Abstract
Herpesvirus infections can lead to a number of severe clinical manifestations, particularly when involving the central nervous system (CNS), causing encephalitis and meningitis. However, understanding of the host factors conferring increased susceptibility to these diseases and their complications remains incomplete. Previous studies have uncovered defects in the innate Toll-like receptor 3 pathway and production of type I interferon (IFN-I) in children and adults that predispose them to herpes simplex encephalitis. More recently, there is accumulating evidence for an important role of IFN-independent cell-autonomous intrinsic mechanisms, including small nucleolar RNAs, RNA lariat metabolism, and autophagy, in restricting herpesvirus replication and conferring protection against CNS infection. The present review first describes clinical manifestations of HSV infection with a focus on neurological complications and then summarizes the host–pathogen interactions and innate immune pathways responsible for sensing herpesviruses and triggering antiviral responses and immunity. Next, we review the current landscape of inborn errors of immunity and the underlying genetic defects and disturbances of cellular immune pathways that confer increased susceptibility to HSV infection in CNS. Ultimately, we discuss some of the present outstanding unanswered questions relating to inborn errors of immunity and HSV CNS infection together with some perspectives and future directions for research in the pathogenesis of these severe diseases in humans.
1. Herpes Simplex Virus Infections of the Central Nervous System
Herpes simplex virus types 1 and 2 (HSV-1/-2) are ancient human pathogens with a wide spectrum of disease manifestations. These double-stranded (ds)DNA viruses have been remarkably successful from an evolutionary point of view: computational models have shown that HSV-1 is likely the result of codivergence from chimpanzee herpesvirus around 6 Ma, while HSV-2 has arisen from a cross-species transmission event of the same virus 1.6 Ma [1]. It should, therefore, come as no surprise that both HSVs are prevalent in all parts of the world with an estimated two thirds of the global population being infected with at least one of these viruses [2]. The key to understanding this success lies in the ability of both viruses to have two distinct replication cycles: lytic and latent.
Upon primary infection, HSV will start its lytic replication cycle in epithelial cells, causing cold sores (mostly HSV-1) or genital sores (HSV-2). Eventually, the virus will invade sensory neurons, traveling retrogradely to the soma and establishing latency, particularly in the trigeminal and sacral ganglia for HSV-1 and HSV-2, respectively. From here, the virus can occasionally reactivate and travel anterogradely to the primary infection site, causing recurrence of the sores or genital herpes and enabling transmission to the next host. However, on rare occasions, HSV will instead invade the central nervous system (CNS), either during primary infection or at reactivation, thereby giving rise to herpes simplex encephalitis (HSE), brainstem encephalitis, or aseptic meningitis [3].
HSE is the most common form of sporadic viral encephalitis, accounting for 10–20% of all cases, with HSV-1 causing >90% of HSE [4]. There are two age peaks of the disease, one between 5 and 30 years of age and one over 50 years of age [4]. The incidence of HSE is estimated to be around 1–2/250,000 per year, with neither geographical nor seasonal bias [5,6]. Patients usually present with fever, confusion, decreased consciousness, and nuchal rigidity [4,7], and the diagnosis is made by detection of pleocytosis and HSV DNA in the cerebrospinal fluid [8]. Before effective antiviral treatment was developed in the form of acyclovir, mortality approached 70%, and even with adequate antiviral therapy, it can still be as high as 25%, with the majority of survivors suffering from temporary or permanent neurological sequelae, including anterograde amnesia and cognitive deficits [9].
Brainstem encephalitis, also called rhombencephalitis, caused by HSV is extremely rare with only a few dozen published cases. Patients from case reports have been described as having a fever and varying neurological symptoms, including abnormal ocular movements, cranial nerve deficits, and headaches [10]. Acyclovir treatment appears to be beneficial, despite the low number of patients described [10].
Aseptic meningitis is defined by inflammation of the meninges without bacterial infection. In most cases, the exact etiology cannot be determined [11,12]. In the cases caused by viruses, enteroviruses, varicella zoster virus, and HSV-2 are the most common agents [6,12]. The most common symptoms include headache, nausea, nuchal rigidity, and fever [6]. Mortality is generally low, but a significant proportion of patients have unfavorable outcomes nonetheless [6]. In rare cases, patients suffer from recurrent episodes of viral meningitis, known as Mollaret’s meningitis, named after the French neurologist Pierre Mollaret, who first described this condition [13]. The most common etiology in these patients is HSV-2, and the condition is usually self-limiting, although half of patients experience transient neurological impairment [14]. Acyclovir and valacyclovir are thought of as effective, although definitive trial data are for antivirals as prophylaxis and treatment are lacking [14].
It is this conundrum of the ubiquity of HSV in the global population and its rare but serious CNS manifestations that has spurred research for decades to understand the interplay between HSV and host immunity.
2. Immunity to Herpes Simplex Virus
HSV has been successfully transmitting and adapting to human immunity for millions of years, while still retaining its virulence. So far, 284 viral open reading frames (ORFs) have been identified, the transcripts and protein products of which modulate and help the virus evade the adaptive and innate immune responses as well as cell-intrinsic defenses [15,16,17]. As HSV-1 and HSV-2 share 83% homology in their genes, and several of their proteins share biological functions [18], we will for, the most part, not discriminate between the two viruses in the following sections.
2.1. Interferon-Dependent Anti-HSV Immunity
Secretion of type I interferons (IFN-I) is a central part of anti-HSV immunity (Figure 1). In the extracellular and endosomal compartments, HSV is recognized by at least three Toll-like receptors (TLR), all of which eventually induce the expression of IFN-I. TLR3 is activated by dsRNA species to signal through interferon regulatory factor (IRF)3, IRF7, or nuclear factor (NF)-kB [19,20]. TLR2 recognizes HSV glycoprotein B (gB) and signals through myeloid differentiation primary response (MyD)88 and NF-κB [21]. TLR9 recognizes DNA with unmethylated CpG motifs, such as the HSV genome, and signals through either MyD88 and NF-κB or via MyD88 and IRF7 [22,23].
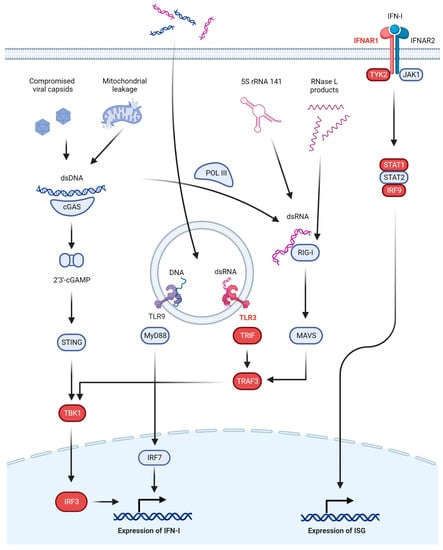
Figure 1.
Interferon-dependent antiviral immunity to herpes simplex virus. Major pathways involved in innate anti-HSV immunity based on IFN-I are shown. In the endosomal compartment, dsRNA is recognized by TLR3 and dsDNA by TLR9. In the cytosol, dsDNA is recognized by cGAS to produce 2′3′-cGAMP and activate the STING pathway or by POL III to produce RNA. dsRNA in the cytosol is recognized by RIG-I to activate the MAVS pathway. These pathways (except for TLR9) converge at TBK1, finally phosphorylating IRF3 and activating IFN-I production. IFN-I acts through its receptor IFNAR to activate ISGs and drive cellular antiviral programs. Proteins in which defects have been linked to HSE are shown in red.
In the cytosol, multiple receptors exist to detect viral DNA and other danger signals, such as dsRNA species. Notable here is the cyclic GMP-AMP synthase–stimulator of interferon genes (cGAS-STING) axis. cGAS recognizes dsDNA from the viral genome or mitochondrial DNA leaked into the cytosol [24] to produce 2′3′-cyclic GMP-AMP (2′3′-cGAMP) as a second messenger to activate STING on the endoplasmic reticulum [25,26,27]. STING then signals through serine/threonine protein kinase 1 (formerly known as TANK-binding kinase 1, TBK1) and IRF3 to induce IFN-I [16]. Alternative dsDNA sensors exist, including DNA-dependent activators of IRFs (DAI) [28] and RNA polymerase III (POL III) [29,30], but the cGAS-STING signaling axis has been recognized as a major contributor to dsDNA-induced antiviral signaling, at least in mouse studies [31,32], although this is likely tissue and cell-type dependent. The presence of dsRNA in the cytosol produced during viral replication by POL III recognizing dsDNA [29,30] or through combined upregulation and increased nuclear export of 5S rRNA 141 (RNA5SP141) [33] will activate retinoic acid inducible gene (RIG)-I (and, to a lesser degree, melanoma differentiation-associated gene (MDA)5) and start signaling through mitochondrial antiviral-signaling protein (MAVS), TBK1, and IRF3 to induce IFN-I [34,35,36,37]. Alternatively, dsRNA can stimulate protein kinase R (PKR), which, through phosphorylation of eukaryotic initiation factor (eIF)2α, will halt translation of host and viral protein alike [38], or it can activate the oligoadenylate synthetase (OAS)-RNase L system which degrades RNA in the cytosol and inhibits viral replication [39]. Finally, small self-RNA products from RNase L activity can also serve as RIG-I ligands, further amplifying the antiviral response [40].
