Feeding on a Bartonella henselae Infected Host Triggers Temporary Changes in the Ctenocephalides felis Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Cat Bartonella henselae Infection and C. felis Feeding
2.2. PCR Assays
2.3. Library Preparation and Sequencing
2.4. Data Analysis
3. Results
3.1. NGS Filtering
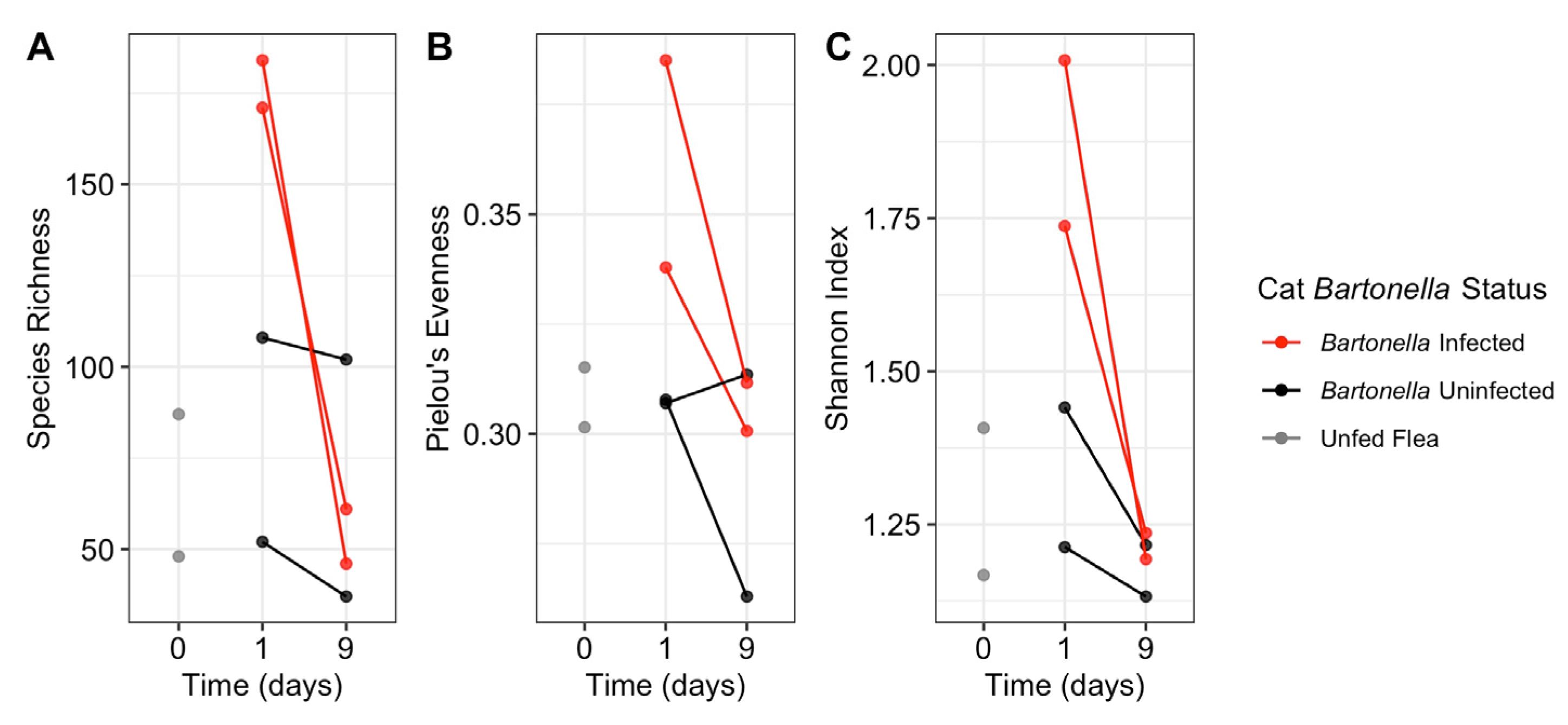
3.2. Length of Feeding
3.3. Host Bartonella Status
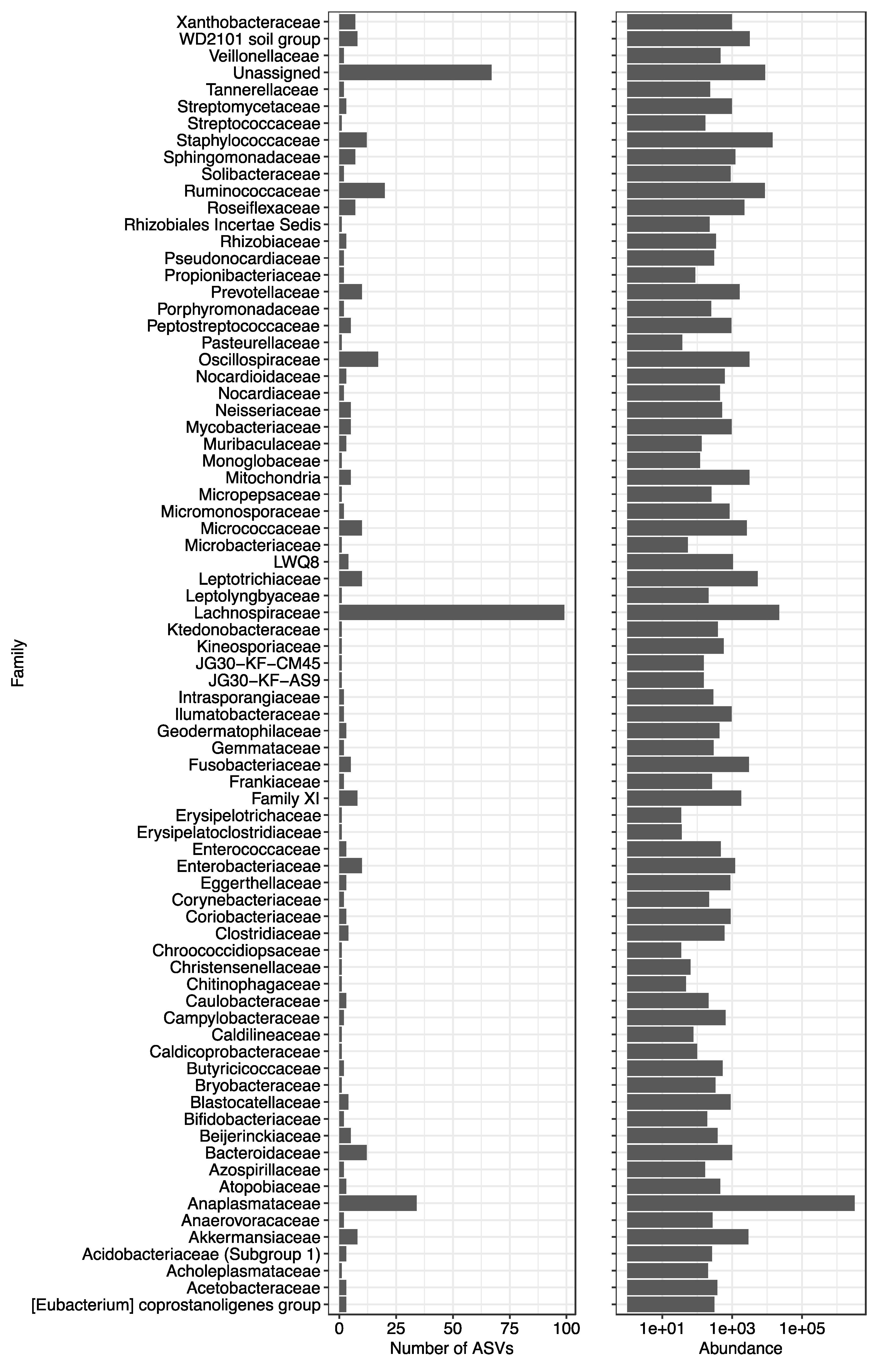
3.4. Bacterial Detection
3.5. Flea Cox1 Haplotypes
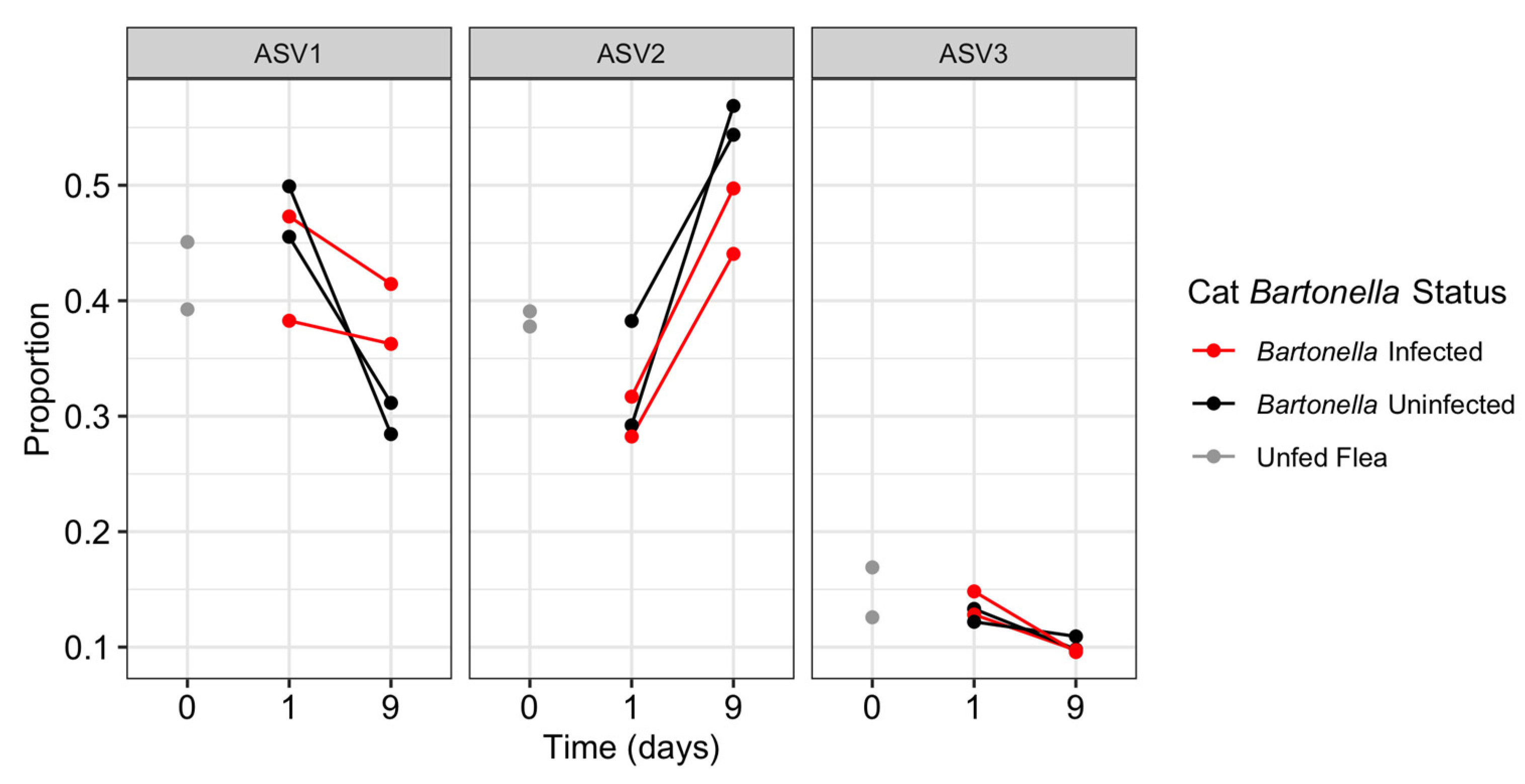
3.6. Differential Sequence Abundance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rust, M.K. The Biology and Ecology of Cat Fleas and Advancements in Their Pest Management: A Review. Insects 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Boulouis, H.-J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella Spp. in Pets and Effect on Human Health. Emerg. Infect. Dis. 2006, 12, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lashnits, E.; Neupane, P.; Bradley, J.M.; Richardson, T.; Maggi, R.G.; Breitschwerdt, E.B. Comparison of Serological and Mo-lecular Assays for Bartonella Species in Dogs with Hemangiosarcoma. Pathogens 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rolain, J.-M. Bartonella infection: Treatment and drug resistance. Future Microbiol. 2010, 5, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Richardson, T.; Breitschwerdt, E.B.; Miller, J.C. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species within human clinical samples. J. Microbiol. Methods 2020, 176, 106022. [Google Scholar] [CrossRef]
- Fromm, K.; Dehio, C. The Impact of Bartonella VirB/VirD4 Type IV Secretion System Effectors on Eukaryotic Host Cells. Front. Microbiol. 2021, 12, 3582. [Google Scholar] [CrossRef]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, Dynamics, and Control of Emerging Vector-Borne Zoonotic Diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef]
- Adegoke, A.; Kumar, D.; Bobo, C.; Rashid, M.; Durrani, A.; Sajid, M.; Karim, S. Tick-Borne Pathogens Shape the Native Microbiome Within Tick Vectors. Microorganisms 2020, 8, 1299. [Google Scholar] [CrossRef]
- Dorigatti, I.; McCormack, C.; Nedjati-Gilani, G.; Ferguson, N.M. Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol. 2017, 34, 102–113. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Ryan, P.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from Aedes transmitted arboviruses. Gates Open Res. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, E.J.; Billeter, S.A.; Jett, L.A.; Meinersmann, R.J.; Barr, M.C.; Diniz, P.P.; Oakley, B.B. Assessing Cat Flea Microbiomes in Northern and Southern California by 16S rRNA Next-Generation Sequencing. Vector-Borne Zoonotic Dis. 2018, 18, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.L.; Hii, S.-F.; Chong, R.; Webb, C.E.; Traub, R.; Brown, G.; Šlapeta, J. Evaluation of the bacterial microbiome of two flea species using different DNA-isolation techniques provides insights into flea host ecology. FEMS Microbiol. Ecol. 2015, 91, fiv134. [Google Scholar] [CrossRef] [PubMed]
- Leulmi, H.; Socolovschi, C.; Laudisoit, A.; Houemenou, G.; Davoust, B.; Bitam, I.; Raoult, D.; Parola, P. Detection of Rickettsia felis, Rickettsia typhi, Bartonella Species and Yersinia pestis in Fleas (Siphonaptera) from Africa. PLoS Negl. Trop. Dis. 2014, 8, e3152. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.E.; Macaluso, K.R. Ecology of Rickettsia felis: A Review. J. Med. Entomol. 2009, 46, 723–736. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Brockway, C.; Shrewsberry, D.L.; Plumer, M.; Sevdalis, S.E.; Beckmann, J.F.; Krueger, L.M.; Macaluso, K.R.; Azad, A.F.; et al. Evolution of Wolbachia mutualism and reproductive parasitism: Insight from two novel strains that co-infect cat fleas. PeerJ 2020, 8, e10646. [Google Scholar] [CrossRef]
- Khoo, J.; Kurtti, T.; Husin, N.; Beliavskaia, A.; Lim, F.; Zulkifli, M.; Al-Khafaji, A.; Hartley, C.; Darby, A.; Hughes, G.; et al. Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines. Microorganisms 2020, 8, 988. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Genet. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef]
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The Relative Importance of Innate Immune Priming in Wolbachia-Mediated Dengue Interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.A.; Radulovic, S.; Jaworski, D.C.; Azad, A.F. Acquisition of the Cat Scratch Disease Agent Bartonella henselae by Cat Fleas (Siphonaptera: Pulicidae). J. Med. Entomol. 1996, 33, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Bouhsira, E.; Franc, M.; Boulouis, H.-J.; Jacquiet, P.; Raymond-Letron, I.; Liénard, E. Assessment of Persistence of Bartonella henselae in Ctenocephalides felis. Appl. Environ. Microbiol. 2013, 79, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- André, M.R.; Neupane, P.; Lappin, M.; Herrin, B.; Smith, V.; Williams, T.I.; Collins, L.; Bai, H.; Jorge, G.L.; Balbuena, T.S.; et al. Using Proteomic Approaches to Unravel the Response of Ctenocephalides felis felis to Blood Feeding and Infection With Bartonella henselae. Front. Cell. Infect. Microbiol. 2022, 12, 18. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Manvell, C.; Berman, H.; Callahan, B.; Breitschwerdt, E.; Swain, W.; Ferris, K.; Maggi, R.; Lashnits, E. Identification of microbial taxa present in Ctenocephalides felis (cat flea) reveals widespread co-infection and associations with vector phylogeny. Parasites Vectors 2022, 15, 1–17. [Google Scholar] [CrossRef]
- Lawrence, A.L.; Webb, C.E.; Clark, N.J.; Halajian, A.; Mihalca, A.D.; Miret, J.; D’Amico, G.; Brown, G.; Kumsa, B.; Modrý, D.; et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker’s guide to world domination. Int. J. Parasitol. 2019, 49, 321–336. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package 2020. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 3 May 2021).
- Wagner, B.D.; Grunwald, G.K.; Zerbe, G.O.; Mikulich-Gilbertson, S.K.; Robertson, C.E.; Zemanick, E.T.; Harris, J.K. On the Use of Diversity Measures in Longitudinal Sequencing Studies of Microbial Communities. Front. Microbiol. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella infections in cats and dogs including zoonotic aspects. Parasites Vectors 2018, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D. Immunity of fleas (Order Siphonaptera). Dev. Comp. Immunol. 2019, 98, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Greene, W.K.; Macnish, M.G.; Rice, K.L.; Thompson, R.A. Identification of genes associated with blood feeding in the cat flea, Ctenocephalides felis. Parasites Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.T.; Morgan, E.R.; Woods, D.; Shaw, S.E. Real-time and multiplex real-time polymerase chain reactions for the detection of Bartonella henselae within cat flea, Ctenocephalides felis, samples. Med. Veter. Entomol. 2010, 24, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.J.L.; Brown, T.P.; Kathy, L.O.; Foil, L.D.; Finkelstein, J.L.; Brown, T.P.; Reilly, K.L.O.; Wedincamp, J. Studies on the Growth of Bartonella henselae in the Cat Flea (Siphonaptera: Pulicidae). J. Med. Entomol. 2002, 39, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Morick, D.; Krasnov, B.R.; Khokhlova, I.S.; Gutiérrez, R.; Gottlieb, Y.; Harrus, S. Vertical nontransovarial transmission of Bartonella in fleas. Mol. Ecol. 2013, 22, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Hirunkanokpun, S.; Thepparit, C.; Foil, L.D.; Macaluso, K.R. Horizontal transmission of Rickettsia felis between cat fleas, Ctenocephalides felis. Mol. Ecol. 2011, 20, 4577–4586. [Google Scholar] [CrossRef]
- Jameson, P.; Greene, C.; Regnery, R.; Dryden, M.; Marks, A.; Brown, J.; Cooper, J.; Glaus, B.; Greene, R. Prevalence of Bartonella henselae Antibodies in Pet Cats throughout Regions of North America. J. Infect. Dis. 1995, 172, 1145–1149. [Google Scholar] [CrossRef]
- Dreher-Lesnick, S.M.; Ceraul, S.M.; Gillespie, J.J.; Anderson, J.M.; Jochim, R.C.; Valenzuela, J.G.; Azad, A.F. Analysis of Rickettsia typhi-infected and uninfected cat flea (Ctenocephalides felis) midgut cDNA libraries: Deciphering molecular pathways involved in host response to R. typhi infection. Insect Mol. Biol. 2010, 19, 229–241. [Google Scholar] [CrossRef]
- Thepparit, C.; Hirunkanokpun, S.; Popov, V.L.; Foil, L.D.; Macaluso, K.R. Dissemination of bloodmeal acquired Rickettsia felis in cat fleas, Ctenocephalides felis. Parasites Vectors 2013, 6, 149. [Google Scholar] [CrossRef]
- Rennoll, S.A.; Rennoll-Bankert, K.E.; Guillotte, M.L.; Lehman, S.S.; Driscoll, T.P.; Beier-Sexton, M.; Rahman, M.S.; Gillespie, J.J.; Azad, A.F. The cat flea (Ctenocephalides felis) immune deficiency signaling pathway regulates Rickettsia typhi infection. Infect. Immun. 2018, 86, 11. [Google Scholar] [CrossRef]
- de Leon, A.V.-P.; Schneider, M.G.; Jahnes, B.C.; Sadowski, V.; Camuy-Vélez, L.A.; Duan, J.; Sabree, Z.L. Genetic Drift and Host-Adaptive Features Likely Underlie the Cladogenesis of Insect-Associated Lachnospiraceae. Genome Biol. Evol. 2022, 14, evac086. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, Bacterial, and Archaeal Diversity in the Digestive Tract of Several Beetle Larvae (Coleoptera). BioMed. Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Stewart, P.E. Microbiomes of Blood-Feeding Arthropods: Genes Coding for Essential Nutrients and Relation to Vector Fitness and Pathogenic Infections. A Review. Microorganisms 2021, 9, 2433. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Zhang, X.-Y.; Zhou, X.-J.; Chen, K.-L.; Masoudi, A.; Liu, J.-Z.; Zhang, Y.-K. Bacterial microbiota analysis demonstrates that ticks can acquire bacteria from habitat and host blood meal. Exp. Appl. Acarol. 2022, 87, 81–95. [Google Scholar] [CrossRef] [PubMed]


| Sample | Host Cat B. henselae Status | ITS qPCR (Ct) |
|---|---|---|
| 3711-24 h | Infected | + (31.61) |
| 3711-9 d | Infected | + (32.48) |
| 3320-24 h | Infected | + (38.13) |
| 3320-9 d | Infected | + (41.48) |
| 3363-24 h | Naïve | − (N/A) |
| 3363-9 d | Naïve | − (N/A) |
| 3508-24 h | Naïve | − (N/A) |
| 3508-9 d | Naïve | − (N/A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, C.; Lashnits, E.; Neupane, P.; Herrin, B.H.; Lappin, M.; André, M.R.; Breitschwerdt, E.B. Feeding on a Bartonella henselae Infected Host Triggers Temporary Changes in the Ctenocephalides felis Microbiome. Pathogens 2023, 12, 366. https://doi.org/10.3390/pathogens12030366
Moore C, Lashnits E, Neupane P, Herrin BH, Lappin M, André MR, Breitschwerdt EB. Feeding on a Bartonella henselae Infected Host Triggers Temporary Changes in the Ctenocephalides felis Microbiome. Pathogens. 2023; 12(3):366. https://doi.org/10.3390/pathogens12030366
Chicago/Turabian StyleMoore, Charlotte, Erin Lashnits, Pradeep Neupane, Brian H. Herrin, Michael Lappin, Marcos Rogério André, and Edward B. Breitschwerdt. 2023. "Feeding on a Bartonella henselae Infected Host Triggers Temporary Changes in the Ctenocephalides felis Microbiome" Pathogens 12, no. 3: 366. https://doi.org/10.3390/pathogens12030366
APA StyleMoore, C., Lashnits, E., Neupane, P., Herrin, B. H., Lappin, M., André, M. R., & Breitschwerdt, E. B. (2023). Feeding on a Bartonella henselae Infected Host Triggers Temporary Changes in the Ctenocephalides felis Microbiome. Pathogens, 12(3), 366. https://doi.org/10.3390/pathogens12030366








