Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease
Abstract
1. Introduction
2. Results
2.1. Literature Review
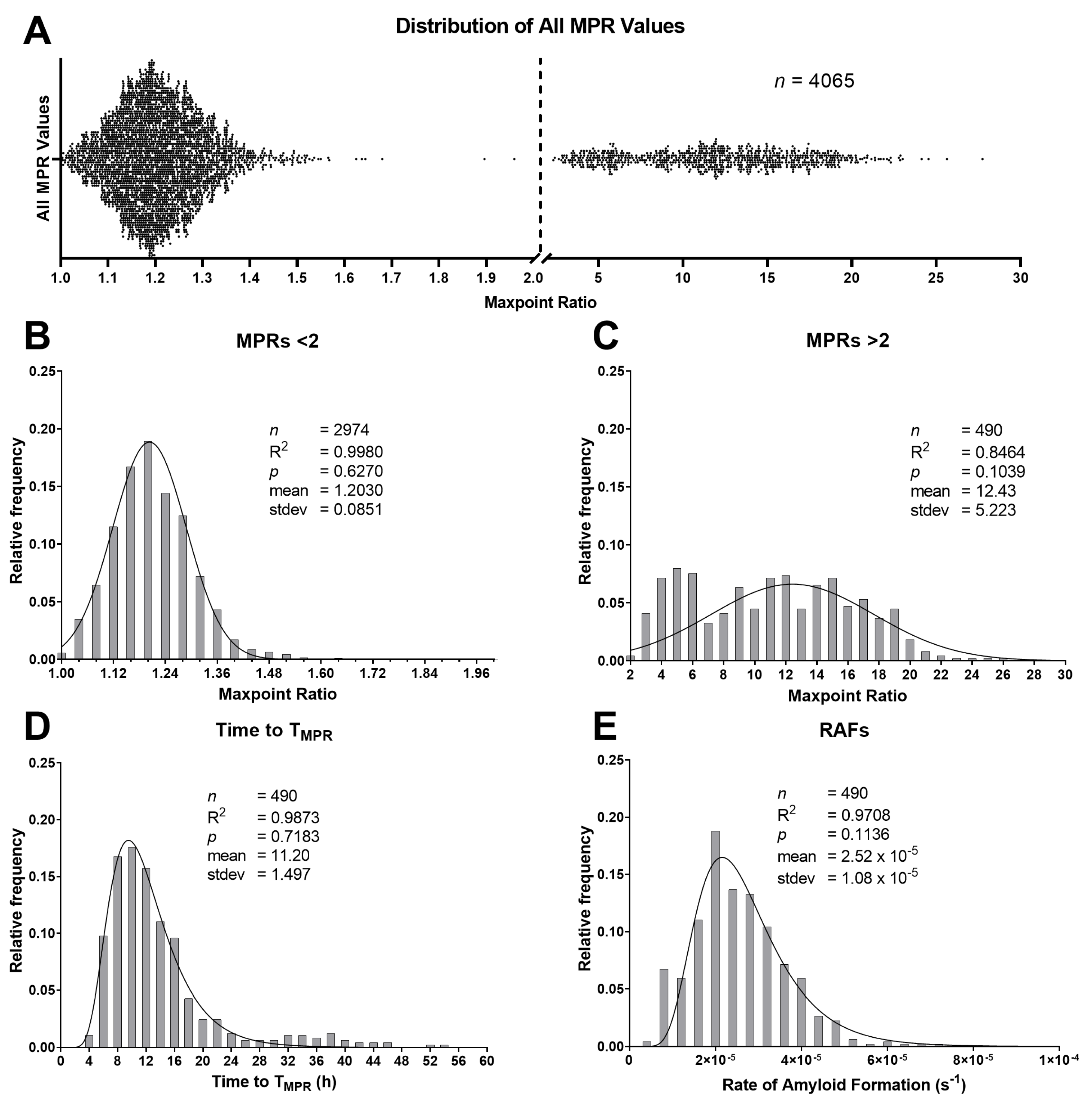
2.2. Maxpoint Ratio, Rate of Amyloid Formation, and Time-to-Threshold Distributions
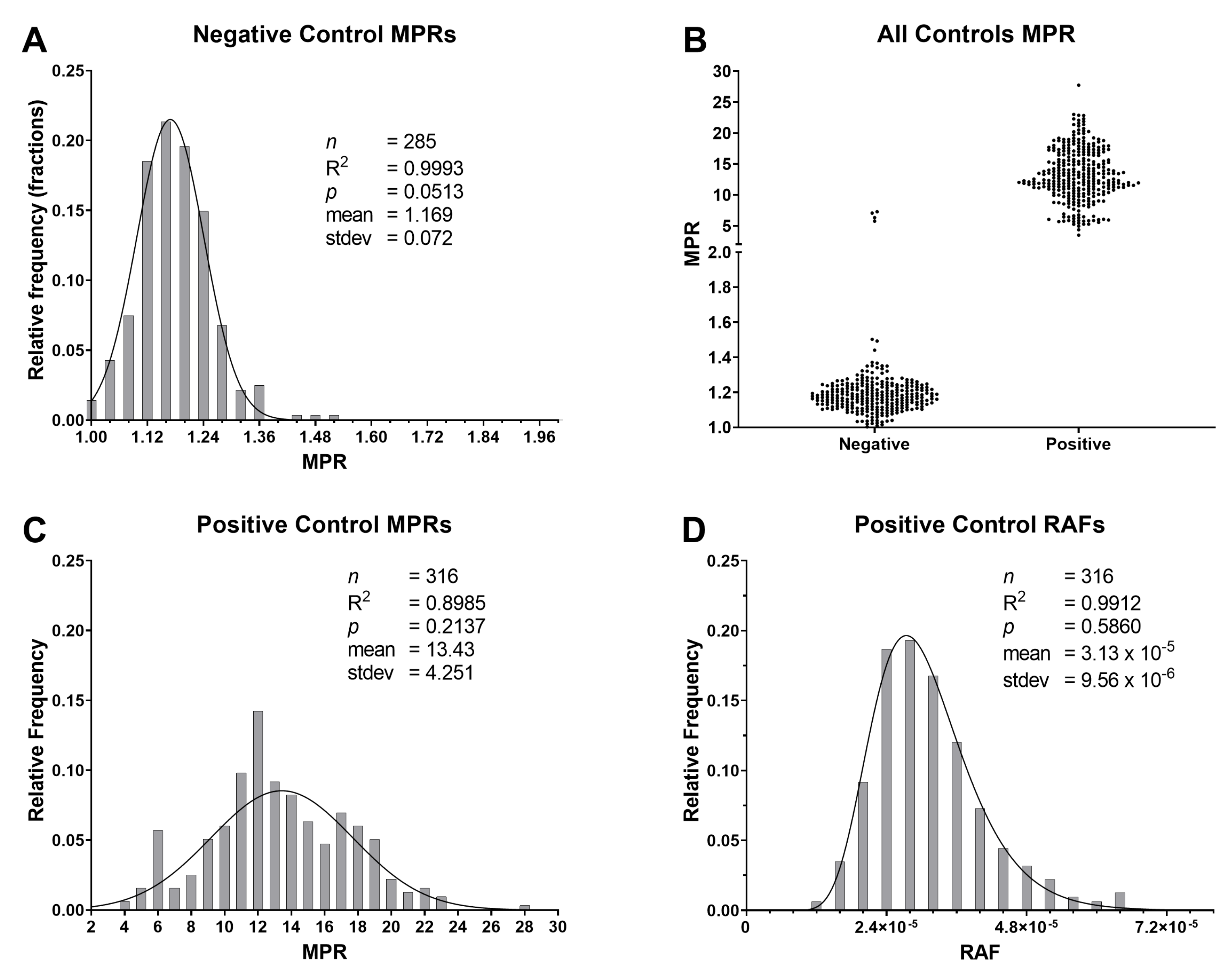
2.3. T and Rate of Amyloid Formation Determinations
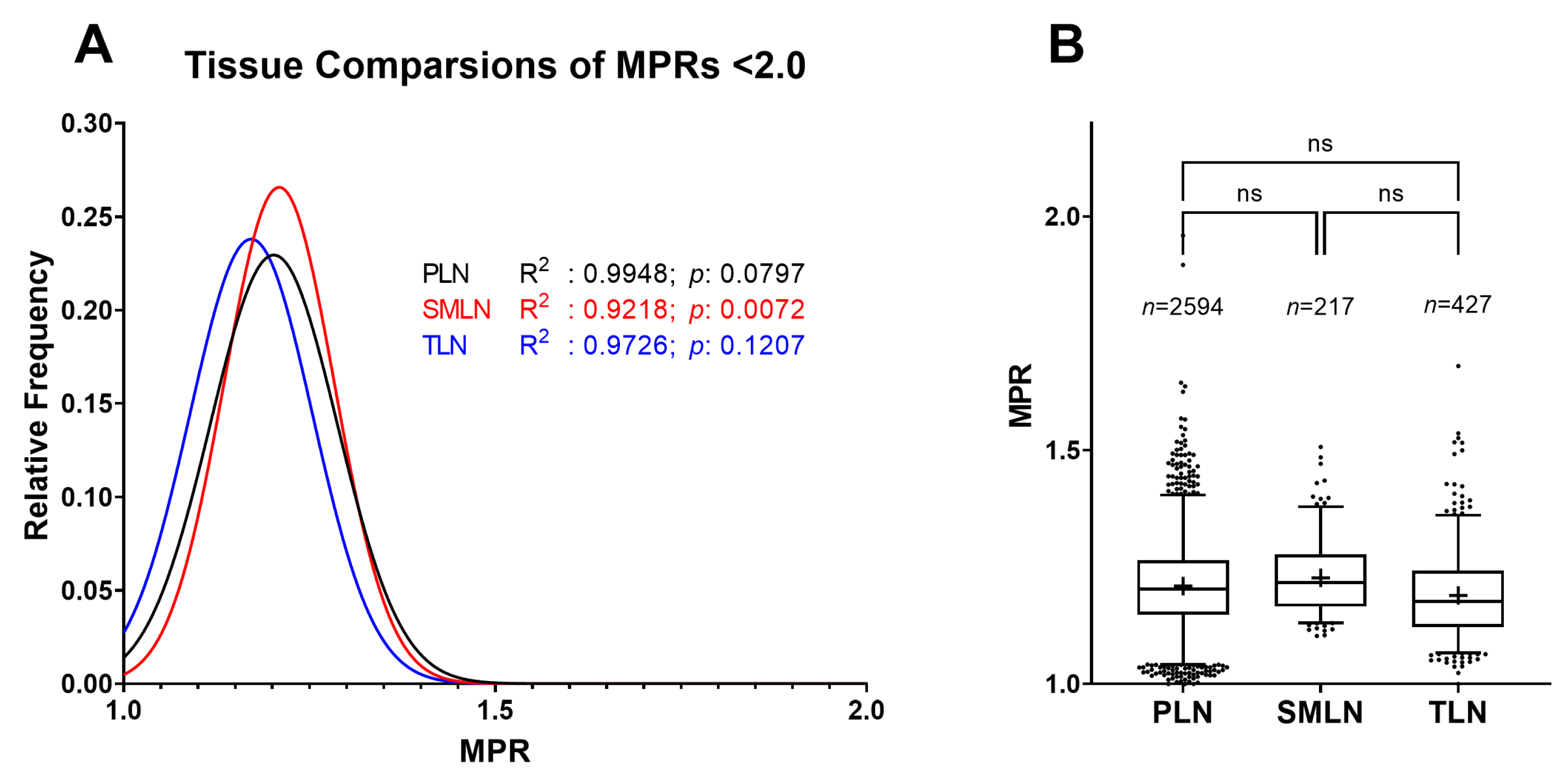
2.4. Distribution Differences between Lymph Nodes
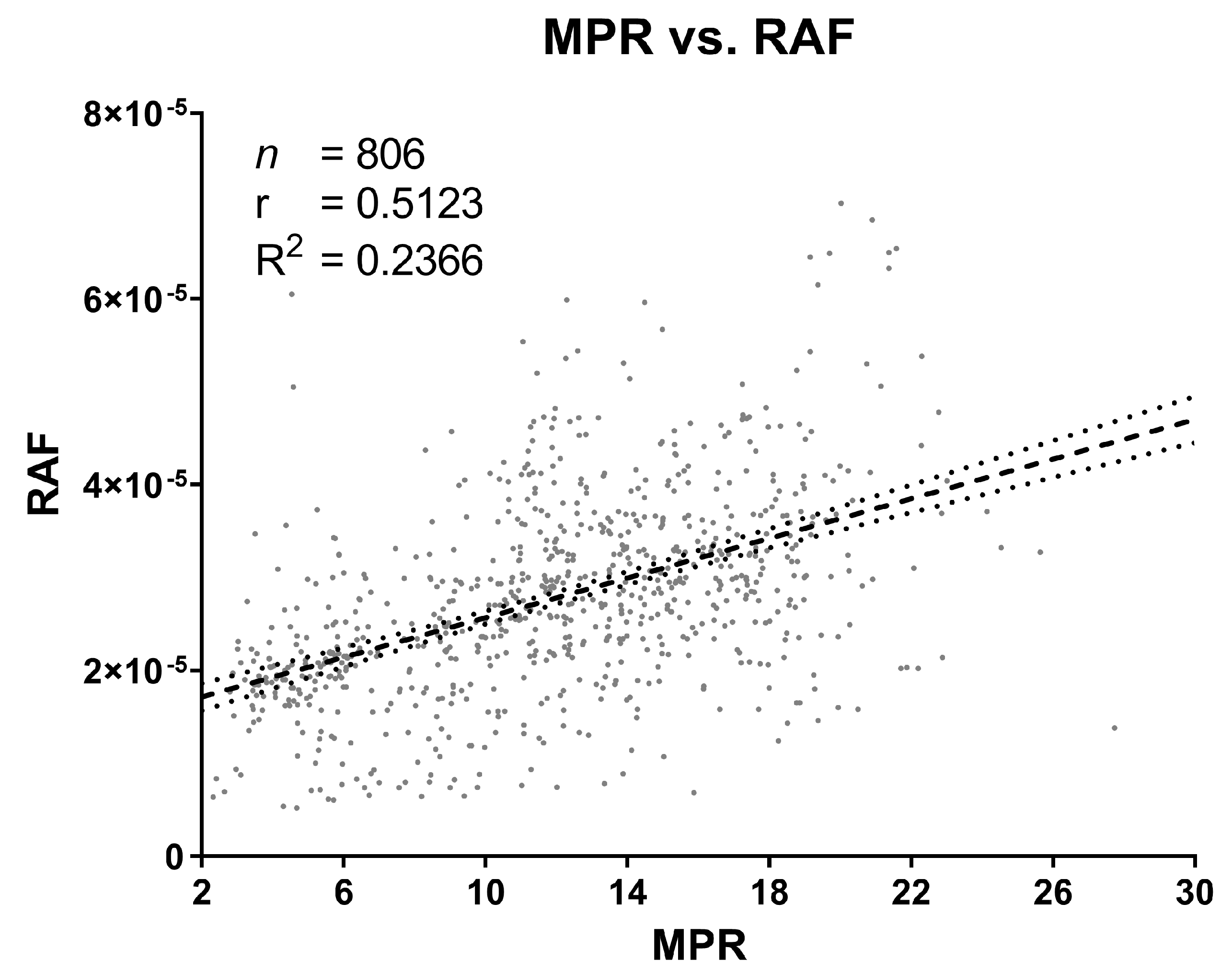
2.5. Correlation between MPR and RAF
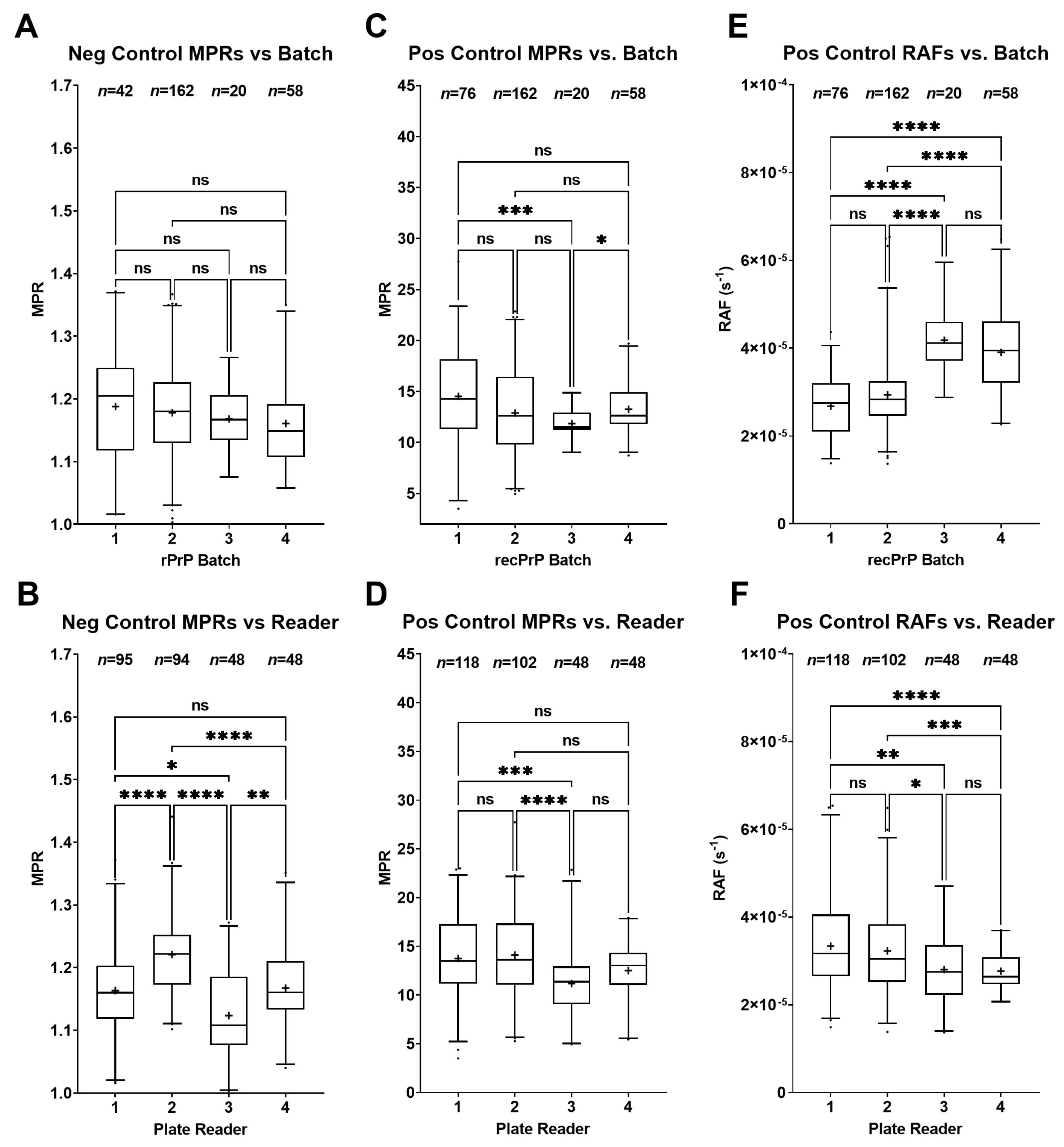
2.6. Machine and Batch Effects on MPR and RAF
2.7. Comparison of RT-QuIC to ELISA
3. Discussion
4. Materials and Methods
4.1. Literature Review
4.2. Source Population and Sample Processing
4.3. RT-QuIC Methods and recPrP Preparation
4.4. Maxpoint Ratio and Rate of Amyloid Formation Calculations
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwabenlander, M.D.; Rowden, G.R.; Li, M.; LaSharr, K.; Hildebrand, E.C.; Stone, S.; Seelig, D.M.; Jennelle, C.S.; Cornicelli, L.; Wolf, T.M.; et al. Comparison of Chronic Wasting Disease Detection Methods and Procedures: Implications for Free-Ranging White-Tailed Deer (Odocoileus virginianus) Surveillance and Management. J. Wildl. Dis. 2021, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, R.; Sano, K.; Satoh, K.; Nishida, N. Real-time quaking-induced conversion. Prion 2011, 5, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Orrù, C.D.; Groveman, B.R.; Hughson, A.G.; Manca, M.; Raymond, L.D.; Raymond, G.J.; Campbell, K.J.; Anson, K.J.; Kraus, A.; Caughey, B. RT-QuIC assays for prion disease detection and diagnostics. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1658, pp. 185–203. [Google Scholar]
- Orrú, C.D.; Groveman, B.R.; Hughson, A.G.; Zanusso, G.; Coulthart, M.B.; Caughey, B. Rapid and sensitive RT-QuIC detection of human creutzfeldt-jakob disease using cerebrospinal fluid. MBio 2015, 6, e02451-14. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.P.; Orrú, C.D.; Hughson, A.G.; Caughey, B.; Graça, T.; Zhuang, D.; Madsen-Bouterse, S.A.; Knowles, D.P.; Schneider, D.A. Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion. J. Gen. Virol. 2016, 97, 803–812. [Google Scholar] [CrossRef]
- Hwang, S.; West Greenlee, M.H.; Balkema-Buschmann, A.; Groschup, M.H.; Nicholson, E.M.; Greenlee, J.J. Real-time quaking-induced conversion detection of bovine spongiform encephalopathy prions in a subclinical steer. Front. Vet. Sci. 2018, 4, 19. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef]
- Metrick, M.A., 2nd; Ferreira, N.d.C.; Saijo, E.; Kraus, A.; Newell, K.; Zanusso, G.; Vendruscolo, M.; Ghetti, B.; Caughey, B. A single ultrasensitive assay for detection and discrimination of tau aggregates of Alzheimer and Pick diseases. Acta Neuropathol. Commun. 2020, 8, 22. [Google Scholar] [CrossRef]
- Prusiner, S.B.; Groth, D.F.; Bolton, D.C.; Kent, S.B.; Hood, L.E. Purification and structural studies of a major scrapie prion protein. Cell 1984, 38, 127–134. [Google Scholar] [CrossRef]
- Prusiner, S.B. Prions. In National Academy of Sciences of the United States of America; National Academy of Sciences: Washington, DC, USA, 1998; Volume 95, pp. 13363–13383. [Google Scholar]
- Williams, E.S.; Young, S. Chronic wasting disease of captive mule deer: A spongiform encephalopathy. J. Wildl. Dis. 1980, 16, 89–98. [Google Scholar] [CrossRef]
- Mysterud, A.; Edmunds, D.R. A review of chronic wasting disease in North America with implications for Europe. Eur. J. Wildl. Res. 2019, 65, 1–13. [Google Scholar] [CrossRef]
- Sohn, H.J.; Kim, J.H.; Choi, K.S.; Nah, J.J.; Joo, Y.S.; Jean, Y.H.; Ahn, S.W.; Kim, O.K.; Kim, D.Y.; Balachandran, A. A case of chronic wasting disease in an elk imported to Korea from Canada. J. Vet. Med. Sci. 2002, 64, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Picasso-Risso, C.; Schwabenlander, M.D.; Rowden, G.; Carstensen, M.; Bartz, J.C.; Larsen, P.A.; Wolf, T.M. Assessment of Real-Time Quaking-Induced Conversion (RT-QuIC) Assay, Immunohistochemistry and ELISA for Detection of Chronic Wasting Disease under Field Conditions in White-Tailed Deer: A Bayesian Approach. Pathogens 2022, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Holz, C.L.; Darish, J.R.; Straka, K.; Grosjean, N.; Bolin, S.; Kiupel, M.; Sreevatsan, S. Evaluation of Real-Time Quaking-Induced Conversion, ELISA, and Immunohistochemistry for Chronic Wasting Disease Diagnosis. Front. Vet. Sci. 2021, 8, 824815. [Google Scholar] [CrossRef] [PubMed]
- Bongianni, M.; Orrù, C.; Groveman, B.R.; Sacchetto, L.; Fiorini, M.; Tonoli, G.; Triva, G.; Capaldi, S.; Testi, S.; Ferrari, S.; et al. Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 2017, 74, 155–162. [Google Scholar] [CrossRef]
- Li, M.; Schwabenlander, M.D.; Rowden, G.R.; Schefers, J.M.; Jennelle, C.S.; Carstensen, M.; Seelig, D.; Larsen, P.A. RT-QuIC detection of CWD prion seeding activity in white-tailed deer muscle tissues. Sci. Rep. 2021, 11, 16759. [Google Scholar] [CrossRef]
- Haley, N.J.; Henderson, D.M.; Donner, R.; Wyckoff, S.; Merrett, K.; Tennant, J.; Hoover, E.A.; Love, D.; Kline, E.; Lehmkuhl, A.D.; et al. Management of chronic wasting disease in ranched elk: Conclusions from a longitudinal three-year study. Prion 2020, 14, 76–87. [Google Scholar] [CrossRef]
- McNulty, E.; Nalls, A.V.; Mellentine, S.; Hughes, E.; Pulscher, L.; Hoover, E.A.; Mathiason, C.K. Comparison of conventional, amplification and bio-assay detection methods for a chronic wasting disease inoculum pool. PLoS ONE 2019, 14, e0216621. [Google Scholar] [CrossRef]
- Favole, A.; Mazza, M.; Vallino Costassa, E.; D’Angelo, A.; Lombardi, G.; Marconi, P.; Crociara, P.; Berrone, E.; Gallo, M.; Palmitessa, C.; et al. Early and Pre-Clinical Detection of Prion Seeding Activity in Cerebrospinal Fluid of Goats using Real-Time Quaking-Induced Conversion Assay. Sci. Rep. 2019, 9, 6173. [Google Scholar] [CrossRef]
- Xiao, K.; Shi, Q.; Zhou, W.; Gao, C.; Chen, C.; Zhang, B.Y.; Wang, J.; Dong, X.P. Evaluation of the effect of various main elements on the PrP Sc detection by real-time quaking-induced conversion assay. Int. J. Mol. Med. 2018, 42, 3231–3237. [Google Scholar]
- Haley, N.J.; Henderson, D.M.; Wyckoff, S.; Tennant, J.; Hoover, E.A.; Love, D.; Kline, E.; Lehmkuhl, A.; Thomsen, B. Chronic wasting disease management in ranched elk using rectal biopsy testing. Prion 2018, 12, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Race, B.; Williams, K.; Orrú, C.D.; Hughson, A.G.; Lubke, L.; Chesebro, B. Lack of Transmission of Chronic Wasting Disease to Cynomolgus Macaques. J. Virol. 2018, 92, e00550-18. [Google Scholar] [CrossRef]
- Henderson, D.M.; Tennant, J.M.; Haley, N.J.; Denkers, N.D.; Mathiason, C.K.; Hoover, E.A. Detection of chronic wasting disease prion seeding activity in deer and elk feces by real-time quaking-induced conversion. J. Gen. Virol. 2017, 98, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Greenlee, J.J.; Nicholson, E.M. Use of bovine recombinant prion protein and real-time quaking-induced conversion to detect cattle transmissible mink encephalopathy prions and discriminate classical and atypical L- and H-Type bovine spongiform encephalopathy. PLoS ONE 2017, 12, e0172391. [Google Scholar] [CrossRef] [PubMed]
- Nalls, A.V.; McNulty, E.; Hoover, C.E.; Pulscher, L.A.; Hoover, E.A.; Mathiason, C.K. Infectious Prions in the Pregnancy Microenvironment of Chronic Wasting Disease-Infected Reeves’ Muntjac Deer. J. Virol. 2017, 91, e00501-17. [Google Scholar] [CrossRef] [PubMed]
- Haley, N.J.; Rielinger, R.; Davenport, K.A.; O’Rourke, K.; Mitchell, G.; Richt, J.A. Estimating chronic wasting disease susceptibility in cervids using real-time quaking-induced conversion. J. Gen. Virol. 2017, 98, 2882–2892. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Nichols, T.; Lehmkuhl, A.; Thomsen, B.; Main, R.; Halbur, P.; Dutta, S.; Kanthasamy, A.G. Ante-mortem detection of chronic wasting disease in recto-anal mucosa-associated lymphoid tissues from elk (Cervus elaphus nelsoni) using real-time quaking-induced conversion (RT-QuIC) assay: A blinded collaborative study. Prion 2017, 11, 415–430. [Google Scholar] [CrossRef]
- Haley, N.J.; Siepker, C.; Hoon-Hanks, L.L.; Mitchell, G.; Walter, W.D.; Manca, M.; Monello, R.J.; Powers, J.G.; Wild, M.A.; Hoover, E.A.; et al. Seeded amplification of chronic wasting disease prions in nasal brushings and recto-anal mucosa-associated lymphoid tissues from elk by real-time quaking-induced conversion. J. Clin. Microbiol. 2016, 54, 1117–1126. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Hannaoui, S.; John, T.R.; Dudas, S.; Czub, S.; Gilch, S. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS ONE 2016, 11, e0166187. [Google Scholar] [CrossRef]
- Hoover, C.E.; Davenport, K.A.; Henderson, D.M.; Pulscher, L.A.; Mathiason, C.K.; Zabel, M.D.; Hoover, E.A. Detection and quantification of CWD prions in fixed paraffin embedded tissues by real-time quaking-induced conversion. Sci. Rep. 2016, 6, 25098. [Google Scholar] [CrossRef]
- Henderson, D.M.; Davenport, K.A.; Haley, N.J.; Denkers, N.D.; Mathiason, C.K.; Hoover, E.A. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J. Gen. Virol. 2015, 96, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Bongianni, M.; Tonoli, G.; Ferrari, S.; Hughson, A.G.; Groveman, B.R.; Fiorini, M.; Pocchiari, M.; Monaco, S.; Caughey, B.; et al. A Test for Creutzfeldt–Jakob Disease Using Nasal Brushings. N. Engl. J. Med. 2014, 371, 519–529. [Google Scholar] [CrossRef]
- Haley, N.J.; Carver, S.; Hoon-Hanks, L.L.; Henderson, D.M.; Davenport, K.A.; Bunting, E.; Gray, S.; Trindle, B.; Galeota, J.; LeVan, I.; et al. Detection of chronic wasting disease in the lymph nodes of free-ranging cervids by real-time quaking-induced conversion. J. Clin. Microbiol. 2014, 52, 3237–3243. [Google Scholar] [CrossRef]
