The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Eimeria spp. Oocysts Isolation
2.2. Alcoholic Plant Extracts
2.3. Experimental Design
2.4. Statistical Analysis and Ontologies
3. Results
3.1. Analysis of Plant Extracts
3.2. In Vitro Antiparasitic Activity of APE against Eimeria spp. Oocysts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Singh, N.K.; Singh, H.; Joachim, A.; Rath, S.S.; Blake, D.P. Discrimination, molecular characterisation and phylogenetic comparison of porcine Eimeria spp. in India. Vet. Parasitol. 2018, 255, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Daugschies, A.; Imarom, S.; Ganter, M.; Bollwahn, W. Prevalence of Eimeria spp. in Sows at Piglet-producing Farms in Germany. J. Vet. Med. Ser. B 2004, 51, 135–139. [Google Scholar] [CrossRef]
- Abdu, S.; Gashaw, A. Production system dynamism and parasitic interaction of swine in and around Holetta, Ethiopia. Ethiop. Vet. J. 2010, 14, 71–82. [Google Scholar]
- Karamon, J.; Ziomko, I.; Cencek, T. Prevalence of Isospora suis and Eimeria spp. in suckling piglets and sows in Poland. Vet. Parasitol. 2007, 147, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kagira, J.M.; Githigia, S.M.; Nganga, J.C.; Kanyari, P.W.N.; Maingi, N.; Gachohi, J. Prevalence of gastrointestinal protozoa and association with risk factors in free-range pigs in Kenya. J. Protozool. Res. 2010, 20, 1–9. [Google Scholar]
- Zhang, W.J.; Xu, L.H.; Liu, Y.Y.; Xiong, B.Q.; Zhang, Q.L.; Li, F.C.; Zhao, J. Prevalence of coccidian infection in suckling piglets in China. Vet. Parasitol. 2012, 190, 51–55. [Google Scholar] [CrossRef]
- McDonald, V.; Shirley, M.W. Past and future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef]
- Eckert, J.; Braun, R.; Shirley, M.W.; Coudert, P. COST 89/820: Biotechnology: Guidelines on Techniques in Coccidiosis Research; European Commission: Luxembourg, 1995; pp. 103–118.
- Joachim, A.; Schwarz, L. Coccidia of swine: Eimeria species, Cystoisospora (syn. Isospora) suis. In Encyclopedia of Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mai, K.; Sharman, P.A.; Walker, R.A.; Katrib, M.; Souza, D.D.; McConville, M.J.; Wallach, M.G.; Belli, S.I.; Ferguson, D.J.; Smith, N.C. Oocyst wall formation and composition in coccidian parasites. Mem. Inst. Oswaldo Cruz 2009, 104, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.X.; Li, B.M.; Zhang, Q.; Lin, B.Z.; Ge, L.P.; Wang, C.Y.; Cao, W. Disinfection effectiveness of slightly acidic electrolysed water in swine barns. J. Appl. Microbiol. 2013, 115, 703–710. [Google Scholar] [CrossRef]
- Junior, J.S.G.; Bogado, A.L.G.; Da Cunha, T.C.B.; Garcia, J.L. In vitro evaluation of the disinfection efficacy on Eimeria tenella unsporulated oocysts isolated from broilers. Rev. Bras. Parasitol. Veterinária 2007, 16, 67–71. [Google Scholar]
- Bajwa, R.S.; Gill, B.S. Effect of irradiation (gamma rays) on the biology of Eimeria tenella oocysts. Ann. Rech. Vet. 1977, 8, 181–186. [Google Scholar]
- Williams, R.B. Laboratory tests of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet. Rec. 1997, 141, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Daugschies, A.; Böse, R.; Marx, J.; Teich, K.; Friedhoff, K.T. Development and application of a standardized assay for chemical disinfection of coccidia oocysts. Vet. Parasitol. 2002, 103, 299–308. [Google Scholar] [CrossRef]
- Liou, C.T.; Wang, J.S.; Ooi, H.K. Effect of ozone treatment on Eimeria colchici oocysts. J. Parasitol. 2002, 88, 159–162. [Google Scholar] [CrossRef]
- Li, M.H.; Ooi, H.K. Effect of chromium compounds on sporulation of Eimeria piriformis oocysts. Exp. Anim. 2008, 57, 79–83. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Tzora, A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Ehtisham-ul-Haque, S. In vitro Efficacy of Herbal Extracts against Eimeria tenella. Int. J. Agric. Biol. 2015, 17, 848–850. [Google Scholar] [CrossRef]
- Gadelhaq, S.M.; Arafa, W.M.; Abolhadid, S.M. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet. Parasitol. 2018, 251, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Alnassan, A.A.; Thabet, A.; Daugschies, A.; Bangoura, B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 3913–3915. [Google Scholar] [CrossRef]
- Fatemi, A.; Razavi, S.M.; Asasi, K.; Torabi Goudarzi, M. Effects of Artemisia annua extracts on sporulation of Eimeria oocysts. Parasitol. Res. 2015, 114, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Remmal, A.; Achahbar, S.; Bouddine, L.; Chami, N.; Chami, F. In vitro destruction of Eimeria oocysts by essential oils. Vet. Parasitol. 2011, 182, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Papatsiros, V.G. Impact of animal health management on organic pig farming in Greece. Biotechnol. Anim. Husb. 2011, 27, 115–125. [Google Scholar] [CrossRef]
