Potassium Phosphonate Induces Resistance in Sweet Chestnut against Ink Disease Caused by Phytophthora Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Phytophthora Isolates
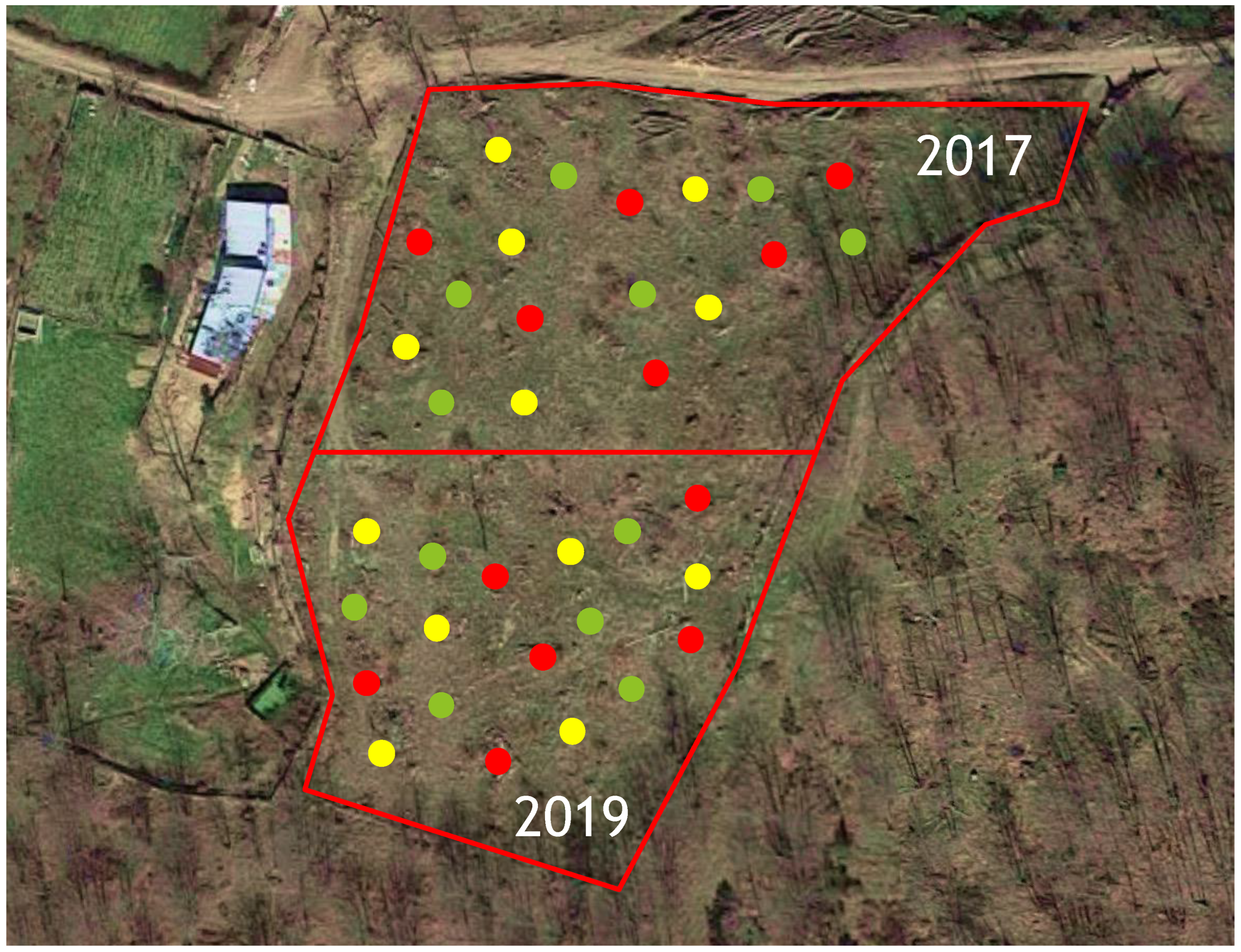
2.2. Study Site and Experimental Design
2.3. Stem Injection of K-Phosphonate
2.4. Stem Inoculation of Phytophthora
2.5. Statistical Analyses
3. Results
3.1. Isolation and Identification of Phytophthora Species
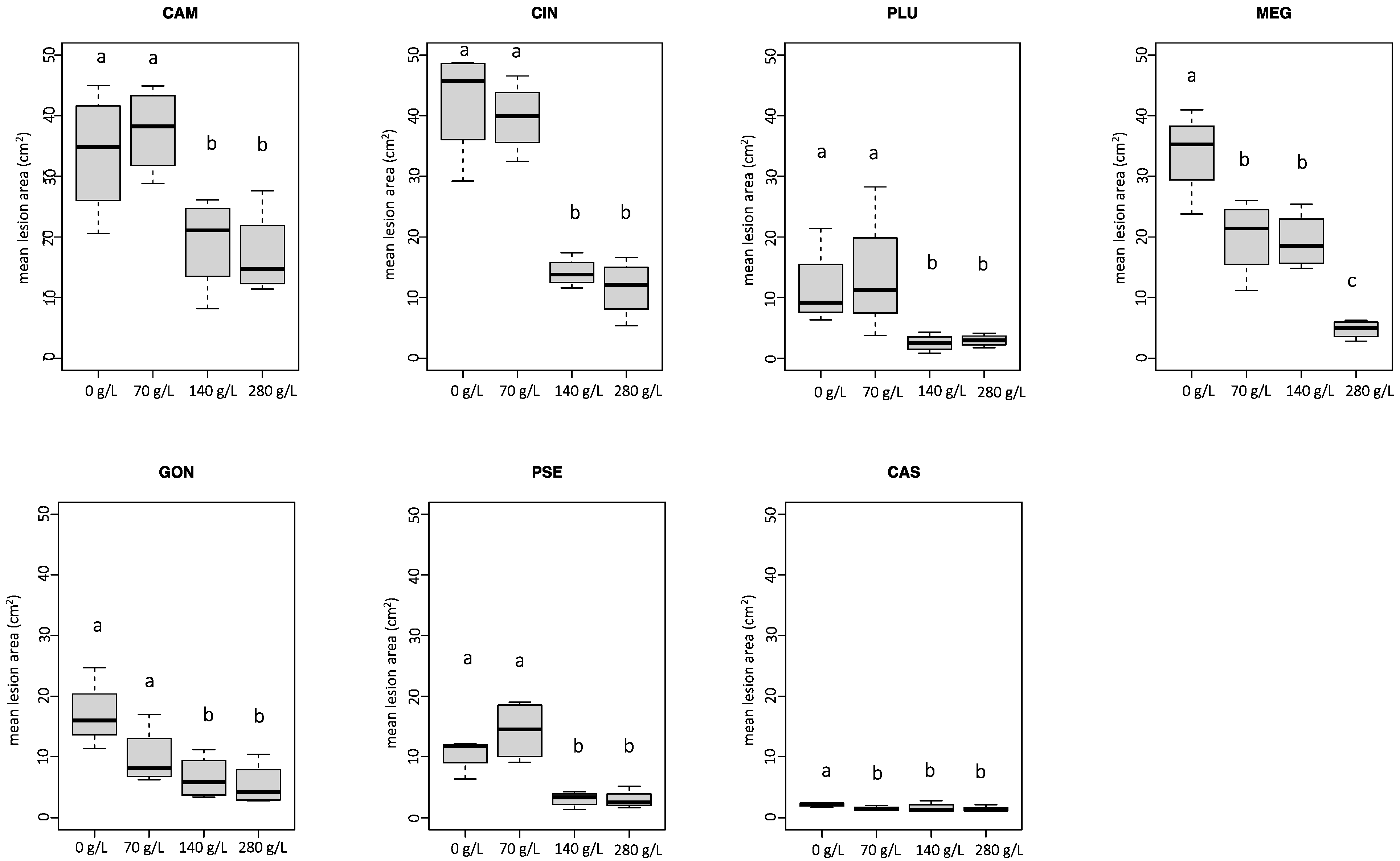
3.2. Experiment 1
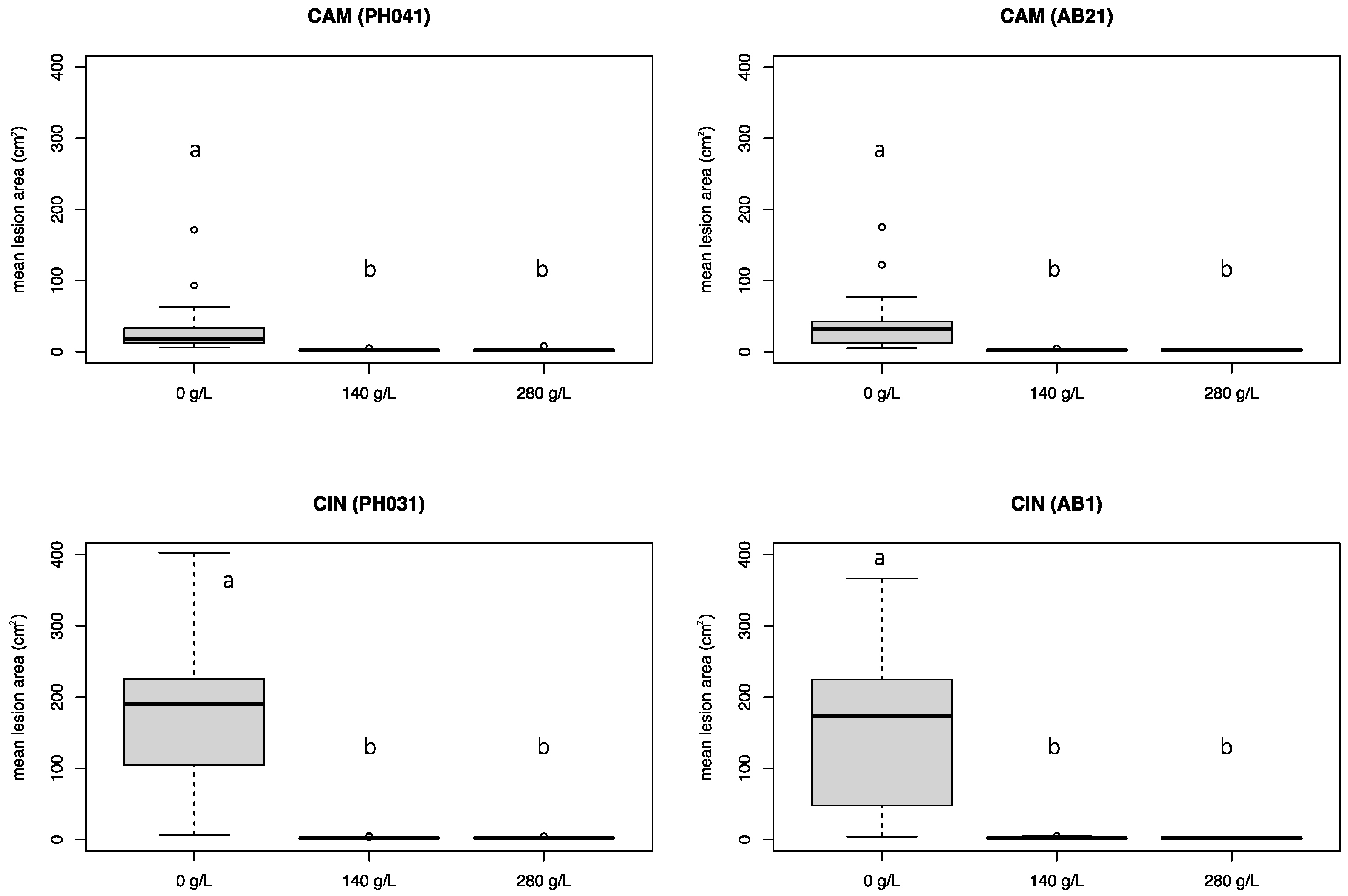
3.3. Experiment 2
3.4. Experiment 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea sativa in Europe: Distribution, habitat, usage and threats. Eur. Atlas For. Tree Species 2016, 78–79. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Costa, R.; Ramos-Cabrer, A.M.; Ribeiro, C.; Serra da Silva, C.; Manzano, G.; Barreneche, T. Variation in grafted European chestnut and hybrids by microsatellites reveals two main origins in the Iberian Peninsula. Tree Genet. Genomes 2010, 6, 701–715. [Google Scholar] [CrossRef]
- Pezzi, G.; Maresi, G.; Conedera, M.; Ferrari, C. Woody species composition of chestnut stands in the Northern Apennines: The result of 200 years of changes in land use. Landsc. Ecol. 2011, 26, 1463–1476. [Google Scholar] [CrossRef]
- Roces-Díaz, J.V.; Vayreda, J.; Banqué-Casanovas, M.; Cusó, M.; Anton, M.; Bonet, J.A.; Brotons, L.; De Cáceres, M.; Herrando, S.; de Aragón, J.M.; et al. Assessing the distribution of forest ecosystem services in a highly populated Mediterranean region. Ecol. Indic. 2018, 93, 986–997. [Google Scholar] [CrossRef]
- Prospero, S.; Forster, B. Chestnut gall wasp (Dryocosmus kuriphilus) infestations: New opportunities for the chestnut blight fungus Cryphonectria parasitica. New Dis. Rep. 2011, 23, 2044-0588. [Google Scholar] [CrossRef]
- Visentin, I.; Gentile, S.; Valentino, D.; Gonthier, P.; Tamietti, G.; Cardinale, F. Gnomoniopsis castanea sp. nov. (Gnomoniaceae, Diaporthales) as a causal agent of nut rot in sweet chestnut. J. Plant Pathol. 2012, 94, 411–419. [Google Scholar] [CrossRef]
- Krebs, P.; Tinner, W.; Conedera, M. Del castagno e della castanicoltura nelle contrade insubriche: Tentativo di una sintesi eco-storica. Arch. Stor. Ticin. 2014, 4–37. [Google Scholar] [CrossRef]
- Seijo, F.; Millington, J.D.; Gray, R.; Sanz, V.; Lozano, J.; García-Serrano, F.; Sangüesa-Barreda, G.; Camarero, J.J. Forgetting fire: Traditional fire knowledge in two chestnut forest ecosystems of the Iberian Peninsula and its implications for European fire management policy. Land Use Policy 2015, 47, 130–144. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil-and airborne Phytophthora species in forests and woodlands. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.M.; Natili, G.; Anselmi, N.; Vannini, A. Recovery and pathogenicity of Phytophthora species associated with a resurgence of ink disease in Castanea sativa in Italy. Plant Pathol. 2001, 50, 90–96. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with Ink Disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Bryner, S.F.; Rigling, D.; Brunner, P.C. Invasion history and demographic pattern of Cryphonectria hypovirus 1 across European populations of the chestnut blight fungus. Ecol. Evol. 2012, 2, 3227–3241. [Google Scholar] [CrossRef]
- Fonseca, T.F.; Abreu, C.G.; Parresol, B.R. Soil compaction and chestnut ink disease. For. Pathol. 2004, 34, 273–283. [Google Scholar] [CrossRef]
- Martins, L.; Castro, J.; Macedo, W.; Marques, C.; Abreu, C. Assessment of the spread of chestnut ink disease using remote sensing and geostatistical methods. Eur. J. Plant Pathol. 2007, 119, 159–164. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.M. Ink disease in chestnuts: Impact on the European chestnut. For. Snow Landsc. Res. 2001, 76, 345–350. [Google Scholar]
- Vannini, A.; Natili, G.; Thomidis, T.; Belli, C.; Morales-Rodriguez, C. Anthropogenic and landscape features are associated with ink disease impact in Central Italy. For. Pathol. 2021, 51, 1–8. [Google Scholar] [CrossRef]
- Černý, K.; Gregorová, B.; Strnadová, V.; Tomšovský, M.; Holub, V.; Gabrielová, Š. Phytophthora cambivora causing ink disease of sweet chestnut recorded in the Czech Republic. Czech Mycol. 2008, 60, 265–274. [Google Scholar] [CrossRef]
- Perlerou, C.; Tziros, G.; Vettraino, A.M.; Diamandis, S. Phytophthora cryptogea causing ink disease of Castanea sativa newly reported in Greece. Plant Pathol. 2010, 59, 799. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Franceschini, A. First report of Phytophthora pseudosyringae associated with ink disease of Castanea sativa in Italy. Plant Dis. 2010, 94, 1068. [Google Scholar] [CrossRef]
- Jung, T.; Jung, M.H.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef]
- Garbelotto, M.; Schmidt, D. Phosphonate controls sudden oak death pathogen for up to 2 years. Calif. Agric. 2009, 63, 10–17. [Google Scholar] [CrossRef]
- González, M.; Romero, M.Á.; Serrano, M.S.; Sánchez, M.E. Fosetyl-aluminium injection controls root rot disease affecting Quercus suber in southern Spain. Eur. J. Plant Pathol. 2020, 156, 101–109. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Vettraino, A.M.; Vannini, A. Efficacy of biofumigation with Brassica carinata commercial pellets (BioFence) to control vegetative and reproductive structures of Phytophthora cinnamomi. Plant Dis. 2016, 100, 324–330. [Google Scholar] [CrossRef]
- Shearer, B.L.; Tippett, J.T. Jarrah Dieback: The dynamics and management of Phytophthora cinnamomi in the Jarrah (Eucalyptus marginata) forest of South-Western Australia. Research Bulletin n°3. 1989. Department of Conservation and Land management, Western Australia. Available online: https://scholar.google.com/scholar_lookup?title=Jarrah+Dieback:+The+Dynamics+and+Management+of+Phytophthora+cinnamomi+in+the+Jarrah+(Eucalyptus+marginata)+Forest+of+South-Western+Australia&author=Shearer,+B.L.&author=Tippett,+J.T.&publication_year=1989 (accessed on 1 December 2022).
- Fernandez-Escobar, R.; Gallego, F.J.; Benlloch, M.; Membrillo, J.; Infante, J.; Perez de Algaba, A. Treatment of oak decline using pressurized injection capsules of antifungal materials. Eur. J. Plant Pathol. 1999, 29, 29–38. [Google Scholar] [CrossRef]
- Pilbeam, R.A.; Colquhoun, I.J.; Shearer, B.; Hardy, G.S.J. Phosphite concentration: Its effect on phytotoxicity symptoms and colonisation by Phytophthora cinnamomi in three understorey species of Eucalyptus marginata forest. Australas. Plant Pathol. 2000, 29, 86–95. [Google Scholar] [CrossRef]
- Hardy, G.E.S.; Barrett, S.; Shearer, B.L. The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australas. Plant Pathol. 2001, 30, 133–139. [Google Scholar] [CrossRef]
- Tynan, K.M.; Wilkinson, C.J.; Holmes, J.M.; Dell, B.; Colquhoun, I.J.; McComb, J.A.; Hardy, G.S.J. The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Aust. J. Bot. 2001, 49, 761–770. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, A.F.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Shearer, B.L.; Fairman, R.G. A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australas. Plant Pathol. 2007, 36, 78–86. [Google Scholar] [CrossRef]
- Barrett, S.; Rathbone, D. Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecol. 2018, 43, 360–374. [Google Scholar] [CrossRef]
- Guest, D.; Grant, B. The complex action of phosphonates as antifungal agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Smillie, R.; Grant, B.; Guest, D. The Mode of Action of Phosphite: Evidence for both direct and indirect modes of action on 3 Phytophthora spp. in plants. Phytopath. 1989, 79, 921–926. [Google Scholar] [CrossRef]
- Wilkinson, C.J.; Holmes, J.M.; Dell, B.; Tynan, K.M.; McComb, J.A.; Shearer, B.L.; Colquhoun, I.J.; Hardy, G.E.S. Effect of phosphite on in planta zoospore production of Phytophthora cinnamomi. Plant Pathol. 2001, 50, 587–593. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Fleischmann, F.; Humez, M.; Osswald, W. Phosphite Protects Fagus Sylvatica Seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 2014, 9, e87860. [Google Scholar] [CrossRef]
