections of Wuchereria bancrofti. PLoS Negl. Trop. Dis. 2012, 6, e1611. [Google Scholar] [CrossRef]
- Kroidl, I.; Chachage, M.; Mnkai, J.; Nsojo, A.; Berninghoff, M.; Verweij, J.J.; Maganga, L.; Ntinginya, N.E.; Maboko, L.; Clowes, P.; et al. Wuchereria bancrofti infection is linked to systemic activation of CD4 and CD8 T cells. PLoS Negl. Trop. Dis. 2019, 13, e0007623. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunology of lymphatic filariasis. Parasite Immunol. 2014, 36, 338–346. [Google Scholar] [CrossRef]
- Ritter, M.; Osei-Mensah, J.; Debrah, L.B.; Kwarteng, A.; Mubarik, Y.; Debrah, A.; Pfarr, K.; Hoerauf, A.; Layland, L.E. Wuchereria bancrofti-infected individuals harbor distinct IL-10-producing regulatory B and T cell subsets which are affected by anti-filarial treatment. PLoS Negl. Trop. Dis. 2019, 13, e0007436. [Google Scholar] [CrossRef]
- Hertz, I.M.; Rush, A.; Nutman, T.B.; Weil, G.J.; Bennuru, S.; Budge, P.J. Characterization of glycan determinants that mediate recognition of the major Wuchereria bancrofti circulating antigen by diagnostic antibodies. Mol. Biochem. Parasitol. 2020, 240, 111317. [Google Scholar] [CrossRef]
- More, S.; Copeman, D.B. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop. Med. Parasitol. 1990, 41, 403–406. [Google Scholar] [PubMed]
- Weil, G.J.; Jain, D.C.; Santhanam, S.; Malhotra, A.; Kumar, H.; Sethumadhavan, K.V.P.; Liftis, F.; Ghosh, T.K. A Monoclonal Antibody-Based Enzyme Immunoassay for Detecting Parasite Antigenemia in Bancroftian Filariasis. J. Infect. Dis. 1987, 156, 350–355. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.; Bishop, H.; Nascimento, F.S.; Mathison, B.; Bradbury, R.; Da Silva, A. Multiplex TaqMan qPCR assay for specific identification of encapsulated Trichinella species prevalent in North America. Memórias Inst. Oswaldo Cruz 2018, 113, e180305. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.L.; Fahle, G.A.; Fischer, S.; Fedorko, D.F.; Nutman, T.B. Toward Molecular Parasitologic Diagnosis: Enhanced Diagnostic Sensitivity for Filarial Infections in Mobile Populations. J. Clin. Microbiol. 2011, 49, 42–47. [Google Scholar] [CrossRef]
- Ximenes, C.; Brandão, E.; Oliveira, P.; Rocha, A.; Rego, T.; Medeiros, R.; Aguiar-Santos, A.; Ferraz, J.; Reis, C.; Araujo, P.; et al. Detection of Wuchereria bancrofti DNA in paired serum and urine samples using polymerase chain reaction-based systems. Mem. Inst. Oswaldo Cruz 2014, 109, 978–983. [Google Scholar] [CrossRef]
- Tanzania HIV and Malaria Indicator Survey. 2007. Available online: https://dhsprogram.com/pubs/pdf/AIS6/AIS6_05_14_09.pdf (accessed on 23 February 2023).
- World Health Organization. AIDS Epidemic Update. 2007. Available online: https://data.unaids.org/pub/epislides/2007/2007_epiupdate_en.pdf (accessed on 23 February 2023).