The above-mentioned pathways will eventually lead to the upregulation of IFN-I which includes IFN-α (13 subtypes), IFN-β, IFN-ε, IFN-κ, and IFN-ω. Two other classes of IFN exist (IFN-II and IFN-III), but these have only been implicated in defects in anti-HSV immunity when the IFN-I system is also affected due to overlap in the downstream signaling cascades, and they will, therefore, only be mentioned briefly. IFN-I are broad-acting antiviral cytokines that signal through the IFN-α/β receptor (IFNAR), composed of IFNAR1 and IFNAR2. IFNAR recruits Janus kinase (JAK)1 and tyrosine kinase (TYK)2, which, in turn, phosphorylate the signal transducer and activator of transcription (STAT)1 and STAT2, which then, together with IRF9, form the complex IFN-stimulated gene factor (ISGF)3 to induce hundreds of IFN-stimulated genes (ISGs). These ISGs are the main drivers of the antiviral response and include PKR, RNase L, and RIG-I [41]. In the periphery, IFN-β can be produced by almost all cells, whereas specialized leukocytes, plasmacytoid dendritic cells (pDC), are the main IFN-α factories [42,43]. The source of IFN-I in the CNS is less obvious. Almost all CNS cells can produce IFN-I, but an in vivo study has suggested microglia and astrocytes are the primary IFN-I producers upon infection with HSV-1 and that neurons play a minor role in the production of IFN-I upon HSV-1 infection [44].
The importance of the above pathways is perhaps best illustrated by the many immune evasion strategies employed by HSV. For example, infected cell protein (ICP)0 inhibits nuclear translocation of IRF3, which is central to several pathways [45]. Viral protein (VP)1-2 and ICP27 both inhibit STING [46,47], and likewise ICP27 also inhibits STAT1-mediated signaling [48]. The function of PKR is also targeted by Us11, sequestering dsRNA and binding directly to PKR [49,50], as well as by ICP34.5 recruiting protein phosphatase 1α to disinhibit protein translation [51].
A simplified overview of these IFN-I dependent pathways is provided in Figure 1.
2.2. Interferon-Independent Anti-HSV Immunity
2.2.1. Autophagy
Autophagy is an essential mechanism for the cell to dispose of intracellular components by lysosomal degradation. It requires a set of autophagy-related proteins (ATG) and is subdivided based on delivery mechanism into macro-autophagy, micro-autophagy, and chaperone-mediated autophagy, of which macro-autophagy is the best characterized process. Macro-autophagy (hereafter simply ‘autophagy’) is triggered by many different stimuli, including starvation, cellular stress, and infection. Structures can be targeted for degradation by ubiquitination which is recognized by p62 (also known as sequestosome 1 or SQSTM1) or other autophagy receptors. The cargo is engulfed by a double-membrane vesicle termed the autophagosome. Microtubule-associated protein 1 light chain 3 (LC3) plays a key role in the formation and maturation of the autophagosome, and lipidated LC3 (LC3-II) accumulates when autophagy is activated, or lysosomal clearance is blocked [52]. Autophagy plays both anti- and pro-viral roles during the HSV life cycle, and the net effects are often cell-type or tissue-dependent. HSV-1 particles are suggested to be degraded by autophagy [53], and autophagy can facilitate the loading of HSV-1 gB onto MHC-I molecules [54]. Control of HSV-1 in primary neurons is critically dependent on ATG5, whereas this is not the case for mucosal epithelial cells [55]. HSV-1 also encodes two anti-autophagy proteins: ICP34.5 inhibits Beclin-1 which is another essential part of autophagosome maturation [56]; and Us11 inhibits PKR and its ability to stimulate autophagy [57]. Although HSV-2 also seems to modulate autophagy, no viral autophagy-blocking proteins have been identified so far [52], so different roles for autophagy between the two viruses cannot be excluded. Finally, an siRNA-knockdown screen has revealed differential roles of autophagy-related proteins in HSV-1 replication [58].
2.2.2. The Lectin Pathway
The lectin pathway of the complement system recognizes surfaces displaying an array of carbohydrates or acetyl groups. The pattern recognition molecules (collectively referred to as lectins) include mannan-binding lectin (MBL), ficolins 1-3, and collectin 10 and 11. The lectins circulate in complex with MBL-associated serine proteases (MASPs) which activate the rest of the pathway [59]. Both MASP1 and MASP2 are indispensable for activation of the lectin pathway [60], and the lectin pathway has been shown to modulate the immune response to HSV infection in vivo through recognition of viral glycoproteins [61]. HSV counters the complement pathways broadly by expression of its surface protein gC, which blocks C3-activity [62].
3. Inborn Errors of Interferon-Dependent Innate Immunity
As previously stated, IFN-I is central to antiviral immunity. IEI in the pathways activating IFN-I or the IFN-I signaling axis itself have been well documented to cause increased susceptibility to a range of viruses, most notably live-attenuated viral vaccines (LAV), respiratory viruses, and herpesviruses [63]. Specific genetic variants previously described in connection with HSV CNS infections are compiled in Table 1.

Table 1.
List of genetic variants in innate immune pathways associated with heightened susceptibility to HSV CNS infections. In some cases, several variants have been shown to cause deficiency of a specific protein; here, variants from patients in whom HSV-1 or HSV-2 CNS infection was diagnosed are listed.
3.1. TLR3 Signaling
The TLR3 signaling axis has emerged as a non-redundant and essential part of the defense against HSE. In general, cells from patients with these defects show decreased IFN-I responses to HSV-1 and increased cytolytic activity or production of the virus, even impacting the intrinsic defenses of induced pluripotent stem cell (iPSC)-derived neurons [84]. UNC93B1 deficiency in two unrelated patients with HSE was reported to reduce signaling via TLR3 and TLR7-9 due to its role in trafficking these receptors from the ER to the endosomes [64]. Both autosomal recessive (AR) and dominant (AD) defects of TLR3 itself have been reported and exhibit specific susceptibility to HSV-1 [65,66,67]. Defects in downstream signaling adaptors have also been documented, including TRIF, TRAF3, NEMO, TBK1, and IRF3 (Figure 1 and Table 1) [68,69,70,71,72,73,74]. Although other signaling cascades converge on some of these downstream adaptors, including STING and RIG-I-MAVS signaling, TLR3 signaling appears to be the common denominator for all these defects, underscoring its importance in HSV-1 immunity of the CNS. A possible explanation for the central role of TLR3 is its recently proposed function as a rheostat of tonic IFNAR signaling, as discussed below [85].
3.2. IFNAR Signaling
Defects in the IFNAR signaling cascade can have a spectrum of clinical pictures, including severe disseminated infection after LAV administration or during natural viral infections, and is mostly determined by the localization of the specific defect within the signaling cascade [63]. This is partly due to several adaptor proteins, especially in the JAK-STAT signaling, being shared between the receptors for IFN-I and IFN-II.
IFNAR1 deficiency, initially associated with severe disease after LAV [86], was recently linked to a case of childhood HSE [76]. The identified variant encoded a truncated IFNAR1 protein which could not interact with TYK2. This is the first time HSE has been directly attributed to IFNAR deficiency [87]. The explanation could be a broader phenotype for this immunodeficiency, and patients, therefore, usually present with some other severe infectious phenotype, usually heightened susceptibility to LAV [63,86,88,89], as well as a short observation time (the first case of IFNAR deficiency was only described in 2015 [63,88]).
Deficiency of STAT1 was the first reported genetic etiology of HSE [77,90]. The patient had dissemination of the Bacillus Calmette–Guerin mycobacterial vaccine and recurrent HSE which proved lethal in the second episode [77]. This patient was included in a recent study describing a cohort of 32 patients with complete or partial STAT1 deficiency, and it was the only case of HSE, although several other patients had experienced mild HSV-related disease [91]. A related IEI, TYK2 deficiency, is also linked to disseminated mycobacterial disease. At least one patient has been reported with HSV meningitis, two others with aseptic meningitis of unknown etiology, and one patient with HSE [82,92,93].
Deficiency of IRF9 due to a biallelic splice-site variant has been reported in a family with heightened susceptibility to a range of viruses and sepsis [78]. One sibling was admitted to the pediatric intensive care unit for HSV meningoencephalitis at the age of 9 months. At 14 months of age, she died due to complications following a yellow fever virus vaccination [78], mirroring the phenotype of IFNAR deficiency with heightened susceptibility to LAV [63,86,89].
3.3. GTF3A and RNA5SP141 as Guard Mechanisms
Recently, Naesens and colleagues reported a case of HSE in a patient who was compound heterozygous for variants in GTF3A [75]. This gene encodes the transcription factor IIIA (TFIIIA), which is essential for POL III-mediated transcription of 5S rRNA species. Among these is the pseudogene RNA5SP141, which was previously shown by the Gack group to play an essential role in antiviral immunity [33]. The RNA species (5S rRNA 141) is normally expressed at low levels and mainly resides in the nucleus, and the few copies that make it to the cytoplasm are sequestered by the RNA-binding proteins MRPL18 and TST. HSV-infection, through its virion host shutoff (vhs) protein, downregulates most host mRNA, including MRPL18 and TST, but RNA5SP141 is slightly upregulated, and most of it is then exported to the cytoplasm [33]. In a mechanism analogous to the guard theory of plant immunity [94], RNA 5S ribosomal 141 is released from its sequestration and can now induce the RIG-I-mediated activation of IFN-I [33] (Figure 2A,B). Interestingly, both the reported patient and his sister (who also harbored both GTF3A variants but did not experience HSE) had decreased IFN-I responses to HSV-1, and this could be rescued by reconstitution with WT GTF3A [75]. Both siblings also had a CVID-like phenotype [75], and, although rare, HSE is not unheard of among CVID patients, as discussed below [95]. It remains to be clarified if the biallelic GTF3A variants disturb B cell function as well and whether the HSE phenotype manifests exclusively in the context of disturbed B cell function (primary or secondary).
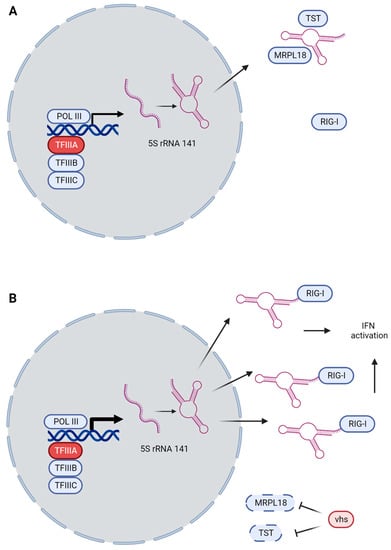
Figure 2.
Guard mechanism of RNA5SP141 and RIG-I. (A) During homeostasis, POL III transcribes RNA5SP141 at low levels, with the product staying mostly in the nucleus. When escaping to the cytosol, the RNA is sequestered by MRPL18 and TST, preventing it from activating RIG-I. (B) Upon HSV-infection, vhs causes global mRNA downregulation, decreasing the amounts of MRPL18 and TST. RNA5SP141 is slightly upregulated, but importantly, the transcript is shifted from the nucleus to the cytoplasm where it, disinhibited by the lack of MRPL18 and TST, can activate RIG-I and induce IFN-dependent immunity. TFIIIA (red, encoded by GTF3A) is part of the transcription factor complex necessary for POL III-transcription of 5S rRNA species.
4. Inborn Errors of Interferon-Independent Innate and Cell-Intrinsic Immunity
4.1. Autophagy
Building on the evidence of a neuron-specific antiviral role of autophagy in murine neurons [55], two cases of recurrent lymphocytic Mollaret’s meningitis caused by HSV-2 have been reported due to monoallelic variants in the essential autophagy genes ATG4A and MAP1LC3B2. Primary fibroblasts from both patients showed markedly decreased autophagy induction upon HSV-2 infection, starvation, or rapamycin treatment, as well as increased HSV-2 replication and cell death, all of which was rescued by reconstitution with WT ATG4A and MAP1LC3B2 [83]. This study is supported by another study demonstrating that the HSV-1 protein ICP34.1 confers neurovirulence by targeting the autophagy protein Beclin-1 for degradation [56]. Altogether it may make sense that neurons, being post-mitotic cells sensitive to hyperinflammation and hypoxia, utilize autophagy in antiviral defenses to restrict excessive IFN-I production and immunopathology [96]. As mentioned above, not much is known about the role of autophagy in the HSV-2 life cycle and antiviral immunity because most HSV research has been carried out using HSV-1. It will, therefore, be interesting to learn if autophagy plays a similar role in neuroprotection against HSV-1 CNS infections in future studies.
4.2. Intrinsic Defenses: SNORA31 and DBR1
In recent years, two studies from the Casanova group have uncovered defects in cell-intrinsic defense systems predisposing to brainstem encephalitis and HSE.
In seven patients from three kindreds with brainstem encephalitis due to either HSV-1, influenza B virus, or norovirus, the group identified biallelic variants in the gene encoding debranching enzyme 1, DBR1 [81], which hydrolyzes intron lariat RNA at its branch point to facilitate its turnover or processing. This enzyme is ubiquitously expressed, but the levels are highest in the spinal cord and brainstem. The group found that the variants caused a defect in DBR1 function due to an enzymatic defect as well as lower protein levels. Patient cells showed an increased HSV-1 replication rate and increased levels of RNA lariats. Together with the fact that HSV-1 has been shown to inhibit splicing or cause alternative splicing of some genes [97], the authors speculate that DBR1 controls brainstem-intrinsic defenses by regulation of lariat metabolism [81].
In five patients with HSE, the group found monoallelic missense mutations in SNORA31 [79]. The gene encodes a small nucleolar RNA, snoRNA31. Fibroblasts and iPSC-derived neurons showed heightened susceptibility to HSV-1, whereas this was not observed in iPSC or B cells, suggesting a cell-type specific role for snoRNA31. The authors demonstrated that snoRNA31 is involved in pseudo-uridylation at U218 of 18S rRNA; however, this was only affected in full knockouts and not in cells carrying monoallelic variants, the latter being the case for all patients. Furthermore, the transcriptome was affected differently in patient cells infected with HSV-1 than in healthy control cells, but the exact mechanism by which snoRNA31 protects against HSV-1 remains elusive [79].
4.3. Lectin Pathway of Complement Activation
IEI of the lectin pathway have been shown to be associated with symptomatic HSV reactivation [61,98]. Bibert and colleagues reported two cases of HSE in adult patients who harbored monoallelic loss-of-function variants in MASP2. Their assays showed that antibody-depleted plasma from patients displayed reduced capacity for HSV-1 neutralization [80]. MASP2 deficiency was first described in 2003 in a patient with severe pneumococcal infections as well as autoimmune and autoinflammatory conditions [99]. Since then, at least ten additional patients with various clinical pictures as well as nine asymptomatic individuals have been identified, casting doubt on the clinical relevance of the lectin pathway in human immunity [59]. MBL deficiency is the prime example of a related controversial condition, originally described as an immunodeficiency with susceptibility to pyogenic infections, albeit with low clinical penetrance [100], only to later have this status revoked due to the high frequency of asymptomatic MBL-deficient individuals [101,102,103]. However, several other proteins act in parallel with MBL, whereas MASP1 and MASP2 are both non-redundant in the lectin pathway, and the report of HSE in MASP2-deficient patients can, therefore, not be discarded on the same grounds. It should be noted, however, that systematic investigations of very large groups of individuals with IEI affecting the entire lectin complement pathway, especially with a focus on viral diseases, are still lacking [59].
5. Inborn Errors of Lymphocyte Function
Patients with inborn errors of lymphocyte function often present with a diverse spectrum of infections.
The most extreme syndrome of lymphocyte dysfunction, severe combined immunodeficiency (SCID), arises from mutations in a number of genes: IL2RG, RAG1, RAG2, ADA, JAK3, and IL7RG. The patients are highly susceptible to lethal infections with a broad range of pathogens. HSV will usually cause recurrent skin infections and meningitis [104]. GATA2 deficiency mainly affects B cells, NK cells, and dendritic cells. The patients will also be at risk of disseminated HSV skin infections and meningitis, as well as hemophagocytic lymphohistiocytosis [104]. Intriguingly, HSE is not commonly found in SCID- or GATA2-deficient patients [104].
Errors in NK cell-mediated immunity show a well-established link to severe manifestations of HSV [105,106]. Of note, the original case of NK cell deficiency was later shown to have GATA2 deficiency [107]. However, reports of HSE in NK cell-deficient patients are few. Almerigogna and colleagues reported a series of five pediatric cases with HSE and impaired NK cell function from 2003 to 2005, two of which had a polymorphism in CD16A (p.Leu48His), although further functional analyses could not be carried out [108]. In a more recent report, Lisco and colleagues reported a male patient with a hemizygous germline variant in IL2RG (located on the X chromosome), which primarily affected his NK cells due to somatic reversion in the other lymphocyte populations [109]. The patient had a long history of severe and refractory human papillomavirus disease but notably also experienced HSE as an adolescent [109].
Common variable immunodeficiency (CVID) is an inborn error of B lymphocyte maturation and the most common immunodeficiency in adults. It is defined by low amounts of IgG and IgA or IgM, corresponding to a lack of proper seroconversion and insufficient response to polysaccharide vaccines without other causes for hypogammaglobulinemia [110]. Only a few cases have linked CVID to HSE or other severe manifestation of HSV infection, and the association remains weak [95,111].
6. Other Severe Phenotypes of HSV Infection
Extreme phenotypes of HSV infection other than HSE have been ascribed a genetic component, albeit with less systematic functional validation, but further studies may prove fruitful in understanding the host–pathogen interaction of HSV.
Neonatal HSV infections can present as skin, eye, and mouth (SEM) infections or a disseminated phenotype involving hepatosplenomegaly. A recent genetic study of 10 infants with severe neonatal HSV (SEM, HSE, or disseminated disease) identified variants in genes known from HSE immunogenetics, including variants in TLR3, TRAF3, IRF3, STAT1, and DBR1, as well as candidate variants in other genes, including MSR1, PRF1, GRB2, RAG2, C6, and C7 [112]. However, functional consequences of dominant disease-causing effects of any of these variants remain to be established.
Eczema herpeticum (EH) is a rare skin manifestation of HSV infection that can afflict patients with skin conditions, especially atopic dermatitis. Compromised epidermal barrier function is central to the pathogenesis and facilitates viral penetration deep into the skin [113]. Genetic studies have mainly focused on the general pathogenesis of atopic dermatitis, and there are only a few reports associating genetic variants with EH [113]. In 49 patients with recurrent EH, variants were found in eight genes: RBBP8NL, TRIM15, CLEC7A, SIDT2, FRMD3, TPSG1, GSTZ1, and SP110 [114]. Of the identified variants, RBBP8NL and SIDT2 could be linked to enhanced viral replication, restricted IFN-κ expression (an IFN-I originally named after its high expression in keratinocytes [115]), and the expression of genes related to keratinocyte differentiation [114]. While SIDT2 is required for IL-1β expression during HSV-1 infection, RBBP8NL appears to restrict the expression of IL-1β. In addition to IFN-κ and IL-1β, IFN-γ plays an important role in protection against EH. Indeed, targeted deep sequencing in 121 EH patients revealed rare missense variants in the IFN-γ receptor 1 (IFNGR1), leading to decreased STAT1 signaling upon IFN-γ stimulation [116]. Finally, the importance of impaired barrier function in EH pathogenesis is reflected by loss-of-function variants in Filaggrin (FLG), detected in about 25% of EH patients and rarely in healthy individuals [117]. Filaggrin is important for the structural integrity of keratin filaments, while its degradation products, urocanic acid and pyrrolidone carboxylic acid, contribute to natural skin moisturization and acidification [118]. The impaired restriction of HSV-1 observed in FLG knockdown in human keratinocytes has been suggested to be due to reduced acidification [119].
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory, life-threatening disorder characterized by persistent fever, splenomegaly with cytopenia, hypertriglyceridemia, and hypofibrinogenemia and the presence of hemophagocytosis in the bone marrow [120]. Histiocytes exert hemophagocytic activity in the reticulo-endothelial system, which can be triggered by congenital defects in T and NK cell immunity (primary HLH) or secondary to infections, rheumatic diseases, and cancer [121]. Often, secondary HLH is triggered by the unchecked replication of viruses, of which the Epstein–Barr virus (EBV) is the most frequent and may be partly explained by mutations in genes relevant to EBV immunity [121]. HLH triggered by HSV-1 is extremely rare in adults but less so in neonates [120]. The literature on the genetic causes of HSV HLH is limited. While some clinical case reports of HSV-1 HLH [122,123,124,125,126] and HSV-2 HLH exist [127], new genetic variants have been discovered in only three studies to date: in a 19-year-old woman suffering from postpartum HSV-1 HLH, a Caspase-8 (CASP8) variant was noted, but a definitive link between the variant and the impaired IFN responses observed in her PBMCs was not established [128]; a novel variant in EP300 was discovered in a patient with Rubinstein–Taybi syndrome and HSV-1 HLH [129]; and finally, an 18-year-old female patient was reported in whom a GATA2 variant was identified postmortem, although the disease-causing potential was not functionally validated [130].
7. Perspectives and Future Directions
CNS infections with HSV are extremely rare events caused by ubiquitous pathogens. These and other very rare infectious diseases have, therefore, been suggested as manifestations of novel IEI [131]. Using patient phenotypes as a steppingstone and harnessing the power of next-generation sequencing have proven immensely successful in defining novel syndromes of immunodeficiency with both broad and specific susceptibility to severe or lethal infections [132,133]. After the first discoveries of STAT1 and UNC93B1 deficiencies as causal of HSE, the focus has mainly been on unraveling other defects in the IFN-I system (Figure 1 and Table 1). Perhaps surprisingly, because HSV is a DNA virus, almost all defects in IFN-I activation have been detected in proteins related to the RNA-sensing TLR3 and RIG-I pathways. It is unlikely only to be due to confirmation bias that researchers focus on the pathways we know are crucial for HSV immunity, as important DNA-detecting pathways, such as cGAS-STING and TLR9, have been known for years, and predicted deleterious variants in genes related to these would have prompted further investigations. Rather, it may simply be that some of these pathways are not only non-redundant in human immunity but also more broadly in homeostasis, applying a strong negative selection on deleterious variants and thus a survivorship bias. Additionally, applying findings in mouse models directly to human immunology can easily mislead [134]. However, the argument of survivorship bias could also have been made for IFN-I signaling defects in general because of their key roles in antiviral immunity, but not only have many different deleterious variants been described, recently, it has also been shown that deleterious variants in IFNAR1 and IFNAR2 are prevalent at an appreciable rate in Western Polynesian and Arctic peoples [89,135]. Studies of these populations could, for the first time, provide insights into the clinical penetrance of severe infections in individuals without functional IFN-I signaling pathways, including HSV CNS infections.
Although neurons may not be the main source of IFN-I in the CNS, lesions of the TLR3 pathway, nevertheless, lead to impaired viral control, predisposing to HSE. Interestingly, these patients do not suffer from disseminated mucocutaneous viral disease in the periphery, as is the case for patients with general lymphocyte defects [104]. One way to reconcile these observations, is the hypothesis that TLR3 is a rheostat of tonic IFN-I signaling in the CNS. Tonic IFNAR signaling is well described in mice, with implications for hematopoietic stem cells, immune functions, NK cell proliferation, and bone remodeling, but the regulation of this basal level of IFN-I has not been fully elucidated [136]. Indeed, it was recently shown that patients with lesions of the TLR3-signaling pathway had significantly decreased levels of basal IFN-β as well as several ISGs [85]. The authors posit that the lack of tonic IFNAR signaling in TLR3- and IFNAR-signaling-deficient patients leads to unchecked early viral replication, predisposing to the development of HSE. It is, however, not yet clear what ligand, if any, maintains this constitutive TLR3 activation in healthy individuals [85] (Figure 3).
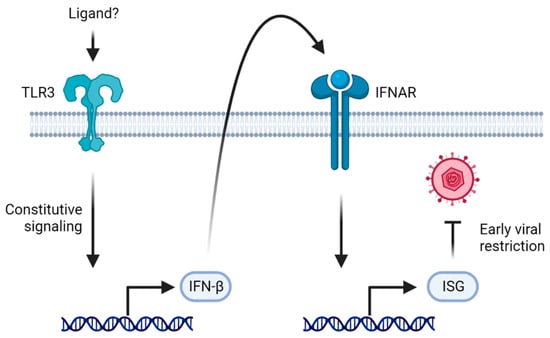
Figure 3.
TLR3 as a rheostat of tonic IFNAR signaling. In healthy individuals, TLR3 signals constitutively, leading to a basal level of IFN-β which activates IFNAR signaling. The resulting basal level of ISGs being expressed restricts early viral replication. In patients deficient in the TLR3-IFNAR-signaling axis, the virus replicates at a higher rate early during the infection, predisposing to HSE.
In recent years, other parts of the immune system, such as cell autonomous innate immune mechanisms, have been implicated as non-redundant in anti-HSV immunity, including autophagy, small nucleolar RNAs, and RNA lariat metabolism (Figure 4). A large body of literature already exists on how autophagy works, but specific knowledge on which role it plays in anti-HSV immunity in humans, including differences between HSV-1 and HSV-2, is still needed. Autophagy also regulates the levels of many proteins, including several in the IFN-I system [137], blurring the lines between strictly autophagy-mediated immunity and IFN-I. Autophagy defects have, however, already been implicated not only in Mollaret’s meningitis but also in poliomyelitis, implying a non-redundant role in neuronal protection against specific neurotropic viruses [83,138].
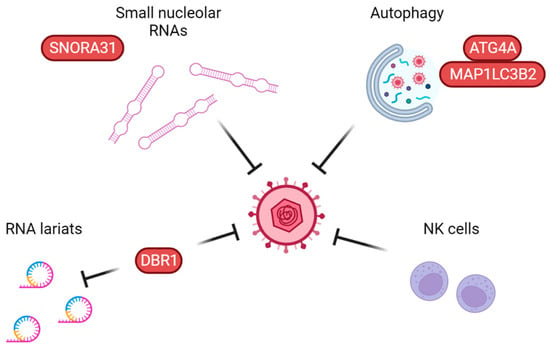
Figure 4.
Proposed non-redundant anti-herpes simplex virus pathways working independently of type I interferons. Small nucleolar RNAs, including snoRNA31, have been shown to exert a direct antiviral effect on HSV replication. Autophagy defects have been linked to two adult cases of Mollaret’s meningitis. NK cell deficiency has been identified in a few cases of pediatric HSE. Finally, DBR1 debranches RNA lariats and restricts HSV replication, although the exact antiviral mechanism is not yet fully understood. Proteins in which defects have been linked to HSE are shown in red.
Advances in our basic understanding of immunology is crucial to the discovery of novel immunodeficiencies. Without the discovery of RNA5SP141-mediated RIG-I activation, the connection between GTF3A and HSE is unlikely to have been made [33,75]. However, the opposite is also true, as evidenced by the body of literature, testifying to the ability of the discoveries of novel IEI in humans to teach us as yet uncovered principles of antiviral immunity in humans. With the characterization of variants in DBR1 and SNORA31 [79,81], translational immunology not only identifies which genes are central to immunity against which pathogens in natura but also provides basic immunology and virology with a challenge to understand novel mechanisms of neuroprotection in humans. Based on recent developments in the investigation of IEI in HSV immunity, it seems plausible that we will learn of new basic cellular processes involved in antiviral defenses and promoting severe disease and CNS infection when defective or dysregulated. Such pathways may include DNA sensing, cell death pathways, inflammasome activation, integrated stress responses, ubiquitination, and more. A guiding principle seems to be the quest to balance powerful antiviral neuroprotective immune responses aimed at eradicating the viral threat, while at the same time limiting inflammation and neuropathology and restoring homeostasis [96,139]. It will be exciting to follow the next technological advances and major discoveries, which may provide insights into the complexity of regulatory signaling networks in the immune system, and along this path, allow the discovery of novel IEI, the understanding of which may help diagnose patients and improve prophylaxis and treatment of severe herpesvirus infections of the CNS.
Author Contributions
T.H.M. conceived and developed the idea together with M.K.S. M.K.S. and T.H.M. wrote the first draft with contributions from M.W. M.K.S. prepared figures. All authors have read and agreed to the published version of the manuscript.
Funding
T.H.M. is funded by The Independent Research Fund Denmark (4004-00047B), The Lundbeck Foundation (R268-2016-3927), The Novo Nordisk Foundation (NNF21OC0067157), Innovation Fund Denmark (8056-00010B), and an EU HORIZON Europe grant (ID 101057100; UNDINE). M.K.S. and M.W. are supported by PhD scholarships from the Faculty of Health, Aarhus University, Aarhus, Denmark.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created using BioRender.com (BioRender, Canada, accessed on 18 January 2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wertheim, J.O.; Smith, M.D.; Smith, D.M.; Scheffler, K.; Kosakovsky Pond, S.L. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol. Biol. Evol. 2014, 31, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Grinde, B. Herpesviruses: Latency and reactivation—Viral strategies and host response. J. Oral Microbiol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Spear, P.G. Infections with herpes simplex viruses (2). N. Engl. J. Med. 1986, 314, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Jmor, F.; Emsley, H.C.; Fischer, M.; Solomon, T.; Lewthwaite, P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol. J. 2008, 5, 134. [Google Scholar] [CrossRef]
- Bodilsen, J.; Storgaard, M.; Larsen, L.; Wiese, L.; Helweg-Larsen, J.; Lebech, A.M.; Brandt, C.; Ostergaard, C.; Nielsen, H.; DASGIB Study Group. Infectious meningitis and encephalitis in adults in Denmark: A prospective nationwide observational cohort study (DASGIB). Clin. Microbiol. Infect. 2018, 24, 1102.e1–1102.e5. [Google Scholar] [CrossRef]
- Studahl, M.; Hagberg, L.; Rekabdar, E.; Bergstrom, T. Herpesvirus DNA detection in cerebral spinal fluid: Differences in clinical presentation between alpha-, beta-, and gamma-herpesviruses. Scand. J. Infect. Dis. 2000, 32, 237–248. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Kleinschmidt-DeMasters, B.K.; Weinberg, A.; Tyler, K.L. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J. Clin. Virol. 2002, 25 (Suppl. S1), S5–S11. [Google Scholar] [CrossRef]
- Whitley, R.J.; Alford, C.A.; Hirsch, M.S.; Schooley, R.T.; Luby, J.P.; Aoki, F.Y.; Hanley, D.; Nahmias, A.J.; Soong, S.J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 1986, 314, 144–149. [Google Scholar] [CrossRef]
- Jubelt, B.; Mihai, C.; Li, T.M.; Veerapaneni, P. Rhombencephalitis/brainstem encephalitis. Curr. Neurol. Neurosci. Rep. 2011, 11, 543–552. [Google Scholar] [CrossRef]
- Shukla, B.; Aguilera, E.A.; Salazar, L.; Wootton, S.H.; Kaewpoowat, Q.; Hasbun, R. Aseptic meningitis in adults and children: Diagnostic and management challenges. J. Clin. Virol. 2017, 94, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, I.; Sellier, P.; Lopes, A.; Morgand, M.; Makovec, T.; Delcey, V.; Champion, K.; Simoneau, G.; Green, A.; Mouly, S.; et al. Etiologies and Management of Aseptic Meningitis in Patients Admitted to an Internal Medicine Department. Medicine 2016, 95, e2372. [Google Scholar] [CrossRef]
- Mollaret, P. La meningite endothelio-leucocytaire multirecurrente benigne: Syndrome nouveau ou maladie nouvelle? Presentation de deux malades. Bull. Soc. Med. Hop. Paris 1944, 60, 121–122. [Google Scholar]
- Shalabi, M.; Whitley, R.J. Recurrent benign lymphocytic meningitis. Clin. Infect. Dis. 2006, 43, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Melchjorsen, J.; Matikainen, S.; Paludan, S.R. Activation and evasion of innate antiviral immunity by herpes simplex virus. Viruses 2009, 1, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Verzosa, A.L.; McGeever, L.A.; Bhark, S.J.; Delgado, T.; Salazar, N.; Sanchez, E.L. Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms. Front. Immunol. 2021, 12, 644664. [Google Scholar] [CrossRef]
- Whisnant, A.W.; Jürges, C.S.; Hennig, T.; Wyler, E.; Prusty, B.; Rutkowski, A.J.; L’Hernault, A.; Djakovic, L.; Göbel, M.; Döring, K.; et al. Integrative functional genomics decodes herpes simplex virus 1. Nat. Commun. 2020, 11, 2038. [Google Scholar] [CrossRef]
- Dolan, A.; Jamieson, F.E.; Cunningham, C.; Barnett, B.C.; McGeoch, D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998, 72, 2010–2021. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Doyle, S.; Vaidya, S.; O’Connell, R.; Dadgostar, H.; Dempsey, P.; Wu, T.; Rao, G.; Sun, R.; Haberland, M.; Modlin, R.; et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 2002, 17, 251–263. [Google Scholar] [CrossRef]
- Cai, M.; Li, M.; Wang, K.; Wang, S.; Lu, Q.; Yan, J.; Mossman, K.L.; Lin, R.; Zheng, C. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-kappaB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS ONE 2013, 8, e54586. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Sorensen, L.N.; Malmgaard, L.; Ank, N.; Baines, J.D.; Chen, Z.J.; Paludan, S.R. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007, 81, 13315–13324. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sato, S.; Ishii, K.J.; Coban, C.; Hemmi, H.; Yamamoto, M.; Terai, K.; Matsuda, M.; Inoue, J.; Uematsu, S.; et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004, 5, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Macmillan, J.B.; Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Ablasser, A.; Bauernfeind, F.; Hartmann, G.; Latz, E.; Fitzgerald, K.A.; Hornung, V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009, 10, 1065–1072. [Google Scholar] [CrossRef]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.J.; Sparrer, K.M.J.; van Gent, M.; Lassig, C.; Huang, T.; Osterrieder, N.; Hopfner, K.P.; Gack, M.U. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat. Immunol. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzozka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef]
- Bartok, E.; Hartmann, G. Immune Sensing Mechanisms that Discriminate Self from Altered Self and Foreign Nucleic Acids. Immunity 2020, 53, 54–77. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef]
- Pindel, A.; Sadler, A. The role of protein kinase R in the interferon response. J. Interferon Cytokine Res. 2011, 31, 59–70. [Google Scholar] [CrossRef]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef]
- Malathi, K.; Dong, B.; Gale, M., Jr.; Silverman, R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 2007, 448, 816–819. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Lopusna, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vaegter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef]
- Lin, R.; Noyce, R.S.; Collins, S.E.; Everett, R.D.; Mossman, K.L. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 2004, 78, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Bodda, C.; Reinert, L.S.; Fruhwurth, S.; Richardo, T.; Sun, C.; Zhang, B.C.; Kalamvoki, M.; Pohlmann, A.; Mogensen, T.H.; Bergstrom, P.; et al. HSV1 VP1-2 deubiquitinates STING to block type I interferon expression and promote brain infection. J. Exp. Med. 2020, 217, e20191422. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.; Jensen, S.B.; Miettinen, J.J.; Luecke, S.; Prabakaran, T.; Reinert, L.S.; Mettenleiter, T.; Chen, Z.J.; Knipe, D.M.; Sandri-Goldin, R.M.; et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J. 2016, 35, 1385–1399. [Google Scholar] [CrossRef]
- Johnson, K.E.; Song, B.; Knipe, D.M. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology 2008, 374, 487–494. [Google Scholar] [CrossRef]
- Poppers, J.; Mulvey, M.; Khoo, D.; Mohr, I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 2000, 74, 11215–11221. [Google Scholar] [CrossRef]
- Peters, G.A.; Khoo, D.; Mohr, I.; Sen, G.C. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 2002, 76, 11054–11064. [Google Scholar] [CrossRef]
- He, B.; Gross, M.; Roizman, B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Lussignol, M.; Esclatine, A. Herpesvirus and Autophagy: “All Right, Everybody Be Cool, This Is a Robbery!”. Viruses 2017, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Talloczy, Z.; Virgin, H.W.T.; Levine, B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2006, 2, 24–29. [Google Scholar] [CrossRef] [PubMed]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippe, R.; et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Yordy, B.; Iijima, N.; Huttner, A.; Leib, D.; Iwasaki, A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe 2012, 12, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Orvedahl, A.; Alexander, D.; Talloczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lussignol, M.; Queval, C.; Bernet-Camard, M.F.; Cotte-Laffitte, J.; Beau, I.; Codogno, P.; Esclatine, A. The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J. Virol. 2013, 87, 859–871. [Google Scholar] [CrossRef]
- Mauthe, M.; Langereis, M.; Jung, J.; Zhou, X.; Jones, A.; Omta, W.; Tooze, S.A.; Stork, B.; Paludan, S.R.; Ahola, T.; et al. An siRNA screen for ATG protein depletion reveals the extent of the unconventional functions of the autophagy proteome in virus replication. J. Cell Biol. 2016, 214, 619–635. [Google Scholar] [CrossRef]
- Garcia-Laorden, M.I.; Hernandez-Brito, E.; Munoz-Almagro, C.; Pavlovic-Nesic, S.; Rua-Figueroa, I.; Briones, M.L.; Rajas, O.; Borderias, L.; Payeras, A.; Lorente, L.; et al. Should MASP-2 Deficiency Be Considered a Primary Immunodeficiency? Relevance of the Lectin Pathway. J. Clin. Immunol. 2020, 40, 203–210. [Google Scholar] [CrossRef]
- Heja, D.; Harmat, V.; Fodor, K.; Wilmanns, M.; Dobo, J.; Kekesi, K.A.; Zavodszky, P.; Gal, P.; Pal, G. Monospecific inhibitors show that both mannan-binding lectin-associated serine protease-1 (MASP-1) and -2 Are essential for lectin pathway activation and reveal structural plasticity of MASP-2. J. Biol. Chem. 2012, 287, 20290–20300. [Google Scholar] [CrossRef]
- Gadjeva, M.; Paludan, S.R.; Thiel, S.; Slavov, V.; Ruseva, M.; Eriksson, K.; Lowhagen, G.B.; Shi, L.; Takahashi, K.; Ezekowitz, A.; et al. Mannan-binding lectin modulates the response to HSV-2 infection. Clin. Exp. Immunol. 2004, 138, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.F.; Friedman, H.M.; Cohen, G.H.; Eisenberg, R.J.; Hammer, C.H.; Frank, M.M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 1986, 137, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.J.A.; Randall, R.E.; Hambleton, S. Genetic Lesions of Type I Interferon Signalling in Human Antiviral Immunity. Trends Genet. 2021, 37, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Casrouge, A.; Zhang, S.Y.; Eidenschenk, C.; Jouanguy, E.; Puel, A.; Yang, K.; Alcais, A.; Picard, C.; Mahfoufi, N.; Nicolas, N.; et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 2006, 314, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef]
- Lim, H.K.; Seppanen, M.; Hautala, T.; Ciancanelli, M.J.; Itan, Y.; Lafaille, F.G.; Dell, W.; Lorenzo, L.; Byun, M.; Pauwels, E.; et al. TLR3 deficiency in herpes simplex encephalitis: High allelic heterogeneity and recurrence risk. Neurology 2014, 83, 1888–1897. [Google Scholar] [CrossRef]
- Guo, Y.; Audry, M.; Ciancanelli, M.; Alsina, L.; Azevedo, J.; Herman, M.; Anguiano, E.; Sancho-Shimizu, V.; Lorenzo, L.; Pauwels, E.; et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exp. Med. 2011, 208, 2083–2098. [Google Scholar] [CrossRef]
- Sancho-Shimizu, V.; Perez de Diego, R.; Lorenzo, L.; Halwani, R.; Alangari, A.; Israelsson, E.; Fabrega, S.; Cardon, A.; Maluenda, J.; Tatematsu, M.; et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J. Clin. Investig. 2011, 121, 4889–4902. [Google Scholar] [CrossRef]
- Perez de Diego, R.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A.; Plancoulaine, S.; Picard, C.; Herman, M.; Cardon, A.; Durandy, A.; Bustamante, J.; et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 2010, 33, 400–411. [Google Scholar] [CrossRef]
- Herman, M.; Ciancanelli, M.; Ou, Y.H.; Lorenzo, L.; Klaudel-Dreszler, M.; Pauwels, E.; Sancho-Shimizu, V.; Perez de Diego, R.; Abhyankar, A.; Israelsson, E.; et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 2012, 209, 1567–1582. [Google Scholar] [CrossRef]
- Niehues, T.; Reichenbach, J.; Neubert, J.; Gudowius, S.; Puel, A.; Horneff, G.; Lainka, E.; Dirksen, U.; Schroten, H.; Doffinger, R.; et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J. Allergy Clin. Immunol. 2004, 114, 1456–1462. [Google Scholar] [CrossRef]
- Puel, A.; Reichenbach, J.; Bustamante, J.; Ku, C.L.; Feinberg, J.; Doffinger, R.; Bonnet, M.; Filipe-Santos, O.; de Beaucoudrey, L.; Durandy, A.; et al. The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation. Am. J. Hum. Genet. 2006, 78, 691–701. [Google Scholar] [CrossRef]
- Audry, M.; Ciancanelli, M.; Yang, K.; Cobat, A.; Chang, H.H.; Sancho-Shimizu, V.; Lorenzo, L.; Niehues, T.; Reichenbach, J.; Li, X.X.; et al. NEMO is a key component of NF-kappaB- and IRF-3-dependent TLR3-mediated immunity to herpes simplex virus. J. Allergy Clin. Immunol. 2011, 128, 610–617. [Google Scholar] [CrossRef]
- Andersen, L.L.; Mork, N.; Reinert, L.S.; Kofod-Olsen, E.; Narita, R.; Jorgensen, S.E.; Skipper, K.A.; Honing, K.; Gad, H.H.; Ostergaard, L.; et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J. Exp. Med. 2015, 212, 1371–1379. [Google Scholar] [CrossRef]
- Naesens, L.; Muppala, S.; Acharya, D.; Nemegeer, J.; Bogaert, D.; Lee, J.H.; Staes, K.; Debacker, V.; De Bleser, P.; De Bruyne, M.; et al. GTF3A mutations predispose to herpes simplex encephalitis by disrupting biogenesis of the host-derived RIG-I ligand RNA5SP141. Sci. Immunol. 2022, 7, eabq4531. [Google Scholar] [CrossRef]
- Bastard, P.; Manry, J.; Chen, J.; Rosain, J.; Seeleuthner, Y.; AbuZaitun, O.; Lorenzo, L.; Khan, T.; Hasek, M.; Hernandez, N.; et al. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Investig. 2021, 131, e139980. [Google Scholar] [CrossRef]
- Dupuis, S.; Jouanguy, E.; Al-Hajjar, S.; Fieschi, C.; Al-Mohsen, I.Z.; Al-Jumaah, S.; Yang, K.; Chapgier, A.; Eidenschenk, C.; Eid, P.; et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003, 33, 388–391. [Google Scholar] [CrossRef]
- Bravo Garcia-Morato, M.; Calvo Apalategi, A.; Bravo-Gallego, L.Y.; Blazquez Moreno, A.; Simon-Fuentes, M.; Garmendia, J.V.; Mendez Echevarria, A.; Del Rosal Rabes, T.; Dominguez-Soto, A.; Lopez-Granados, E.; et al. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J. Allergy Clin. Immunol. 2019, 144, 309–312.e310. [Google Scholar] [CrossRef]
- Lafaille, F.G.; Harschnitz, O.; Lee, Y.S.; Zhang, P.; Hasek, M.L.; Kerner, G.; Itan, Y.; Ewaleifoh, O.; Rapaport, F.; Carlile, T.M.; et al. Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis. Nat. Med. 2019, 25, 1873–1884. [Google Scholar] [CrossRef]
- Bibert, S.; Piret, J.; Quinodoz, M.; Collinet, E.; Zoete, V.; Michielin, O.; Menasria, R.; Meylan, P.; Bihl, T.; Erard, V.; et al. Herpes simplex encephalitis in adult patients with MASP-2 deficiency. PLoS Pathog. 2019, 15, e1008168. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Clark, N.E.; Freije, C.A.; Pauwels, E.; Taggart, A.J.; Okada, S.; Mandel, H.; Garcia, P.; Ciancanelli, M.J.; Biran, A.; et al. Inborn Errors of RNA Lariat Metabolism in Humans with Brainstem Viral Infection. Cell 2018, 172, 952–965.e918. [Google Scholar] [CrossRef]
- Kreins, A.Y.; Ciancanelli, M.J.; Okada, S.; Kong, X.F.; Ramirez-Alejo, N.; Kilic, S.S.; El Baghdadi, J.; Nonoyama, S.; Mahdaviani, S.A.; Ailal, F.; et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J. Exp. Med. 2015, 212, 1641–1662. [Google Scholar] [CrossRef]
- Hait, A.S.; Olagnier, D.; Sancho-Shimizu, V.; Skipper, K.A.; Helleberg, M.; Larsen, S.M.; Bodda, C.; Moldovan, L.I.; Ren, F.; Brinck Andersen, N.S.; et al. Defects in LC3B2 and ATG4A underlie HSV2 meningitis and reveal a critical role for autophagy in antiviral defense in humans. Sci. Immunol. 2020, 5, 54. [Google Scholar] [CrossRef]
- Lafaille, F.G.; Pessach, I.M.; Zhang, S.Y.; Ciancanelli, M.J.; Herman, M.; Abhyankar, A.; Ying, S.W.; Keros, S.; Goldstein, P.A.; Mostoslavsky, G.; et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012, 491, 769–773. [Google Scholar] [CrossRef]
- Gao, D.; Ciancanelli, M.J.; Zhang, P.; Harschnitz, O.; Bondet, V.; Hasek, M.; Chen, J.; Mu, X.; Itan, Y.; Cobat, A.; et al. TLR3 controls constitutive IFN-beta antiviral immunity in human fibroblasts and cortical neurons. J. Clin. Investig. 2021, 131, eabc2691. [Google Scholar] [CrossRef]
- Hernandez, N.; Bucciol, G.; Moens, L.; Le Pen, J.; Shahrooei, M.; Goudouris, E.; Shirkani, A.; Changi-Ashtiani, M.; Rokni-Zadeh, H.; Sayar, E.H.; et al. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J. Exp. Med. 2019, 216, 2057–2070. [Google Scholar] [CrossRef]
- Meyts, I. Null IFNAR1 and IFNAR2 alleles are surprisingly common in the Pacific and Arctic. J. Exp. Med. 2022, 219, e20220491. [Google Scholar] [CrossRef]
- Duncan, C.J.; Mohamad, S.M.; Young, D.F.; Skelton, A.J.; Leahy, T.R.; Munday, D.C.; Butler, K.M.; Morfopoulou, S.; Brown, J.R.; Hubank, M.; et al. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci. Transl. Med. 2015, 7, 307ra154. [Google Scholar] [CrossRef]
- Duncan, C.J.A.; Skouboe, M.K.; Howarth, S.; Hollensen, A.K.; Chen, R.; Borresen, M.L.; Thompson, B.J.; Stremenova Spegarova, J.; Hatton, C.F.; Staeger, F.F.; et al. Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J. Exp. Med. 2022, 219, e20212427. [Google Scholar] [CrossRef]
- Dupuis, S.; Dargemont, C.; Fieschi, C.; Thomassin, N.; Rosenzweig, S.; Harris, J.; Holland, S.M.; Schreiber, R.D.; Casanova, J.L. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 2001, 293, 300–303. [Google Scholar] [CrossRef]
- Le Voyer, T.; Sakata, S.; Tsumura, M.; Khan, T.; Esteve-Sole, A.; Al-Saud, B.K.; Gungor, H.E.; Taur, P.; Jeanne-Julien, V.; Christiansen, M.; et al. Genetic, Immunological, and Clinical Features of 32 Patients with Autosomal Recessive STAT1 Deficiency. J. Immunol. 2021, 207, 133–152. [Google Scholar] [CrossRef]
- Sarrafzadeh, S.A.; Mahloojirad, M.; Casanova, J.L.; Badalzadeh, M.; Bustamante, J.; Boisson-Dupuis, S.; Pourpak, Z.; Nourizadeh, M.; Moin, M. A New Patient with Inherited TYK2 Deficiency. J. Clin. Immunol. 2020, 40, 232–235. [Google Scholar] [CrossRef]
- Mork, N.; Kofod-Olsen, E.; Sorensen, K.B.; Bach, E.; Orntoft, T.F.; Ostergaard, L.; Paludan, S.R.; Christiansen, M.; Mogensen, T.H. Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun. 2015, 16, 552–566. [Google Scholar] [CrossRef]
- Van der Biezen, E.A.; Jones, J.D. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 1998, 23, 454–456. [Google Scholar] [CrossRef]
- Borish, L.; Ayars, A.G.; Kirkpatrick, C.H. Common variable immunodeficiency presenting as herpes simplex virus encephalitis. J. Allergy Clin. Immunol. 2011, 127, 541–543. [Google Scholar] [CrossRef]
- Paludan, S.R.; Mogensen, T.H. Constitutive and latent immune mechanisms exert ‘silent’ control of virus infections in the central nervous system. Curr. Opin. Immunol. 2021, 72, 158–166. [Google Scholar] [CrossRef]
- Tang, S.; Patel, A.; Krause, P.R. Herpes simplex virus ICP27 regulates alternative pre-mRNA polyadenylation and splicing in a sequence-dependent manner. Proc. Natl. Acad. Sci. USA 2016, 113, 12256–12261. [Google Scholar] [CrossRef]
- Seppanen, M.; Lokki, M.L.; Lappalainen, M.; Hiltunen-Back, E.; Rovio, A.T.; Kares, S.; Hurme, M.; Aittoniemi, J. Mannose-binding lectin 2 gene polymorphism in recurrent herpes simplex virus 2 infection. Hum. Immunol. 2009, 70, 218–221. [Google Scholar] [CrossRef]
- Stengaard-Pedersen, K.; Thiel, S.; Gadjeva, M.; Moller-Kristensen, M.; Sorensen, R.; Jensen, L.T.; Sjoholm, A.G.; Fugger, L.; Jensenius, J.C. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N. Engl. J. Med. 2003, 349, 554–560. [Google Scholar] [CrossRef]
- Notarangelo, L.; Casanova, J.L.; Fischer, A.; Puck, J.; Rosen, F.; Seger, R.; Geha, R. International Union of Immunological Societies Primary Immunodeficiency diseases classification, c. Primary immunodeficiency diseases: An update. J. Allergy Clin. Immunol. 2004, 114, 677–687. [Google Scholar] [CrossRef]
- Verdu, P.; Barreiro, L.B.; Patin, E.; Gessain, A.; Cassar, O.; Kidd, J.R.; Kidd, K.K.; Behar, D.M.; Froment, A.; Heyer, E.; et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum. Mol. Genet. 2006, 15, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.L.; Abel, L. Human genetics of infectious diseases: Unique insights into immunological redundancy. Semin. Immunol. 2018, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Jouanguy, E.; Beziat, V.; Mogensen, T.H.; Casanova, J.L.; Tangye, S.G.; Zhang, S.Y. Human inborn errors of immunity to herpes viruses. Curr. Opin. Immunol. 2020, 62, 106–122. [Google Scholar] [CrossRef]
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef]
- Mace, E.M.; Hsu, A.P.; Monaco-Shawver, L.; Makedonas, G.; Rosen, J.B.; Dropulic, L.; Cohen, J.I.; Frenkel, E.P.; Bagwell, J.C.; Sullivan, J.L.; et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood 2013, 121, 2669–2677. [Google Scholar] [CrossRef]
- Almerigogna, F.; Fassio, F.; Giudizi, M.G.; Biagiotti, R.; Manuelli, C.; Chiappini, E.; Galli, L.; Romagnani, S.; De Martino, M. Natural killer cell deficiencies in a consecutive series of children with herpetic encephalitis. Int. J. Immunopathol. Pharmacol. 2011, 24, 231–238. [Google Scholar] [CrossRef]
- Lisco, A.; Hsu, A.P.; Dimitrova, D.; Proctor, D.M.; Mace, E.M.; Ye, P.; Anderson, M.V.; Hicks, S.N.; Grivas, C.; Hammoud, D.A.; et al. Treatment of Relapsing HPV Diseases by Restored Function of Natural Killer Cells. N. Engl. J. Med. 2021, 385, 921–929. [Google Scholar] [CrossRef]
- Salzer, U.; Warnatz, K.; Peter, H.H. Common variable immunodeficiency: An update. Arthritis Res. Ther. 2012, 14, 223. [Google Scholar] [CrossRef]
- Lourdes, L.S.; Daily, K.C. Common variable immunodeficiency syndrome in an adult. Lancet 2014, 383, 926. [Google Scholar] [CrossRef]
- Cummings, L.; Tucker, M.; Gibson, M.; Myers, A.; Pastinen, T.; Johnston, J.; Farrow, E.; Sampath, V. Rare Genetic Variants in Immune Genes and Neonatal Herpes Simplex Viral Infections. Pediatrics 2021, 147, e20200687. [Google Scholar] [CrossRef]
- Hodara, E.; Ong, P.Y. The Genetics of Eczema Herpeticum. Clin. Rev. Allergy Immunol. 2022, 63, 390–397. [Google Scholar] [CrossRef]
- Bin, L.; Malley, C.; Taylor, P.; Preethi Boorgula, M.; Chavan, S.; Daya, M.; Mathias, M.; Shankar, G.; Rafaels, N.; Vergara, C.; et al. Whole genome sequencing identifies novel genetic mutations in patients with eczema herpeticum. Allergy 2021, 76, 2510–2523. [Google Scholar] [CrossRef]
- Fox, L.E.; Locke, M.C.; Lenschow, D.J. Context Is Key: Delineating the Unique Functions of IFNα and IFNβ in Disease. Front. Immunol. 2020, 11, 606874. [Google Scholar] [CrossRef]
- Gao, L.; Bin, L.; Rafaels, N.M.; Huang, L.; Potee, J.; Ruczinski, I.; Beaty, T.H.; Paller, A.S.; Schneider, L.C.; Gallo, R.; et al. Targeted deep sequencing identifies rare loss-of-function variants in IFNGR1 for risk of atopic dermatitis complicated by eczema herpeticum. J. Allergy Clin. Immunol. 2015, 136, 1591–1600. [Google Scholar] [CrossRef]
- Gao, P.S.; Rafaels, N.M.; Hand, T.; Murray, T.; Boguniewicz, M.; Hata, T.; Schneider, L.; Hanifin, J.M.; Gallo, R.L.; Gao, L.; et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J. Allergy Clin. Immunol. 2009, 124, 507–513.e7. [Google Scholar] [CrossRef]
- Eaaswarkhanth, M.; Xu, D.; Flanagan, C.; Rzhetskaya, M.; Hayes, M.G.; Blekhman, R.; Jablonski, N.G.; Gokcumen, O. Atopic Dermatitis Susceptibility Variants in Filaggrin Hitchhike Hornerin Selective Sweep. Genome Biol. Evol. 2016, 8, 3240–3255. [Google Scholar] [CrossRef]
- Kim, B.E.; Bin, L.; Ye, Y.M.; Ramamoorthy, P.; Leung, D.Y.M. IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with Th2 cytokines to enhance HSV-1 replication. J. Investig. Dermatol. 2013, 133, 2678–2685. [Google Scholar] [CrossRef]
- Ishii, E. Hemophagocytic Lymphohistiocytosis in Children: Pathogenesis and Treatment. Front. Pediatr. 2016, 4, 47. [Google Scholar] [CrossRef]
- Canna, S.W.; Marsh, R.A. Pediatric hemophagocytic lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef]
- McKeone, D.J.; DeMartini, T.K.M.; Kavanagh, R.P.; Halstead, E.S. Case Report: Rapid Recognition and Immune Modulation of Secondary HLH Due to Disseminated HSV Infection. Front. Pediatr. 2021, 9, 681055. [Google Scholar] [CrossRef]
- Takehara, H.; Hirohata, K.; Mutoh, H.; Irisa, C.; Kakiuchi, S.; Nishimura, R.; Oka, A.; Takahashi, N. Critically Severe Case of Neonatal Herpes with High Viral Load and Hemophagocytic Syndrome. Tohoku J. Exp. Med. 2019, 247, 149–152. [Google Scholar] [CrossRef]
- States, V.A.; Kapp, M.E. Herpes simplex virus-1 triggered hemophagocytic lymphohistiocytosis in a patient with granulomatosis with polyangiitis. Autops. Case Rep. 2022, 12, e2021395. [Google Scholar] [CrossRef]
- Yabushita, T.; Yoshioka, S.; Koba, Y.; Ono, Y.; Hiramoto, N.; Tabata, S.; Itou, M.; Shimizu, N.; Tomii, K.; Ishikawa, T. Successful Treatment of Herpes Simplex Virus (HSV)-1-associated Hemophagocytic Lymphohistiocytosis (HLH) with Acyclovir: A Case Report and Literature Review. Intern. Med. 2017, 56, 2919–2923. [Google Scholar] [CrossRef]
- Yamada, K.; Yamamoto, Y.; Uchiyama, A.; Ito, R.; Aoki, Y.; Uchida, Y.; Nagasawa, H.; Kimura, H.; Ichiyama, T.; Fukao, T.; et al. Successful treatment of neonatal herpes simplex-type 1 infection complicated by hemophagocytic lymphohistiocytosis and acute liver failure. Tohoku J. Exp. Med. 2008, 214, 1–5. [Google Scholar] [CrossRef]
- Kurosawa, S.; Sekiya, N.; Fukushima, K.; Ikeuchi, K.; Fukuda, A.; Takahashi, H.; Chen, F.; Hasegawa, H.; Katano, H.; Hishima, T.; et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect. Dis. 2019, 19, 65. [Google Scholar] [CrossRef]
- Freytag, M.R.; Jorgensen, S.E.; Thomsen, M.M.; Al-Mousawi, A.; Hait, A.S.; Olagnier, D.; Bay, J.T.; Helleberg, M.; Mogensen, T.H. Postpartum Disseminated Herpes Simplex Virus Type 1 Infection With Hemophagocytic Lymphohistiocytosis and Fulminant Neonatal Herpes Infection. J. Infect. Dis. 2022, 225, 157–162. [Google Scholar] [CrossRef]
- Saettini, F.; Radaelli, S.; Ocello, L.; Ferrari, G.M.; Corti, P.; Dell’Acqua, F.; Ippolito, D.; Foresti, S.; Gervasini, C.; Badolato, R.; et al. Secondary hemophagocytic lymphohystiocytosis in a Rubinstein Taybi syndrome patient. Pediatr. Hematol. Oncol. 2022, 39, 74–79. [Google Scholar] [CrossRef]
- Spinner, M.A.; Ker, J.P.; Stoudenmire, C.J.; Fadare, O.; Mace, E.M.; Orange, J.S.; Hsu, A.P.; Holland, S.M. GATA2 deficiency underlying severe blastomycosis and fatal herpes simplex virus-associated hemophagocytic lymphohistiocytosis. J. Allergy Clin. Immunol. 2016, 137, 638–640. [Google Scholar] [CrossRef]
- Casanova, J.L. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E7128–E7137. [Google Scholar] [CrossRef]
- Casanova, J.L.; Conley, M.E.; Seligman, S.J.; Abel, L.; Notarangelo, L.D. Guidelines for genetic studies in single patients: Lessons from primary immunodeficiencies. J. Exp. Med. 2014, 211, 2137–2149. [Google Scholar] [CrossRef]
- Casanova, J.L.; Abel, L. From rare disorders of immunity to common determinants of infection: Following the mechanistic thread. Cell 2022, 185, 3086–3103. [Google Scholar] [CrossRef]
- Gros, P.; Casanova, J.L. Reconciling Mouse and Human Immunology at the Altar of Genetics. Annu. Rev. Immunol. 2022, 219, e20220028. [Google Scholar] [CrossRef]
- Bastard, P.; Hsiao, K.C.; Zhang, Q.; Choin, J.; Best, E.; Chen, J.; Gervais, A.; Bizien, L.; Materna, M.; Harmant, C.; et al. A loss-of-function IFNAR1 allele in Polynesia underlies severe viral diseases in homozygotes. J. Exp. Med. 2022, 219, e20220028. [Google Scholar] [CrossRef]
- Gough, D.J.; Messina, N.L.; Clarke, C.J.; Johnstone, R.W.; Levy, D.E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 2012, 36, 166–174. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, M.L.; Zhao, J. Crosstalk between Autophagy and Type I Interferon Responses in Innate Antiviral Immunity. Viruses 2019, 11, 132. [Google Scholar] [CrossRef]
- Brinck Andersen, N.S.; Jorgensen, S.E.; Skipper, K.A.; Larsen, S.M.; Heinz, J.; Thomsen, M.M.; Farahani, E.; Cai, Y.; Hait, A.S.; Kay, L.; et al. Essential role of autophagy in restricting poliovirus infection revealed by identification of an ATG7 defect in a poliomyelitis patient. Autophagy 2021, 17, 2449–2464. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).