- Mircean, V.; Cozma, V.; Gyorke, A. Diagnostic Coproscopic in Bolile Parazitare la Animale (Coproparasitological Diagnostic in Parasitic Diseases in Animals); Risoprint: Cluj-Napoca, Romania, 2011; pp. 23–35. [Google Scholar]
- Felici, M.; Tugnoli, B.; Piva, A.; Grilli, E. In Vitro Assessment of Anticoccidials: Methods and Molecules. Animals 2021, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Bǎieş, M.H.; Gherman, C.; Boros, Z.; Olah, D.; Vlase, A.M.; Cozma-Petrut, A.; Györke, A.; Miere, D.; Vlase, L.; Crişan, G.; et al. The Effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the Embryogenesis of Ascaris suum Eggs during an In Vitro Experimental Study. Pathogens 2022, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Debbou-Iouknane, N.; Nerín, C.; Amrane-Abider, M.; Ayad, A. In vitro anticoccidial effects of Olive Leaf (Olea europaea L. var. Chemlal) extract against broiler chickens Eimeria oocysts. Vet. Zootech. 2021, 79, 1–8. [Google Scholar]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced Pharm Bull 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Ramírez, C.; Ibarra, F.; Pérez, H.I.; Manjarrez, N.; Salgado, H.J.; Ortega, L. Assessment and determination of LC50 of carvacrol and salicylic acid analogues with acaricide activity in larvae and adult ticks of Rhipicephalus (Boophilus) microplus. Parasite Epidemiol. Control 2016, 1, 72–77. [Google Scholar] [CrossRef]
- Habibi, H.; Firouzi, S.; Nili, H.; Razavi, M.; Asadi, S.L.; Daneshi, S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: In vitro and in vivo study. J Parasit. Dis. 2016, 40, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.C.; Danforth, H.D.; Augustine, P.C. Dietary modulation of avian coccidiosis. Int. J. Parasitol. 1998, 28, 1131–1140. [Google Scholar] [CrossRef]
- Mo, P.; Ma, Q.; Zhao, X.; Cheng, N.; Tao, J.; Li, J. Apoptotic effects of antimalarial artemisinin on the second generation merozoites of Eimeria tenella and parasitized host cells. Vet. Parasitol. 2014, 206, 297–303. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Abudabos, A. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ. Sci. Pollut. Res. 2019, 26, 14194–14199. [Google Scholar] [CrossRef]