- Nielsen, N.O.; Simonsen, P.; Magnussen, P.; Magesa, S.; Friis, H. Cross-sectional relationship between HIV, lymphatic filariasis and other parasitic infections in adults in coastal northeastern Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 543–550. [Google Scholar] [CrossRef]
- Simon, G. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: Scientific links. Int. J. Infect. Dis. 2016, 42, 54–57. [Google Scholar] [CrossRef]
- Yegorov, S.; Joag, V.; Galiwango, R.M.; Good, S.V.; Mpendo, J.; Tannich, E.; Boggild, A.K.; Kiwanuka, N.; Bagaya, B.S.; Kaul, R. Schistosoma mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-I pathways. Nat. Commun. 2019, 10, 2296. [Google Scholar] [CrossRef]
- Kroidl, I.; Saathoff, E.; Maganga, L.; Makunde, W.H.; Hoerauf, A.; Geldmacher, C.; Clowes, P.; Maboko, L.; Hoelscher, M. Effect of Wuchereria bancrofti infection on HIV incidence in southwest Tanzania: A prospective cohort study. Lancet 2016, 388, 1912–1920. [Google Scholar] [CrossRef]
- Kroidl, I.; Clowes, P.; Mwalongo, W.; Maganga, L.; Maboko, L.; Kroidl, A.L.; Geldmacher, C.; Machibya, H.; Hoelscher, M.; Saathoff, E. Low Specificity of Determine HIV1/2 RDT Using Whole Blood in South West Tanzania. PLoS ONE 2012, 7, e39529. [Google Scholar] [CrossRef]
- Albers, A.; Sartono, E.; Wahyuni, S.; Yazdanbakhsh, M.; Maizels, R.M.; Klarmann-Schulz, U.; Pfarr, K.; Hoerauf, A. Real-time PCR detection of the HhaI tandem DNA repeat in pre- and post-patent Brugia malayi Infections: A study in indonesian transmigrants. Parasites Vectors 2014, 7, 146. [Google Scholar] [CrossRef]
- Colebunders, R.; Mandro, M.; Mokili, J.L.; Mucinya, G.; Mambandu, G.; Pfarr, K.; Reiter-Owona, I.; Hoerauf, A.; Tepage, F.; Levick, B.; et al. Risk factors for epilepsy in Bas-Uélé Province, Democratic Republic of the Congo: A case–control study. Int. J. Infect. Dis. 2016, 49, 1–8. [Google Scholar] [CrossRef]
- Arnold, K.; Venegas, A.; Houseweart, C.; Fuhrman, J. Discrete transcripts encode multiple chitinase isoforms in Brugian microfilariae. Mol. Biochem. Parasitol. 1996, 80, 149–158. [Google Scholar] [CrossRef]
- Bossonario, P.A.; Ferreira, M.R.L.; Andrade, R.L.D.P.; de Sousa, K.D.L.; Bonfim, R.O.; Saita, N.M.; Monroe, A.A. Fatores de risco à infecção pelo HIV entre adolescentes e jovens: Revisão sistemática. Rev. Lat.-Am. Enferm. 2022, 30. [Google Scholar] [CrossRef]
- Prodger, J.L.; Kaul, R. The biology of how circumcision reduces HIV susceptibility: Broader implications for the prevention field. AIDS Res. Ther. 2017, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Rauniyar, R.; Gupta, B.P. Microfilaria persistent foci during post MDA and the risk assessment of resurgence in India. Trop. Med. Health 2018, 46, 25. [Google Scholar] [CrossRef] [PubMed]
- Aboagye-Antwi, F.; Kwansa-Bentum, B.; Dadzie, S.K.; Ahorlu, C.K.; Appawu, M.A.; Gyapong, J.; Wilson, M.D.; Boakye, D.A. Transmission indices and microfilariae prevalence in human population prior to mass drug administration with ivermectin and albendazole in the Gomoa District of Ghana. Parasites Vectors 2015, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, E.P.; Derua, A.Y.; Magesa, S.M.; Pedersen, E.M.; Stensgaard, A.-S.; Malecela, M.N.; Kisinza, W.N. Lymphatic filariasis control in Tanga Region, Tanzania: Status after eight rounds of mass drug administration. Parasites Vectors 2014, 7, 507. [Google Scholar] [CrossRef]
- Weil, G.J.; Kastens, W.; Susapu, M.; Laney, S.J.; Williams, S.; King, C.L.; Kazura, J.W.; Bockarie, M.J. The Impact of Repeated Rounds of Mass Drug Administration with Diethylcarbamazine Plus Albendazole on Bancroftian Filariasis in Papua New Guinea. PLoS Negl. Trop. Dis. 2008, 2, e344. [Google Scholar] [CrossRef] [PubMed]
- Endeshaw, T.; Taye, A.; Tadesse, Z.; Katabarwa, M.N.; Shafi, O.; Seid, T.; Richards, F.O. Presence of Wuchereria bancrofti microfilaremia despite 7 years of annual ivermectin monotherapy mass drug administration for onchocerciasis control: A study in north-west Ethiopia. Ann. Trop. Med. Parasitol. 2015, 109, 344–351. [Google Scholar] [CrossRef]
- Iboh, I.C.; Okon, O.E.; Opara, K.N.; Asor, J.E.; Etim, S.E. Lymphatic filariasis among the Yakurr people of Cross River State, Nigeria. Parasites Vectors 2012, 5, 203. [Google Scholar] [CrossRef]
- Omondi, M.A.; Kamassa, E.H.; Katawa, G.; Tchopba, C.N.; Vogelbusch, C.; Parcina, M.; Tchadié, E.P.; Amessoudji, O.M.; Arndts, K.; Karou, S.D.; et al. Hookworm infection associates with a vaginal Type 1/Type 2 immune signature and increased HPV load. Front. Immunol. 2022, 13, 1009968. [Google Scholar] [CrossRef]
- Dreyer, G.; Pimentael, A.; Medeiros, Z.; Béliz, F.; Moura, I.; Coutinho, A.; de Andrade, L.D.; Rocha, A.; da Silva, L.M.; Piessens, W.F. Studies on the periodicity and intravascular distribution of Wuchereria bancrofti microfilariae in paired samples of capillary and venous blood from Recife, Brazil. Trop. Med. Int. Health 1996, 1, 264–272. [Google Scholar] [CrossRef]
| Nematode Species | N | CFA | W. bancrofti MF Count/mL | MF PCR Pos | MF PCR Neg |
|---|---|---|---|---|---|
| W.bancrofti | 1 | pos | 200 | 0 | 1 |
| W.bancrofti | 1 | pos | 1740 | 1 | 0 |
| W.bancrofti | 1 | pos | 2030 | 1 | 0 |
| W.bancrofti | 1 | pos | 2170 | 1 | 0 |
| W.bancrofti | 1 | pos | 2420 | 1 | 0 |
| W.bancrofti | 1 | pos | 4090 | 1 | 0 |
| W.bancrofti | 1 | pos | 4360 | 1 | 0 |
| Mansonellaperstans | 20 | neg | NA | 0 | 20 |
| Loaloa | 6 | neg | NA | 0 | 6 |
| Onchocercavolvulus | 20 | neg | NA | 0 | 20 |
| not infected * | 30 | neg | 0 | 1 | 29 |
| not infected # | 50 | neg | Not determined | 0 | 50 |
| MF PCR Performed | No PCR Performed | ||
|---|---|---|---|
| Characteristic | MF PCR Tested | MF Not Tested | p-Value * |
| Number of samples | 350 | 156 | |
| Sex (%) | |||
| female | 170 (48.6%) | 75 (48.1%) | 0.918 |
| male | 180 (51.4%) | 81 (51.9%) | |
| Age group (years) | |||
| <25 | 117 (33.2%) | 64 (41.0%) | |
| 25–<45 | 110 (31.5%) | 44 (28.2%) | 0.257 |
| ≥45 | 123 (35.2%) | 48 (35.2%) | |
| Median age in years (IQR $) | 32.7 (21.8–56.7) | 28.4.8 (18.7–51.0) | 0.090 |
| SES rank, median (IQR) | 3.04 (0.86–5.84) | 3.93 (1.35–6.14) | 0.088 |
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Exp. | N-Pos | Incidence per 100 PY | IRR | 95% CI | p-Value | IRR | 95% CI | p-Value |
| All MF at survey 1 | 1109 | 22 | 1.98 | ||||||
| Neg * | 1070 | 19 | 1.78 | 1.00 | 1.00 | ||||
| Pos | 39 | 3 | 7.75 | 4.29 | (1.33 to 13.9) | 0.015 | 4.58 | (1.37 to 15.4) | 0.014 |
| Gender | |||||||||
| Female * | 537 | 11 | 2.05 | 1.00 | - | - | 1.00 | - | - |
| Male | 572 | 11 | 1.92 | 0.94 | (0.41 to 2.18) | 0.885 | 0.90 | (0.39 to 2.07) | 0.796 |
| Age | |||||||||
| 14–<25 * | 312 | 6 | 1.92 | 1.00 | - | - | 1.00 | - | - |
| 25–<45 | 338 | 10 | 2.61 | 1.35 | (0.49 to 3.75) | 0.560 | 1.46 | (0.54 to 3.95) | 0.453 |
| ≥45 | 413 | 6 | 1.45 | 0.76 | (0.25 to 2.36) | 0.636 | 0.75 | (0.25 to 2.12) | 0.530 |
| SES rank (per unit) | - | - | - | 0.91 | (0.78 to 1.06) | 0.213 | 0.89 | (0.76 to 1.04) | 0.134 |
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Exp. | N-pos | IR per 100 PY | IRR | 95% CI | p-Value | IRR | 95% CI | p-Value |
| All | 3187 | 38 | 1.19 | ||||||
| Gender | |||||||||
| Female * | 1642 | 22 | 1.34 | 1.00 | - | - | 1.00 | - | - |
| Male | 1546 | 16 | 1.04 | 0.77 | (0.41 to 1.48) | 0.436 | 0.55 | (0.42 to 1.24) | 0.148 |
| Age | |||||||||
| 14–<25 * | 1337 | 10 | 0.75 | 1.00 | - | - | 1.00 | ||
| 25–<45 | 964 | 18 | 1.87 | 2.48 | (1.14 to 5.39) | 0.022 | 1.73 | (0.66 to 4.52) | 0.263 |
| ≥45 | 886 | 10 | 1.13 | 1.51 | (0.63 to 3.62) | 0.361 | 1.27 | (0.45 to 3.59) | 0.657 |
| LF status | |||||||||
| CFA neg * | 2101 | 16 | 0.76 | 1.00 | - | - | 1.00 | - | - |
| CFA + MF neg | 1051 | 19 | 1.81 | 2.36 | (1.21 to 4.59) | 0.012 | 2.12 | (1.71 to 4.20) | 0.030 |
| CFA + MF pos | 35 | 3 | 8.69 | 11.1 | (3.43 to 36.2) | <0.001 | 12.7 | (3.48 to 46.2) | <0.001 |
| Marital status | |||||||||
| Never married * | 1113 | 7 | 0.63 | 1.00 | - | - | 1.00 | - | - |
| Married | 1357 | 19 | 1.40 | 2.22 | (0.93 to 5.29) | 0.073 | 0.94 | (0.27 to 3.22) | 0.922 |
| Separated # | 718 | 12 | 1.67 | 2.64 | (1.04 to 6.73) | 0.042 | 1.20 | (0.35 to 4.10) | 0.772 |
| No. of sex partners | |||||||||
| None * | 285 | 2 | 0.70 | 1.00 | - | - | 1.00 | - | - |
| One | 1487 | 20 | 1.35 | 1.91 | (0.44 to 8.21) | 0.386 | 2.63 | (0.51 to 13.7) | 0.251 |
| Two or more | 378 | 11 | 2.91 | 4.09 | (0.90 to 18.6) | 0.069 | 8.52 | (1.49 to 48.7) | 0.016 |
| No info | 1038 | 5 | 0.48 | 0.69 | (0.13 to 3.56) | 0.656 | 1.29 | (0.24 to 7.01) | 0.771 |
| Circumcised (male) | |||||||||
| No * | 1351 | 15 | 1.11 | 1.00 | - | - | 1.00 | - | - |
| Yes | 195 | 1 | 0.51 | 0.42 | (0.06 to 3.19) | 0.418 | 0.48 | (0.06 to 3.60) | 0.476 |
| N/A (female) | 1642 | 22 | 1.34 | 1.00 | (omitted) | - | 1.00 | (omitted) | - |
| SES rank (per unit) | - | - | - | 0.92 | (0.81 to 1.03) | 0.158 | 0.91 | (0.80 to 1.03) | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnkai, J.; Ritter, M.; Maganga, L.; Maboko, L.; Olomi, W.; Clowes, P.; Minich, J.; Lelo, A.E.; Kariuki, D.; Debrah, A.Y.; et al. Increased HIV Incidence in Wuchereria bancrofti Microfilaria Positive Individuals in Tanzania. Pathogens 2023, 12, 387. https://doi.org/10.3390/pathogens12030387
Mnkai J, Ritter M, Maganga L, Maboko L, Olomi W, Clowes P, Minich J, Lelo AE, Kariuki D, Debrah AY, et al. Increased HIV Incidence in Wuchereria bancrofti Microfilaria Positive Individuals in Tanzania. Pathogens. 2023; 12(3):387. https://doi.org/10.3390/pathogens12030387
Chicago/Turabian StyleMnkai, Jonathan, Manuel Ritter, Lucas Maganga, Leonard Maboko, Willyhelmina Olomi, Petra Clowes, Jessica Minich, Agola Eric Lelo, Daniel Kariuki, Alexander Yaw Debrah, and et al. 2023. "Increased HIV Incidence in Wuchereria bancrofti Microfilaria Positive Individuals in Tanzania" Pathogens 12, no. 3: 387. https://doi.org/10.3390/pathogens12030387
APA StyleMnkai, J., Ritter, M., Maganga, L., Maboko, L., Olomi, W., Clowes, P., Minich, J., Lelo, A. E., Kariuki, D., Debrah, A. Y., Geldmacher, C., Hoelscher, M., Saathoff, E., Chachage, M., Pfarr, K., Hoerauf, A., & Kroidl, I. (2023). Increased HIV Incidence in Wuchereria bancrofti Microfilaria Positive Individuals in Tanzania. Pathogens, 12(3), 387. https://doi.org/10.3390/pathogens12030387