- Kasuga, T.; Hayden, K.J.; Eyre, C.A.; Croucher, P.J.; Schechter, S.; Wright, J.W.; Garbelotto, M. Innate resistance and phosphite treatment affect both the pathogen’s and host’s transcriptomes in the tanoak-Phytophthora ramorum pathosystem. J. Fungi 2021, 7, 198. [Google Scholar] [CrossRef]
- Solla, A.; Moreno, G.; Malewski, T.; Jung, T.; Klisz, M.; Tkaczyk, M.; Siebyla, M.; Pérez, A.; Cubera, E.; Hrynyk, H.; et al. Phosphite spray for the control of oak decline induced by Phytophthora in Europe. For. Ecol. Manag. 2021, 485, 118938. [Google Scholar] [CrossRef]
- Whiley, A.W.; Hargreaves, P.A.; Pegg, K.G.; Doogan, V.J.; Ruddle, L.J.; Saranah, J.B.; Langdon, P.W. Changing sink strengths influence translocation of phosphonate in avocado (Persea americana Mill.) trees. Aust. J. Agric. Res. 1995, 46, 1079–1090. [Google Scholar] [CrossRef]
- Nyoni, M.; Lotze, E.; Mazzola, M.; Wessels, J.P.B.; McLeod, A. Evaluating different approaches in the application of phosphonates for the control of apple root diseases. Austr. Plant Pathol. 2019, 48, 461–472. [Google Scholar] [CrossRef]
- Achary, V.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.; Agrawal, P. Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotech. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef]
- Lim, T.M. Trunk injection with phosphorous acid for controlling Phytophthora on chestnuts: Early promising results. In Proceedings of the 9th Biennial Conference. Australasian Plant Pathology Society, Hobart, Australia, 4–8 July 1993; p. 25. [Google Scholar]
- Gentile, S.; Valentino, D.; Tamietti, G. Control of ink disease by trunk injection of potassium phosphite. J. Plant Pathol. 2009, 91, 565–571. [Google Scholar]
- Dal Maso, E.; Cocking, J.; Montecchio, L. An enhanced trunk injection formulation of potassium phosphite against chestnut ink disease. Arboric. J. 2017, 39, 125–141. [Google Scholar] [CrossRef]
- Brasier, C.M.; Beales, P.A.; Kirk, S.A.; Denman, S.; Rose, J. Phytophthora kernoviae sp. nov., an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in the UK. Mycol. Res. 2005, 109, 853–859. [Google Scholar] [CrossRef]
- Brasier, C.M. Physiology of Reproduction in Phytophthora. PhD. Thesis, University of Hull, Hull, UK, 1967; p. 220. [Google Scholar]
- Scanu, B.; Webber, J.F. Dieback and mortality of Nothofagus in Britain: Ecology, pathogenicity and sporulation potential of the causal agent Phytophthora pseudosyringae. Plant Pathol. 2016, 65, 26–36. [Google Scholar] [CrossRef]
- Scanu, B.; Hunter, G.C.; Linaldeddu, B.T.; Franceschini, A.; Maddau, L.; Jung, T.; Denman, S. A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. For. Pathol. 2014, 44, 1–20. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-Plus; Springer: New York, NY, USA, 2000; pp. 3–56. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Dann, E.; McLeod, A. Phosphonic acid: A long-standing and versatile crop protectant. Pest Manag. Sci. 2020, 77, 2197–2208. [Google Scholar] [CrossRef]
- Shearer, B.L.; Fairman, R.G.; Grant, M.J. Effective concentration of phosphite in controlling Phytophthora cinnamomi following stem injection of Banksia species and Eucalyptus marginata. For. Pathol. 2006, 36, 119–135. [Google Scholar] [CrossRef]
- Scott, P.; Bader, M.K.F.; Williams, N.M. Foliar phosphite application has minor phytotoxic impacts across a diverse range of conifers and woody angiosperms. Physiol. Plant. 2016, 158, 124–134. [Google Scholar] [CrossRef]
- Horner, I.J.; Hough, E.G.; Horner, M.B. Forest efficacy trials on phosphite for control of kauri dieback. N. Z. Plant Prot. 2015, 68, 7–12. [Google Scholar] [CrossRef]
- Guest, D.I.; Pegg, K.C.; Whiley, A.-W. Control of Phytophthora diseases of trees crops using trunk-injected phosphonates. Horticult. Rev. 1995, 17, 299–330. [Google Scholar]
- Pegg, K.G. Trunk injection methodology. Australas. Plant Pathol. 1990, 19, 142–143. [Google Scholar]
- Tattar, T.; Tattar, S. Evidence for the downward movement of materials injected into trees. Arboric. Urban For. 1999, 25, 325–332. [Google Scholar] [CrossRef]
- Manghi, M.C.; Masiol, M.; Calzavara, R.; Graziano, P.L.; Peruzzi, E.; Pavoni, B. The use of phosphonates in agriculture. Chemical, biological properties and legislative issues. Chemosphere 2021, 283, 131187. [Google Scholar] [CrossRef]
- Bastianelli, G.; Morales-Rodríguez, C.; Caccia, R.; Turco, S.; Rossini, L.; Mazzaglia, A.; Thomidis, T.; Vannini, A. Use of phosphonate salts to control chestnut ‘Brown Rot’ by Gnomoniopsis castaneae in fruit orchards of Castanea sativa. Agronomy 2022, 12, 2434. [Google Scholar] [CrossRef]
- Dann, E.K.; Le, D.P. Effects of silicon amendment on soilborne and fruit diseases of avocado. Plants 2017, 6, 51. [Google Scholar] [CrossRef]
- Hunter, S.; Williams, N.; McDougal, R.; Scott, P.; Garbelotto, M. Evidence for rapid adaptive evolution of tolerance to chemical treatments in Phytophthora species and its practical implications. PLoS ONE 2018, 13, 15. [Google Scholar] [CrossRef]
- Khdiar, M.Y.; Burgess, T.I.; Barber, P.A.; Hardy, G.E.S. Calcium chelate is as effective as phosphite in controlling Phytophthora root rot in glasshouse trials. Plant Pathol. 2022, 72, 112–119. [Google Scholar] [CrossRef]
- Bugatti, V.; Vertuccio, L.; Zara, S.; Fancello, F.; Scanu, B.; Gorrasi, G. Green pesticides based on cinnamate anion incorporated in layered double hydroxides and dispersed in pectin matrix. Carbohydr. Polym. 2019, 209, 356–362. [Google Scholar] [CrossRef]
- Evidente, A.; Maddau, L.; Scanu, B.; Andolfi, A.; Masi, M.; Motta, A.; Tuzi, A. Sphaeropsidones, phytotoxic dimedone methyl ethers produced by Diplodia cupressi: A structure−activity relationship study. J. Nat. Prod. 2011, 74, 757–763. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Ritika, B.; Utpal, D. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr. J. Microbiol. Res. 2014, 8, 1749–1762. [Google Scholar] [CrossRef]
- Moon, J.H.; Won, S.J.; Maung, C.E.H.; Choi, J.H.; Choi, S.I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef]
- IPCC Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Available online: https://www.ipcc.ch/sr15/ (accessed on 1 December 2022).
- Brasier, C.M.; Scott, J.K. European oak declines and global warming: A theoretical assessment with special reference to the activity of Phytophthora cinnamomi. Bulletin OEPP/EPPO Bull. 1994, 24, 221–234. [Google Scholar] [CrossRef]
- Dorado, F.J.; Alías, J.C.; Chaves, N.; Solla, A. Warming Scenarios and Phytophthora cinnamomi Infection in Chestnut (Castanea sativa Mill.). Plants 2023, 12, 556. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of phytophthora species from declining mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef]
- Dobrowolski, M.P.; Shearer, B.L.; Colquhoun, I.J.; O’Brien, P.A.; Hardy, G.E.S. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Franceschini, S.; Natili, G.; Paganini, R.; Alicicco, D.; Vuono, G.; Vannini, A. Integrated Control Protocol (ICP) of ink disease of chestnut in central Italy: Principles and future perspectives. Acta Hortic. 2010, 866, 425–430. [Google Scholar] [CrossRef]
- Vannini, A.; Morales-Rodríguez, C. Integrated disease management in tree nut cultivation. In Achieving Sustainable Cultivation of Tree Nuts; Serdar, ü., Fulbright, D., Eds.; Burleigh Dodds Science Pub: Cambridge, UK, 2019; p. 530. [Google Scholar]
- Burgess, T.I.; Scott, J.K.; Mcdougall, K.L.; Stukely, M.J.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Chang. Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef]




| Phytophthora sp. | Isolate Codes | Year | Host | Substrate | GenBank Accessions | Experiment |
|---|---|---|---|---|---|---|
| P. castanetorum | P14 | 2014 | Castanea sativa | Rhizosphere soil | MF036189 | 1 |
| P. cinnamomi | PH031 | 2008 | Castanea sativa | Root | OP918117 | 1, 2, 3 |
| P. cinnamomi | AB1 | 2016 | Quercus suber | Rhizosphere soil | OP918118 | 2, 3 |
| P. gonapodyides | PH038 | 2009 | Castanea sativa | Rhizosphere soil | OQ176729 | 1 |
| P. megasperma | PH178 | 2010 | Castanea sativa | Rhizosphere soil | KP863491 | 1 |
| P. plurivora | PH089 | 2010 | Castanea sativa | Rhizosphere soil | OP918116 | 1 |
| P. pseudosyringae | PH043 | 2009 | Castanea sativa | Root | OP918115 | 1 |
| P. ×cambivora | PH041 | 2009 | Castanea sativa | Collar | OP918113 | 1, 2, 3 |
| P. ×cambivora | AB21 | 2016 | Quercus suber | Rhizosphere soil | OP918114 | 2, 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandano, A.; Serra, S.; Hardy, G.E.S.J.; Scanu, B. Potassium Phosphonate Induces Resistance in Sweet Chestnut against Ink Disease Caused by Phytophthora Species. Pathogens 2023, 12, 365. https://doi.org/10.3390/pathogens12030365
Brandano A, Serra S, Hardy GESJ, Scanu B. Potassium Phosphonate Induces Resistance in Sweet Chestnut against Ink Disease Caused by Phytophthora Species. Pathogens. 2023; 12(3):365. https://doi.org/10.3390/pathogens12030365
Chicago/Turabian StyleBrandano, Andrea, Salvatorica Serra, Giles E. St. J. Hardy, and Bruno Scanu. 2023. "Potassium Phosphonate Induces Resistance in Sweet Chestnut against Ink Disease Caused by Phytophthora Species" Pathogens 12, no. 3: 365. https://doi.org/10.3390/pathogens12030365
APA StyleBrandano, A., Serra, S., Hardy, G. E. S. J., & Scanu, B. (2023). Potassium Phosphonate Induces Resistance in Sweet Chestnut against Ink Disease Caused by Phytophthora Species. Pathogens, 12(3), 365. https://doi.org/10.3390/pathogens12030365