- Nahed, A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; Elbestawy, A.R. Phytochemical control of poultry coccidiosis: A review. Poult. Sci. 2022, 101, 101542. [Google Scholar]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bauri, R.K.; Tigga, M.N.; Kullu, S.S. A review on use of medicinal plants to control parasites. Indian J. Nat. Prod. Resour. 2015, 6, 268–277. [Google Scholar]
- Ahmed, S.A. In vitro effects of aqueous extracts of garlic (Allium sativum) and onion (Allium cepa) on Trichomonas vaginalis. Parasitol. Unit. J. 2010, 3, 45–54. [Google Scholar]
- Udo, E.J.; Abba, A.M. Comparative Study of in-vitro anticoccidial efficacy of Allium sativum and Carica papaya. J. Zool. 2018, 2, 10–14. [Google Scholar]
- Batiha, G.E.S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Rivero-Perez, N. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Titilincu, A.; Santha, B.; Cozma, V. Effects of Polioel 3 on sporulation and infectivity of Eimeria oocysts. Lucr. Ştiinłifice Med. Vet. 2008, 41, 372–378. [Google Scholar]
- Seidavi, A.; Tavakoli, M.; Slozhenkina, M.; Gorlov, I.; Hashem, N.M.; Asroosh, F.; Swelum, A.A. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: A review. Environ. Sci. Pollut. Res. 2011, 28, 47856–47868. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Kostadinović, L.M.; Puvača, N.M.; Popović, S.J.; Urošević, M.I. Phytochemical constituents of selected plants from Apiaceae family and their biological effects in poultry. Food Feed Res. 2016, 43, 35–41. [Google Scholar] [CrossRef]
- Momin, A.H.; Acharya, S.S.; Gajjar, A.V. Coriandrum sativum-review of advances in phytopharmacology. Int. J. Pharm. Sci. Rev. Res. 2012, 3, 1233. [Google Scholar]
- Pop, L.M.; Varga, E.; Coroian, M.; Nedișan, M.E.; Mircean, V.; Dumitrache, M.O.; Györke, A. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites Vectors 2019, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Herrera, I.; Satyal, P.; Setzer, W.N. In-vitro evaluation of 52 commercially-available essential oils against Leishmania amazonensis. Molecules 2019, 24, 1248. [Google Scholar] [CrossRef] [PubMed]
- Rondon, F.C.; Bevilaqua, C.M.; Accioly, M.P.; Morais, S.M.; Andrade-Junior, H.F.; Machado, L.K.; Rodrigues, A.C.M. In vitro effect of Aloe vera, Coriandrum sativum and Ricinus communis fractions on Leishmania infantum and on murine monocytic cells. Vet. Parasitol. 2011, 178, 235–240. [Google Scholar] [CrossRef]
- Obiad, H.M.; Al-Alousi, T.I.; Al-Jboori, A.H. An epidemiologic study on Cryptosporidium spp. in Kirkuk city with some trials for in vitro treating the parasite. In the Second Scientific Conference; Science College, Tikrit University: Tikrit, Iraq, 2012. [Google Scholar]
- Boros, Z.; Baies, M.H.; Gherman, C.; Cozma, V. The effects of Artemisia absinthium (wormwood), Allium sativum (garlic), Cucurbita pepo (pumpkin), and Coriandrum sativum (coriander) on Trichinella spiralis and Trichinella britovi larvae, in vitro study. Sci Parasitol. 2021, 22, 70–78. [Google Scholar]
- Macedo, I.T.F.; Oliveira, L.M.B.D.; Camurça-Vasconcelos, A.L.F.; Ribeiro, W.L.C.; Santos, J.M.L.D.; Morais, S.M.D.; Bevilaqua, C.M.L. In vitro effects of Coriandrum sativum, Tagetes minuta, Alpinia zerumbet and Lantana camara essential oils on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2013, 22, 463–469. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.; Cave, G.W.; Morrison, A.A.; Elsheikha, H.M. Nematocidal effects of a coriander essential oil and five pure principles on the infective larvae of major ovine gastrointestinal nematodes in vitro. Pathogens 2020, 9, 740. [Google Scholar] [CrossRef]
- Kinyua, C.M. Modelling and Application of Response Surface Methodology for Optimization of Weight Gain of Eight Weeks Old Kenbro Served with Pumpkin (Cucurbita pepo L.) Seeds Extract. Ph.D. Thesis, Chuka University, Chuka, Kenya, 2019. [Google Scholar]
- Etewa, S.; Abaza, S. Herbal medicine and parasitic diseases. Parasitol. United J. 2011, 4, 3–14. [Google Scholar]
- Elhadi, I.M.; Koko, W.S.; Dahab, M.M.; El Imam, Y.M.; El Mageed, M.A. Antigiardial activity of some Cucurbita species and Lagenaria siceraria. Lab. Anim. 2013, 3, 8. [Google Scholar]
- Salman, S.S.; Ardalan, N.M. Evaluation of Amygdalin (B17) and Cucurbita pepo (Pumpkin seed) Activity Against Blastocystis from Diarrheic Patients in Baghdad, Iraq: In Vitro Study. Baghdad Sci. J. 2022, 19, 0016–0016. [Google Scholar]
- Grabensteiner, E.; Liebhart, D.; Arshad, N.; Hess, M. Antiprotozoal activities determined in vitro and in vivo of certain plant extracts against Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. Parasitol. Res. 2008, 103, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Microbiol. 2011, 3, 194. [Google Scholar] [PubMed]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Grilli, E. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult. Sci. 2020, 99, 5350–5355. [Google Scholar] [CrossRef] [PubMed]
- Isakakroudi, N.; Talebi, A.; Allymehr, M.; Tavassoli, M. Effects of essential oils combination on sporulation of turkey (Meleagris gallopavo) Eimeria oocysts. Arch. Razi Inst. 2018, 73, 113–120. [Google Scholar]
- Sausserde, R.; Kampuss, K. Composition of carotenoids in calendula (Calendula officinalis L.) flowers. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being”, Jelgava, Latvia, 8–9 May 2014; pp. 13–18. [Google Scholar]
- Ashwlayanvd, K.A.; Verma, M. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. 2018, 6, 149–155. [Google Scholar]
- Nikmehr, B.; Ghaznavi, H.; Rahbar, A.; Sadr, S.; Mehrzadi, S. In vitro anti-leishmanial activity of methanolic extracts of Calendula officinalis flowers, Datura stramonium seeds, and Salvia officinalis leaves. Chin. J. Nat. Med. 2014, 12, 423–427. [Google Scholar] [CrossRef]
- Doligalska, M.; Jóźwicka, K.; Kiersnowska, M.; Mroczek, A.; Pączkowski, C.; Janiszowska, W. Triterpenoid saponins affect the function of P-glycoprotein and reduce the survival of the free-living stages of Heligmosomoides bakeri. Vet. Parasitol. 2011, 179, 144–151. [Google Scholar] [CrossRef]
- Szakiel, A.; Ruszkowski, D.; Grudniak, A.; Kurek, A.; Wolska, K.I.; Doligalska, M.; Janiszowska, W. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis). Planta Med. 2008, 74, 1709–1715. [Google Scholar] [CrossRef]
- Boyko, O.; Brygadyrenko, V. Nematicidal activity of essential oils of medicinal plants. Folia Oecologica 2021, 48, 42–48. [Google Scholar] [CrossRef]
- Von Borell, E.; Sørensen, J.T. Organic livestock production in Europe: Aims, rules and trends with special emphasis on animal health and welfare. Livest. Prod. Sci. 2004, 90, 3–9. [Google Scholar] [CrossRef]
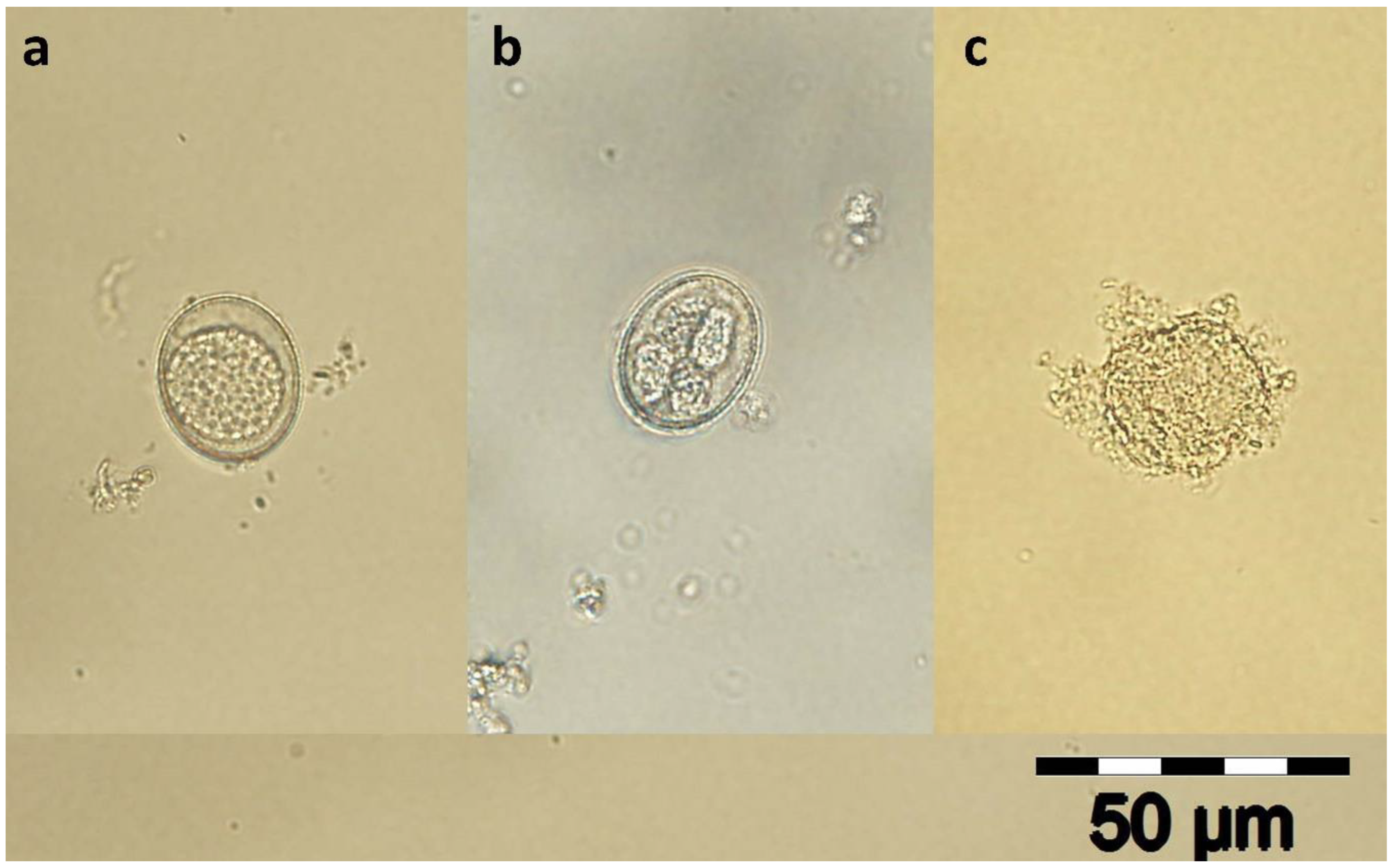
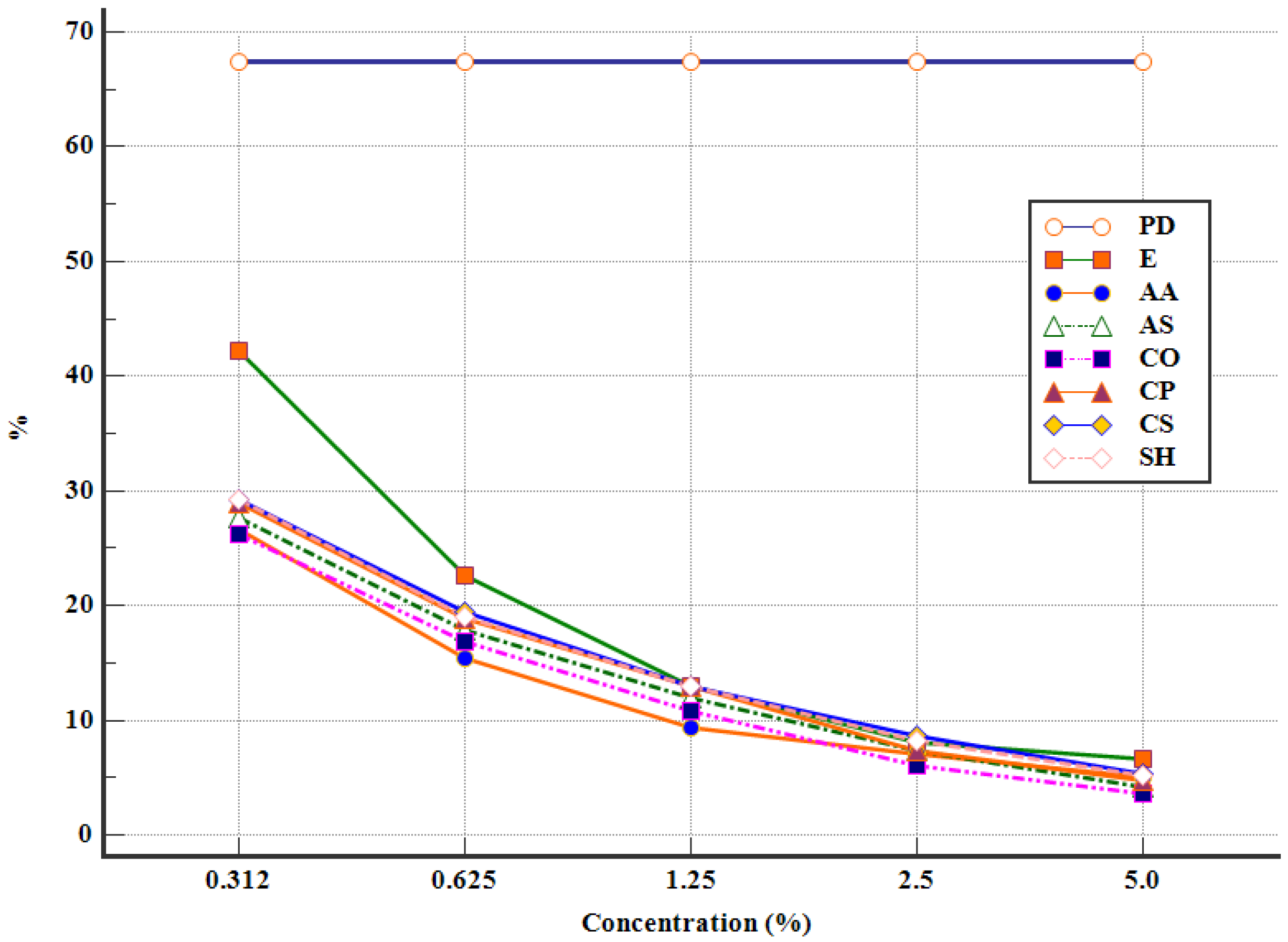
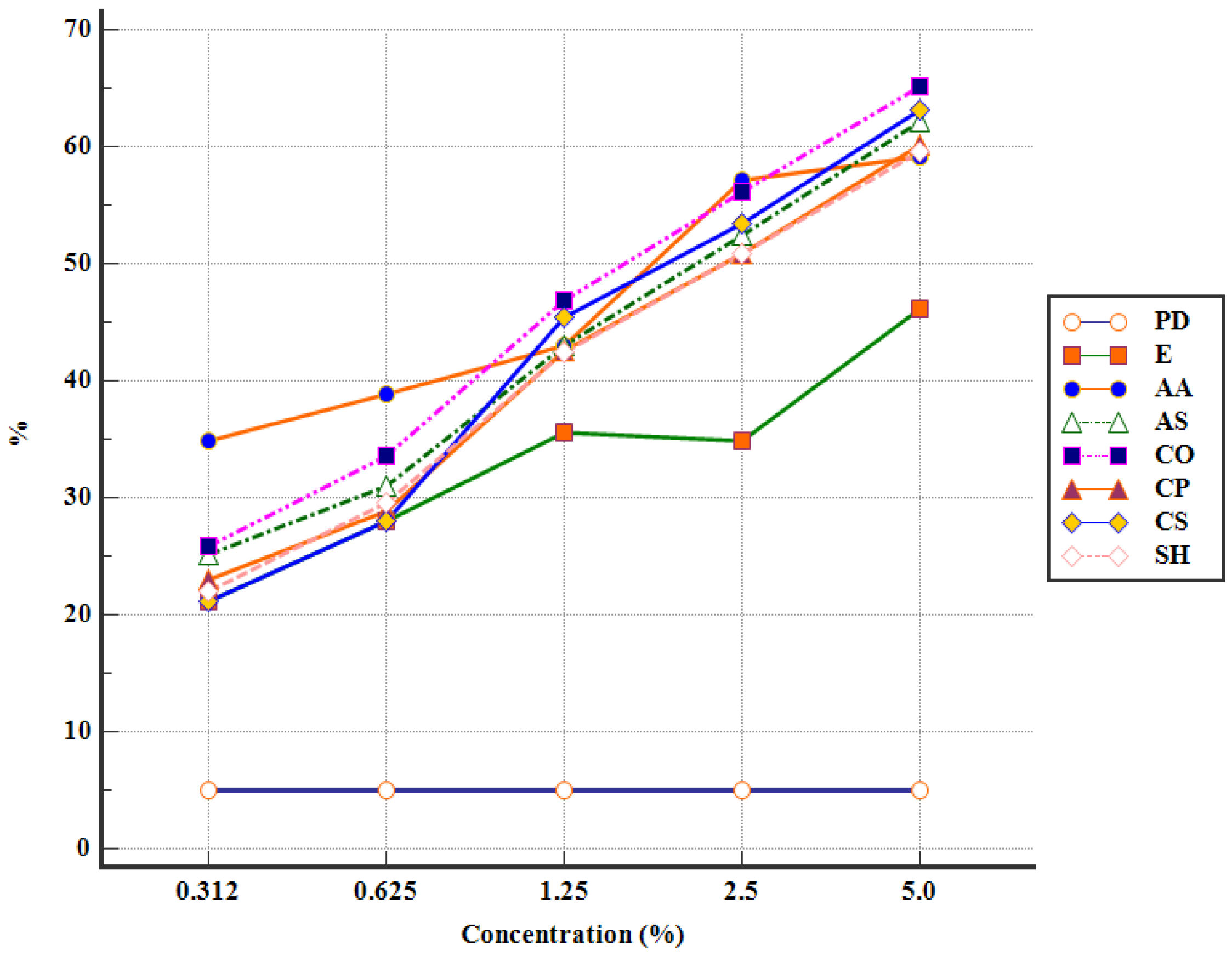
| Groups | Concentration (%) | Abbreviations | Content/Well |
|---|---|---|---|
| Potassium dichromate | 0.625 | PD | 1 mL SOS (0.5 mL OS + 0.5 mL 2.5% PD) + 1 mL DW |
| Ethanol | 35 | E 35 | 1 mL SOS + 1 mL 70% E |
| 17.5 | E 17.5 | 1 mL SOS + 1 mL 35% E | |
| 8.75 | E 8.75 | 1 mL SOS + 1 mL 17.5% E | |
| 4.375 | E 4.375 | 1 mL SOS + 1 mL 8.75% E | |
| 2.187 | E 2.187 | 1 mL SOS + 1 mL 4.37% E | |
| Alcoholic plant extracts | 5 | AS 5, AA 5, CS 5, CP 5, SH 5, CO 5 | 1 mL SOS + 1 mL 10% APE |
| 2.5 | AS 2.5, AA 2.5, CS 2.5, CP 2.5, SH 2.5, CO 2.5 | 1 mL SOS + 1 mL 5% APE | |
| 1.25 | AS 1.25, AA 1.25, CS 1.25, CP 1.25, SH 1.25, CO 1.25 | 1 mL SOS + 1 mL 2.5% APE | |
| 0.625 | AS 0.625, AA 0.625, CS 0.625, CP 0.625, SH 0.625 CO 0.625 | 1 mL SOS + 1 mL 1.25% APE | |
| 0.312 | AS 0.312, AA 0.312, CS 0.312, CP 0.312, SH 0.312, CO 0.312 | 1 mL SOS + 1 mL 0.625% APE |
| Time (Hours) | AS 5 | AA 5 | CS 5 | CP 5 | SH 5 | CO 5 |
|---|---|---|---|---|---|---|
| 24 | 16.44 ± 5.22 a | 9.23 ± 3.05 a | 18.5 ± 3.59 a | 15.6 ± 2.88 a | 15.03 ± 2.72 a | 18.94 ± 1.93 a |
| 48 | 16.12 ± 6.38 a | 6.98 ± 2.04 a | 17.74 ± 3.34 a | 13.05 ± 2.29 a | 14.87 ± 2.54 a | 18.06 ± 2.77 a |
| 72 | 23.78 ± 5.47 a | 13.2 ± 3.09 a | 23.97 ± 3.74 a | 21.97 ± 2.98 a | 20.36 ± 2.88 a | 27.5 ± 2.18 a |
| 96 | 29.38 ± 3.16 ab | 23.96 ± 3.71 ab | 31.27 ± 4.42 ab | 25.36 ± 2.38 ab | 24.21 ± 2.45 b | 35.01 ± 1.93 a |
| AS 2.5 | AA 2.5 | CS 2.5 | CP 2.5 | SH 2.5 | CO 2.5 | |
| 24 | 15.94 ± 4.66 a | 16.03 ± 4.82 a | 18.96 ± 3.68 a | 15.2 ± 2.92 a | 14.1 ± 2.54 a | 18.68 ± 2.07 a |
| 48 | 14.72 ± 4.47 a | 23.46 ± 4.79 a | 18.78 ± 3.49 a | 12.47 ± 2.94 a | 13.03 ± 2.17 a | 17.8 ± 2.55 a |
| 72 | 25.79 ± 6.68 a | 32.12 ± 3.81 a | 25.6 ± 3.24 a | 22.07 ± 2.82 a | 22.64 ± 3.27 a | 29.72 ± 3.14 a |
| 96 | 26.45 ± 6.66 a | 33.82 ± 4.87 a | 28.08 ± 3.29 a | 24.02 ± 3.28 a | 24.15 ± 2.79 a | 32.51 ± 1.72 a |
| AS 1.25 | AA 1.25 | CS 1.25 | CP 1.25 | SH 1.25 | CO 1.25 | |
| 24 | 6.22 ± 3.28 a | 9.44 ± 3.39 a | 9.6 ± 2.29 a | 4.99 ± 1.34 a | 4.49 ± 1.02 a | 8.5 ± 1.51 a |
| 48 | 5.21 ± 3.21 a | 6.85 ± 2.54 a | 8.39 ± 2.7 a | 3.97 ± 1.12 a | 3.72 ± 0.99 a | 8.39 ± 1.81 a |
| 72 | 11.05 ± 3.17 ab | 11.14 ± 2.93 ab | 11.65 ± 2.23 ab | 7.42 ± 1.83 ab | 5.97 ± 1.19 b | 14.18 ± 1.94 a |
| 96 | 10.46 ± 4.28 a | 10.41 ± 2.3 a | 14.31 ± 2.52 a | 10.23 ± 1.73 a | 9.93 ± 1.88 a | 16.31 ± 1.76 a |
| AS 0.625 | AA 0.625 | CS 0.625 | CP 0.625 | SH 0.625 | CO 0.625 | |
| 24 | 4.77 ± 2.32 a | 8.3 ± 3.8 a | 8.84 ± 2.32 a | 3.73 ± 1 a | 3.21 ± 0.8 a | 7.6 ± 1.41 a |
| 48 | 3.33 ± 1.96 a | 12.97 ± 3.24 a | 10.43 ± 2.78 a | 4.31 ± 1.29 a | 3.81 ± 1.05 a | 9.74 ± 1.87 a |
| 72 | 4.14 ± 2.22 ac | 14.17 ± 3.05 a | 5.57 ± 1.88 ac | 1.34 ± 0.46 c | 3.9 ± 0.94 bc | 8.71 ± 1.58 ab |
| 96 | 3.82 ± 2.17 bc | 14.81 ± 2.99 a | 5.94 ± 1.36 bc | 0.8 ± 0.12 c | 1.89 ± 0.3 c | 7.49 ± 1.34 b |
| AS 0.312 | AA 0.312 | CS 0.312 | CP 0.312 | SH 0.312 | CO 0.312 | |
| 24 | 2.4 ± 1.48 ab | 11.01 ± 4.16 a | 5.22 ± 1.89 ab | 1.24 ± 0.42 b | 1.47 ± 0.38 b | 4.75 ± 1.16 ab |
| 48 | 3.58 ± 2.07 b | 14.89 ± 3.44 a | 6.57 ± 1.54 b | 1.85 ± 0.51 b | 2.1 ± 0.62 b | 6.55 ± 1.32 b |
| 72 | 3.31 ± 2.04 bc | 14.92 ± 3.69 a | 1.89 ± 0.65 bc | 0.54 ± 0.12 c | 0.5 ± 0.09 c | 6.57 ± 1.31 b |
| 96 | 5.06 ± 3.22 bc | 17.26 ± 3.09 a | 1.9 ± 0.65 bc | 2.06 ± 0.58 bc | 0.95 ± 0.2 c | 5.74 ± 1 b |
| Time (Hours) | AS (mg/mL) | AA (mg/mL) | CS (mg/mL) | CP (mg/mL) | SH (mg/mL) | CO (mg/mL) |
|---|---|---|---|---|---|---|
| 72 | 28.84 | 31.62 | 28.18 | 33.11 | 35.48 | 24.55 |
| 96 | 21.88 | 18.62 | 20.42 | 23.44 | 23.99 | 16.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bǎieş, M.-H.; Györke, A.; Cotuţiu, V.-D.; Boros, Z.; Cozma-Petruț, A.; Filip, L.; Vlase, L.; Vlase, A.-M.; Crişan, G.; Spînu, M.; et al. The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets. Pathogens 2023, 12, 258. https://doi.org/10.3390/pathogens12020258
Bǎieş M-H, Györke A, Cotuţiu V-D, Boros Z, Cozma-Petruț A, Filip L, Vlase L, Vlase A-M, Crişan G, Spînu M, et al. The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets. Pathogens. 2023; 12(2):258. https://doi.org/10.3390/pathogens12020258
Chicago/Turabian StyleBǎieş, Mihai-Horia, Adriana Györke, Vlad-Dan Cotuţiu, Zsolt Boros, Anamaria Cozma-Petruț, Lorena Filip, Laurian Vlase, Ana-Maria Vlase, Gianina Crişan, Marina Spînu, and et al. 2023. "The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets" Pathogens 12, no. 2: 258. https://doi.org/10.3390/pathogens12020258
APA StyleBǎieş, M.-H., Györke, A., Cotuţiu, V.-D., Boros, Z., Cozma-Petruț, A., Filip, L., Vlase, L., