Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Development and Production of Phyto-Bomat
2.2. Treatment Protocol
2.3. Clinical Cure Rate of Phyto-Bomat
2.4. Bacteriological Cure Rate of Phyto-Bomat
2.4.1. Isolation and Identification of the Causative Agents
2.4.2. Total Bacterial Count and Somatic Cell Count
2.5. Statistical Analysis
3. Results
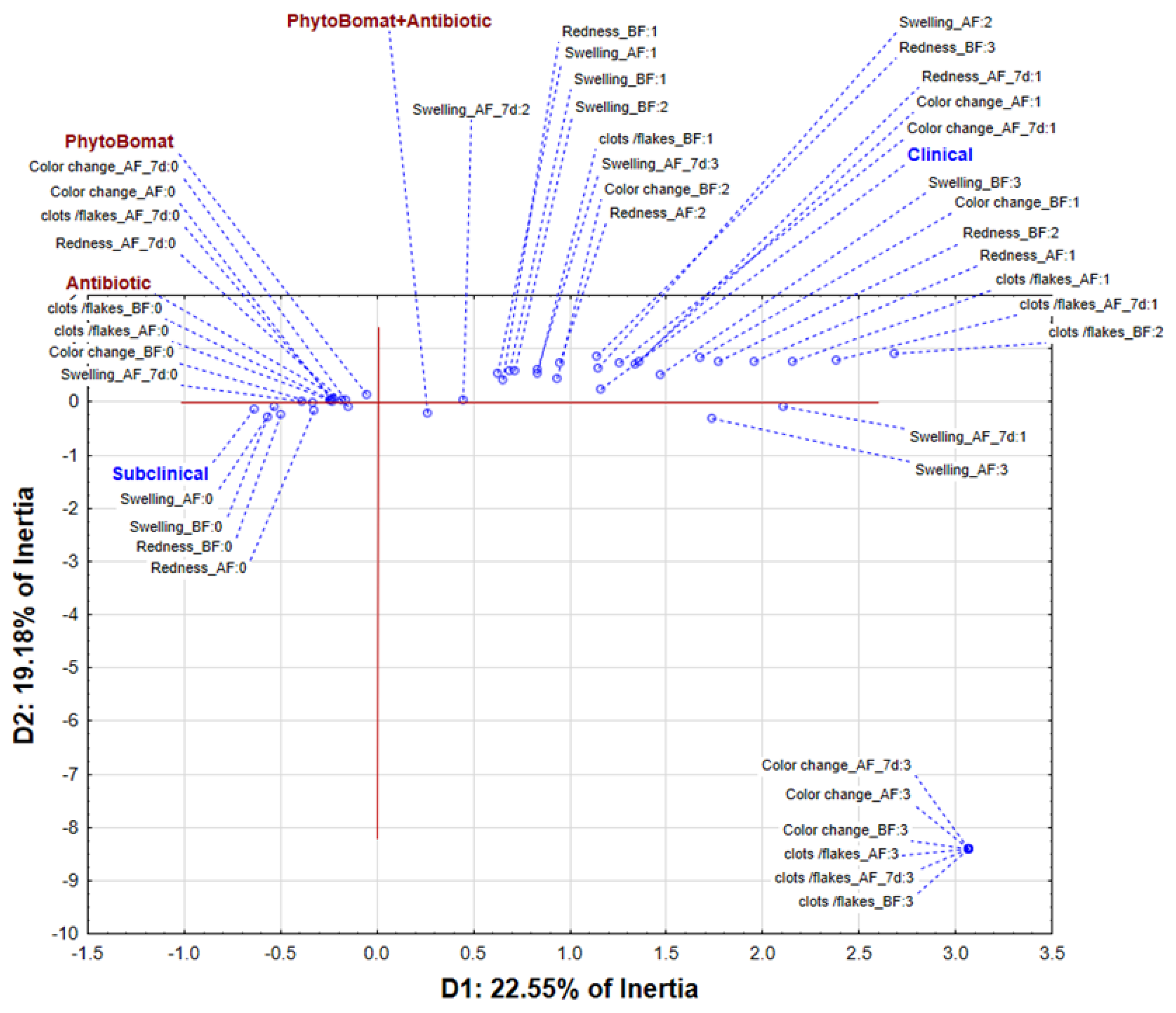
3.1. Clinical Cure Rate of Phyto-Bomat
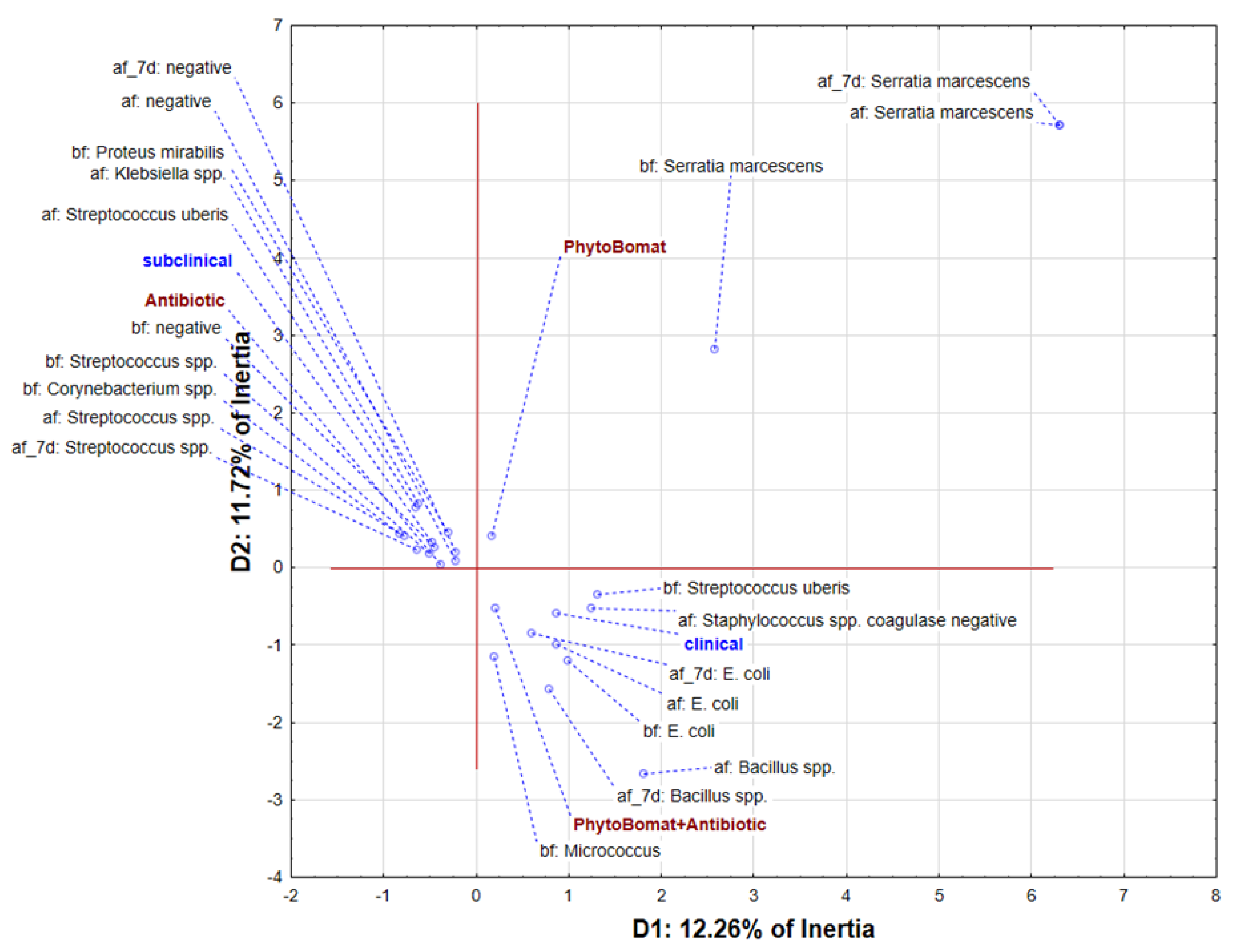
3.2. Bacteriological Cure Rate of Phyto-Bomat
3.2.1. Bacterial Isolation
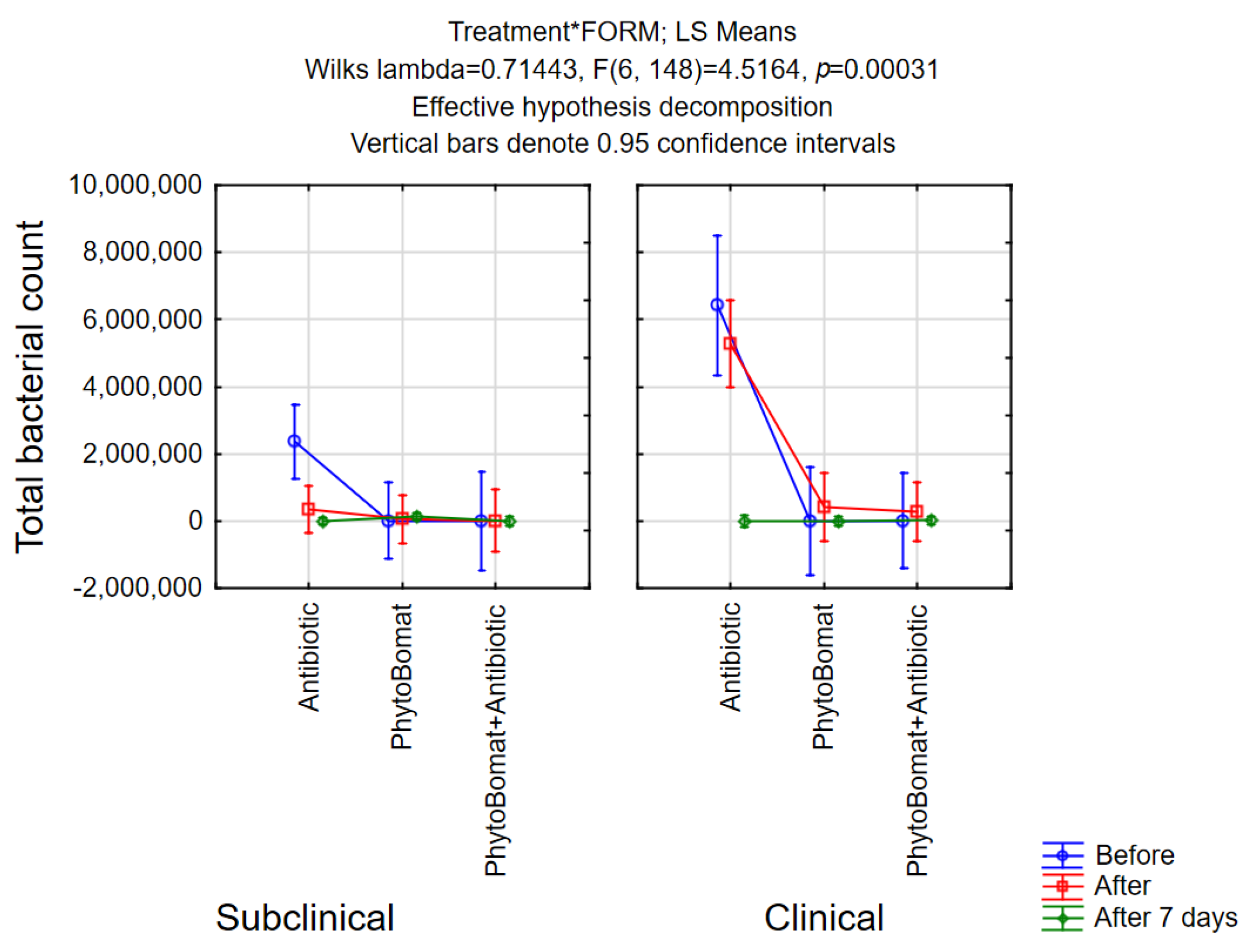
3.2.2. Total Bacterial Count
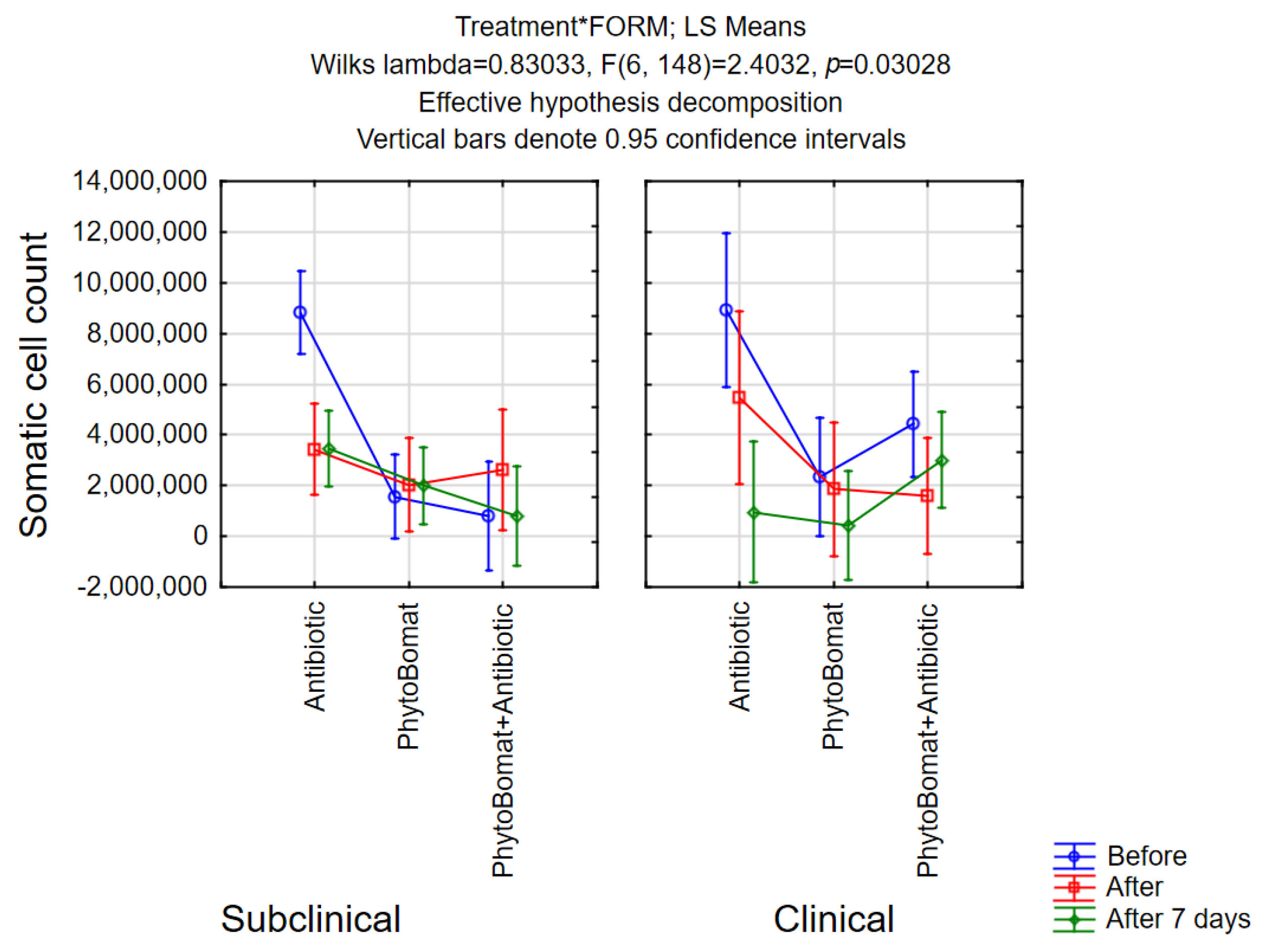
3.2.3. Somatic Cell Count
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D. Comparison of different approaches to antibiotic restriction in food-producing animals: Stratified results from a systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001710. [Google Scholar] [CrossRef]
- Firth, C.L.; Kremer, K.; Werner, T.; Käsbohrer, A. The effects of feeding waste milk containing antimicrobial residues on dairy calf health. Pathogens 2021, 10, 112. [Google Scholar] [CrossRef]
- Lloyd, D.H.; Page, S.W. Antimicrobial stewardship in veterinary medicine. Microbiol. Spectr. 2018, 6, 3–6. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2021, 170, 106103. [Google Scholar] [CrossRef]
- Cooper, B.; Okello, W.O. An economic lens to understanding antimicrobial resistance: Disruptive cases to livestock and wastewater management in Australia. Aust. J. Agric. Resour. Econ. 2021, 65, 900–917. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Veterinary Use (CVMP). Reflection Paper on Antimicrobial resistance in the Environment: Considerations for Current and Future Risk Assessment of Veterinary Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-antimicrobial-resistance-environment-considerations-current-future-risk-assessment_en.pdf (accessed on 25 September 2022).