- Hwang, S.; Greenlee, J.J.; Nicholson, E.M. Real-Time Quaking-Induced Conversion Detection of PrPSc in Fecal Samples From Chronic Wasting Disease Infected White-Tailed Deer Using Bank Vole Substrate. Front. Vet. Sci. 2021, 8, 643754. [Google Scholar] [CrossRef] [PubMed]
- Hoover, C.E.; Davenport, K.A.; Henderson, D.M.; Denkers, N.D.; Mathiason, C.K.; Soto, C.; Zabel, M.D.; Hoover, E.A. Pathways of Prion Spread during Early Chronic Wasting Disease in Deer. J. Virol. 2017, 91, e00077-17. [Google Scholar] [CrossRef] [PubMed]
- Davenport, K.A.; Henderson, D.M.; Bian, J.; Telling, G.C.; Mathiason, C.K.; Hoover, E.A. Insights into Chronic Wasting Disease and Bovine Spongiform Encephalopathy Species Barriers by Use of Real-Time Conversion. J. Virol. 2015, 89, 9524–9531. [Google Scholar] [CrossRef]
- Orrú, C.D.; Yuan, J.; Appleby, B.S.; Li, B.; Li, Y.; Winner, D.; Wang, Z.; Zhan, Y.A.; Rodgers, M.; Rarick, J.; et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci. Transl. Med. 2017, 9, 7785. [Google Scholar] [CrossRef]
- Franceschini, A.; Baiardi, S.; Hughson, A.G.; McKenzie, N.; Moda, F.; Rossi, M.; Capellari, S.; Green, A.; Giaccone, G.; Caughey, B.; et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci. Rep. 2017, 7, 10655. [Google Scholar] [CrossRef]
- Fiorini, M.; Iselle, G.; Perra, D.; Bongianni, M.; Capaldi, S.; Sacchetto, L.; Ferrari, S.; Mombello, A.; Vascellari, S.; Testi, S.; et al. High diagnostic accuracy of RT-QuIC assay in a prospective study of patients with suspected sCJD. Int. J. Mol. Sci. 2020, 21, 880. [Google Scholar] [CrossRef]
- McGuire, L.I.; Peden, A.H.; Orrú, C.D.; Wilham, J.M.; Appleford, N.E.; Mallinson, G.; Andrews, M.; Head, M.W.; Caughey, B.; Will, R.G.; et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 2012, 72, 278–285. [Google Scholar] [CrossRef]
- Moško, T.; Galušková, S.; Matěj, R.; Brůžová, M.; Holada, K. Detection of prions in brain homogenates and csf samples using a second-generation rt-quic assay: A useful tool for retrospective analysis of archived samples. Pathogens 2021, 10, 750. [Google Scholar] [CrossRef]
- Mok, T.H.; Nihat, A.; Luk, C.; Sequeira, D.; Batchelor, M.; Mead, S.; Collinge, J.; Jackson, G.S. Bank vole prion protein extends the use of RT-QuIC assays to detect prions in a range of inherited prion diseases. Sci. Rep. 2021, 11, 5231. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Barbosa, B.J.A.P.; Castrillo, B.B.; Alvim, R.P.; de Brito, M.H.; Gomes, H.R.; Brucki, S.M.D.; Smid, J.; Nitrini, R.; Landemberger, M.C.; Martins, V.R.; et al. Second-Generation RT-QuIC Assay for the Diagnosis of Creutzfeldt-Jakob Disease Patients in Brazil. Front. Bioeng. Biotechnol. 2020, 8, 929. [Google Scholar] [CrossRef]
- Xiao, K.; Shi, Q.; Zhou, W.; Dong, X.P. Different post-mortem brain regions from three Chinese FFI patients induce different reactive profiles both in the first and second generation RT-QuIC assays. Prion 2020, 14, 163–169. [Google Scholar] [CrossRef]
- Cramm, M.; Schmitz, M.; Karch, A.; Mitrova, E.; Kuhn, F.; Schroeder, B.; Raeber, A.; Varges, D.; Kim, Y.S.; Satoh, K.; et al. Stability and Reproducibility Underscore Utility of RT-QuIC for Diagnosis of Creutzfeldt-Jakob Disease. Mol. Neurobiol. 2016, 53, 1896–1904. [Google Scholar] [CrossRef]
- Elder, A.M.; Henderson, D.M.; Nalls, A.V.; Hoover, E.A.; Kincaid, A.E.; Bartz, J.C.; Mathiason, C.K. Immediate and Ongoing Detection of Prions in the Blood of Hamsters and Deer following Oral, Nasal, or Blood Inoculations. J. Virol. 2015, 89, 7421–7424. [Google Scholar] [CrossRef]
- Han, J.Y.; Jang, H.S.; Green, A.J.E.; Choi, Y.P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy Body patients and of a transgenic mouse model of synucleinopathy. Prion 2020, 14, 88–94. [Google Scholar] [CrossRef]
- Sano, K.; Atarashi, R.; Satoh, K.; Ishibashi, D.; Nakagaki, T.; Iwasaki, Y.; Yoshida, M.; Murayama, S.; Mishima, K.; Nishida, N. Prion-Like Seeding of Misfolded α-Synuclein in the Brains of Dementia with Lewy Body Patients in RT-QUIC. Mol. Neurobiol. 2017, 55, 3916–3930. [Google Scholar] [CrossRef]
- Tennant, J.M.; Henderson, D.M.; Wisniewski, T.M.; Hoover, E.A. RT-QuIC detection of tauopathies using full-length tau substrates. Prion 2020, 14, 249–256. [Google Scholar] [CrossRef]
- McNulty, E.E.; Nalls, A.V.; Xun, R.; Denkers, N.D.; Hoover, E.A.; Mathiason, C.K. In vitro detection of haematogenous prions in white-tailed deer orally dosed with low concentrations of chronic wasting disease. J. Gen. Virol. 2020, 101, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Sawada, K.; Yamasaki, T.; Denkers, N.D.; Mathiason, C.K.; Hoover, E.A.; Horiuchi, M. Involvement of N- and C-terminal region of recombinant cervid prion protein in its reactivity to CWD and atypical BSE prions in real-time quaking-induced conversion reaction in the presence of high concentrations of tissue homogenates. Prion 2020, 14, 283–295. [Google Scholar] [CrossRef]
- Tennant, J.M.; Li, M.; Henderson, D.M.; Tyer, M.L.; Denkers, N.D.; Haley, N.J.; Mathiason, C.K.; Hoover, E.A. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS ONE 2020, 15, e0227094. [Google Scholar] [CrossRef]
- Hwang, S.; Greenlee, J.J.; Nicholson, E.M. Role of donor genotype in RT-QuIC seeding activity of chronic wasting disease prions using human and bank vole substrates. PLoS ONE 2020, 15, e0227487. [Google Scholar] [CrossRef] [PubMed]
- Haley, N.J.; Henderson, D.M.; Senior, K.; Miller, M.; Donner, R. Evaluation of Winter Ticks (Dermacentor albipictus) Collected from North American Elk (Cervus canadensis) in an Area of Chronic Wasting Disease Endemicity for Evidence of PrP CWD Amplification Using Real-Time Quaking-Induced Conversion Assay. mSphere 2021, 6, e00515-21. [Google Scholar] [CrossRef]
- Yuan, Q.; Rowden, G.; Wolf, T.M.; Schwabenlander, M.D.; Larsen, P.A.; Bartelt-Hunt, S.L.; Bartz, J.C. Sensitive detection of chronic wasting disease prions recovered from environmentally relevant surfaces. Environ. Int. 2022, 166, 107347. [Google Scholar] [CrossRef]
- Cheng, K.; Vendramelli, R.; Sloan, A.; Waitt, B.; Podhorodecki, L.; Godal, D.; Knox, J.D. Endpoint quaking-induced conversion: A sensitive, specific, and high-throughput method for antemortem diagnosis of creutzfeldt-jacob disease. J. Clin. Microbiol. 2016, 54, 1751–1754. [Google Scholar] [CrossRef]
- Vendramelli, R.; Sloan, A.; Simon, S.L.R.; Godal, D.; Cheng, K. ThermoMixer-aided endpoint quaking-induced conversion (EP-QuIC) permits faster sporadic creutzfeldt-jakob disease (sCJD) identification than real-time quaking-induced conversion (RT-QuIC). J. Clin. Microbiol. 2018, 56, e00423-18. [Google Scholar] [CrossRef]


| Threshold Used | Positivity Determination | Disease | Sample Type | References |
|---|---|---|---|---|
| 20 stdev | Mann–Whitney u-test | AD | Brain | [52] |
| ine 10 stdev | 50% of replicates | BSE | CSF | [21,26] |
| 10 stdev | 25% of replicates | BSE | Brain | [7] |
| ine 20 stdev | 50% of replicates | CJD | Skin | [39] |
| 10 stdev+10% | 50% of replicates | CJD | CSF | [5] |
| 10 stdev | 50% of replicates | CJD | CSF; OM | [17,34,40,41,46] |
| 5 stdev | 50% of replicates | CJD | Brain | [43] |
| 5 stdev | 40% of replicates | CJD | CSF | [44] |
| 3 stdev | 50% of replicates | CJD | CSF | [22,42] |
| >10,000 RFU | 50% of replicates | CJD | CSF | [48] |
| ine 10 stdev | Mann–Whitney u-test | CWD | Muscle; LN | [1,18] |
| 10 stdev | 2/3 of replicates | CWD | Blood; Feces; RAMALT | [19] |
| 10 stdev | 50% of replicates | CWD | Ticks; Feces | [36,57] |
| 10 stdev | 25% of replicates | CWD | Brain | [56] |
| 10 stdev | 1/3 of replicates | CWD | RAMALT | [23,30] |
| 10 stdev | Avg of reps >T | CWD | RAMALT | [28,29] |
| 5 stdev | Mann–Whitney or Wilcoxon signed rank | CWD | Buffy-coat | [53] |
| 5 stdev | Mann–Whitney u-test | CWD | Brain | [20,27,55] |
| 5 stdev | Fisher’s exact test | CWD | Feces | [25] |
| 5 stdev | Unpaired t-test | CWD | LN; Brain | [37] |
| 5 stdev | One sample t-test | CWD | LN; Brain | [32] |
| 5 stdev | 50% of replicates | CWD | Feces | [31] |
| 5 stdev | Avg of reps >T | CWD | Brain; LN | [33,35] |
| 4 stdev | 50% of replicates | CWD | Blood | [49] |
| max signal of pos ctrl | 1/3 of replicates | CWD | Brain | [24] |
| ine 5 stdev | Mann–Whitney or Wilcoxon signed rank | CWD; BSE | Brain | [38] |
| 5 stdev | Avg of reps >T | CWD; BSE | Brain | [54] |
| ine 3 stdev | 50% of replicates | FFI | Brain | [47] |
| ine 30 stdev | 50% of replicates | PD | CSF | [45] |
| 10 stdev+10% | 50% of replicates | PD | Brain | [3,50] |
| >120 RFU | 50% of replicates | PD | Brain | [51] |
| ine 10 stdev | 50% of replicates | Sc | Brain | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowden, G.R.; Picasso-Risso, C.; Li, M.; Schwabenlander, M.D.; Wolf, T.M.; Larsen, P.A. Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease. Pathogens 2023, 12, 309. https://doi.org/10.3390/pathogens12020309
Rowden GR, Picasso-Risso C, Li M, Schwabenlander MD, Wolf TM, Larsen PA. Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease. Pathogens. 2023; 12(2):309. https://doi.org/10.3390/pathogens12020309
Chicago/Turabian StyleRowden, Gage R., Catalina Picasso-Risso, Manci Li, Marc D. Schwabenlander, Tiffany M. Wolf, and Peter A. Larsen. 2023. "Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease" Pathogens 12, no. 2: 309. https://doi.org/10.3390/pathogens12020309
APA StyleRowden, G. R., Picasso-Risso, C., Li, M., Schwabenlander, M. D., Wolf, T. M., & Larsen, P. A. (2023). Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease. Pathogens, 12(2), 309. https://doi.org/10.3390/pathogens12020309