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Cagnardi, P.; Grilli, G.; Villa, R.; Di Cesare, F.; Piccirillo, A. Antimicrobials in farm animals: Impact on the environment and consequent antimicrobial resistance dissemination. Int. J. Health Anim. Sci. Food Saf. 2018, 5, 22–22. [Google Scholar]
- Li, Z.; Hu, Y.; Yang, Y.; Lu, Z.; Wang, Y. Antimicrobial resistance in livestock: Antimicrobial peptides provide a new solution for a growing challenge. Anim. Front. 2018, 8, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Use, E.C.f.M.P.f.V.; Hazards, E.P.o.B.; Murphy, D.; Ricci, A.; Auce, Z.; Beechinor, J.G.; Bergendahl, H.; Breathnach, R.; Bureš, J.; Duarte Da Silva, J.P.; et al. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar]
- Kovacevic, Z.; Blagojevic, B.; Suran, J.; Horvat, O. Mapping knowledge and comprehension of antimicrobial stewardship and biosecurity among veterinary students. PLoS ONE 2020, 15, e0235866. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Loh, B.; Leptihn, S. Will the overuse of antibiotics during the Coronavirus pandemic accelerate antimicrobial resistance of bacteria? Infect. Microbes Dis. 2020, 2, 87–88. [Google Scholar] [CrossRef]
- Avraam, C.; Lambrou, A.S.; Jiang, W.; Siddiqui, S. Antimicrobial Resistance and Livestock Trade for Low and Middle Income Countries: Regional Analysis of Global Coordination Policies. Front. Sustain. Food Syst. 2021, 5, 650315. [Google Scholar] [CrossRef]
- Ajose, D.J.; Oluwarinde, B.O.; Abolarinwa, T.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Combating Bovine Mastitis in the Dairy Sector in an Era of Antimicrobial Resistance: Ethno-veterinary Medicinal Option as a Viable Alternative Approach. Front. Vet. Sci. 2022, 9, 800322. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments-A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S. Indicators of inflammation in the diagnosis of mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Hillerton, J.E.; Kliem, K.E. Effective treatment of Streptococcus uberis clinical mastitis to minimize the use of antibiotics. J. Dairy Sci. 2002, 85, 1009–1014. [Google Scholar] [CrossRef]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Schwarz, S.; Kehrenberg, C.; Walsh, T. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Pașca, C.; Mărghitaș, L.A.; Dezmirean, D.S.; Matei, I.A.; Bonta, V.; Pașca, I.; Chirilă, F.; Cîmpean, A.; Fiț, N.I. Efficacy of natural formulations in bovine mastitis pathology: Alternative solution to antibiotic treatment. J. Vet. Res. 2020, 64, 523–529. [Google Scholar] [CrossRef]
- El-Aziz, A.; Norhan, K.; Ammar, A.M.; El-Naenaeey, E.-s.Y.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and antibiofilm potentials of cinnamon oil and silver nanoparticles against Streptococcus agalactiae isolated from bovine mastitis: New avenues for countering resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Abidin, S.Z.U.; Munem, A.; Khan, R.; Batiha, G.E.S.; Amhad, M.; Zafar, M.; Khalil, A.A.K.; Hetta, H.F.; Mahmoud, M.H.; Sami, A. Ethnoveterinary botanical survey of medicinal plants used in Pashto, Punjabi and Saraiki communities of Southwest Pakistan. Vet. Med. Sci. 2021, 7, 2068–2085. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Rahman, I.U.; Calixto, E.S.; Ali, N.; Ijaz, F. Ethnoveterinary therapeutic practices and conservation status of the medicinal flora of Chamla Valley, Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2019, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Chakale, M.V.; Asong, J.A.; Struwig, M.; Mwanza, M.; Aremu, A.O. Ethnoveterinary Practices and Ethnobotanical Knowledge on Plants Used against Cattle Diseases among Two Communities in South Africa. Plants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Hallier, A.; Noirot, V.; Medina, B.; Leboeuf, L.; Cavret, S. Development of a method to determine essential oil residues in cow milk. J. Dairy Sci. 2013, 96, 1447–1454. [Google Scholar] [CrossRef]
- Tomanić, D.Z.; Stanojević, J.B.; Galić, I.M.; Ružić, Z.N.; Kukurić, T.B.; Tešin, N.B.; Prpa, B.P.; Kovačević, Z.R. Review of trends in essential oils as alternatives to antibiotics in bovine mastitis treatment. Zb. Matice Srp. Prir. Nauk. 2022, 142, 47–60. [Google Scholar] [CrossRef]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New perspective of Origanum vulgare L. and Satureja montana L. essential oils as bovine mastitis treatment alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Kovačević, Z.; Tomanić, D.; Čabarkapa, I.; Šarić, L.; Stanojević, J.; Bijelić, K.; Galić, I.; Ružić, Z.; Erdeljan, M.; Kladar, N. Chemical Composition, Antimicrobial Activity, and Withdrawal Period of Essential Oil-Based Pharmaceutical Formulation in Bovine Mastitis Treatment. Int. J. Environ. Res. Public Health 2022, 19, 16643. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard document M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Local Tolerance of Intramammary Preparations in Cows. Available online: https://www.ema.europa.eu/en/local-tolerance-intramammary-preparations-cows (accessed on 1 June 2022).
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. Institute for Standardization of Serbia: Beograd, Serbia, 2014.
- ISO 13366-1:2008; Milk—Enumeration of Somatic Cells—Part 1: Microscopic Method (Reference Method). Institute for Standardization of Serbia: Beograd, Serbia, 2010.
- National Mastitis Council. Microbiological Procedures for the Diagnosis of Udder Infection, 3rd ed.; National Mastitis Council Inc: New Prague, MN, USA, 2004. [Google Scholar]
- Williamson, J.; Callaway, T.; Rollin, E.; Ryman, V. Association of Milk Somatic Cell Count with Bacteriological Cure of Intramammary Infection—A Review. Agriculture 2022, 12, 1437. [Google Scholar] [CrossRef]
- Piccinini, R.; Binda, E.; Belotti, M.; Casirani, G.; Zecconi, A. Comparison of blood and milk non-specific immune parameters in heifers after calving in relation to udder health. Vet. Res. 2005, 36, 747–757. [Google Scholar] [CrossRef]
- Fratini, F.; Casella, S.; Leonardi, M.; Pisseri, F.; Ebani, V.V.; Pistelli, L.; Pistelli, L. Antibacterial activity of essential oils, their blends and mixtures of their main constituents against some strains supporting livestock mastitis. Fitoterapia 2014, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Damte, D.; Lee, S.-J.; Kim, J.-C.; Park, S.-C. Antimicrobial activity of lemongrass and oregano essential oil against standard antibiotic resistant Staphylococcus aureus and field isolates from chronic mastitis cow. Int. J. Phytomedicine 2012, 4, 134. [Google Scholar]
- Baskaran, S.A.; Kazmer, G.; Hinckley, L.; Andrew, S.; Venkitanarayanan, K. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 2009, 92, 1423–1429. [Google Scholar] [CrossRef]
- Budri, P.E.; Silva, N.C.; Bonsaglia, E.C.; Júnior, A.F.; Júnior, J.A.; Doyama, J.T.; Gonçalves, J.L.; Santos, M.; Fitzgerald-Hughes, D.; Rall, V.L. Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J. Dairy Sci. 2015, 98, 5899–5904. [Google Scholar] [CrossRef]
- Szweda, P.; Zalewska, M.; Pilch, J.; Kot, B.; Milewski, S. Essential oils as potential anti-staphylococcal agents. Acta Vet. -Beograd 2018, 68, 95–107. [Google Scholar]
- Yang, W.-T.; Ke, C.-Y.; Wu, W.-T.; Lee, R.-P.; Tseng, Y.-H. Effective treatment of bovine mastitis with intramammary infusion of Angelica dahurica and Rheum officinale extracts. Evid. -Based Complement. Altern. Med. 2019, 2019, 7242705. [Google Scholar] [CrossRef]
- Cho, B.-W.; Cha, C.-N.; Lee, S.-M.; Kim, M.-J.; Park, J.-Y.; Yoo, C.-Y.; Son, S.-E.; Kim, S.; Lee, H.-J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Kammerer, M.; Le Guenic, M.; Roussel, P.; Linclau, O.; Cartaud, G.; Tainturier, D.; Larrat, M.; Bareille, N.N. Le traitement des mammites cliniques de la vache laitière par des huiles essentielles. Innov. Agron. 2009, 4, 79–83. [Google Scholar]
- Mullen, K.; Anderson, K.; Washburn, S. Effect of 2 herbal intramammary products on milk quantity and quality compared with conventional and no dry cow therapy. J. Dairy Sci. 2014, 97, 3509–3522. [Google Scholar] [CrossRef]
- Hase, P.; Digraskar, S.; Ravikanth, K.; Dandale, M.; Maini, S. Management of subclinical mastitis with mastilep gel and herbal spray (AV/AMS/15). Int. J. Pharm. Pharmacol. 2013, 4, 64–67. [Google Scholar]
- Pinedo, P.; Karreman, H.; Bothe, H.; Velez, J.; Risco, C. Efficacy of a botanical preparation for the intramammary treatment of clinical mastitis on an organic dairy farm. Can. Vet. J. 2013, 54, 479. [Google Scholar]
- Alekish, M.O.; Ismail, Z.B.; Awawdeh, M.S.; Shatnawi, S. Effects of intramammary infusion of sage (Salvia officinalis) essential oil on milk somatic cell count, milk composition parameters and selected hematology and serum biochemical parameters in Awassi sheep with subclinical mastitis. Vet. World 2017, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- McPhee, C.; Anderson, K.; Yeatts, J.; Mason, S.; Barlow, B.; Baynes, R. Hot topic: Milk and plasma disposition of thymol following intramammary administration of a phytoceutical mastitis treatment. J. Dairy Sci. 2011, 94, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S. Treatment of mastitis during lactation. Ir. Vet. J. 2009, 62, S40. [Google Scholar] [CrossRef]
- Abboud, M.; El Rammouz, R.; Jammal, B.; Sleiman, M. In vitro and in vivo antimicrobial activity of two essential oils Thymus vulgaris and Lavandula angustifolia against bovine Staphylococcus and Streptococcus mastitis pathogen. Middle East J. Agric. Res 2015, 4, 975–983. [Google Scholar]
- Knežević, K.; Dobranić, V.; Đuričić, D.; Samardžija, M.; Benić, M.; Getz, I.; Efendić, M.; Cvetnić, L.; Šavorić, J.; Butković, I. Use of somatic cell count in the diagnosis of mastitis and its impacts on milk quality. Vet. Stanica 2021, 52, 751–764. [Google Scholar] [CrossRef]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control-a review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- McDougall, S.; Clausen, L.M.; Hussein, H.M.; Compton, C.W. Therapy of subclinical mastitis during lactation. Antibiotics 2022, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Francoz, D.; Wellemans, V.; Dupré, J.; Roy, J.; Labelle, F.; Lacasse, P.; Dufour, S. Invited review: A systematic review and qualitative analysis of treatments other than conventional antimicrobials for clinical mastitis in dairy cows. J. Dairy Sci. 2017, 100, 7751–7770. [Google Scholar] [CrossRef]
- Ruegg, P. Management of mastitis on organic and conventional dairy farms. J. Anim. Sci. 2009, 87, 43–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanić, D.; Kladar, N.; Radinović, M.; Stančić, I.; Erdeljan, M.; Stanojević, J.; Galić, I.; Bijelić, K.; Kovačević, Z. Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use. Pathogens 2023, 12, 259. https://doi.org/10.3390/pathogens12020259
Tomanić D, Kladar N, Radinović M, Stančić I, Erdeljan M, Stanojević J, Galić I, Bijelić K, Kovačević Z. Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use. Pathogens. 2023; 12(2):259. https://doi.org/10.3390/pathogens12020259
Chicago/Turabian StyleTomanić, Dragana, Nebojša Kladar, Miodrag Radinović, Ivan Stančić, Mihajlo Erdeljan, Jovan Stanojević, Ivan Galić, Katarina Bijelić, and Zorana Kovačević. 2023. "Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use" Pathogens 12, no. 2: 259. https://doi.org/10.3390/pathogens12020259
APA StyleTomanić, D., Kladar, N., Radinović, M., Stančić, I., Erdeljan, M., Stanojević, J., Galić, I., Bijelić, K., & Kovačević, Z. (2023). Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use. Pathogens, 12(2), 259. https://doi.org/10.3390/pathogens12020259






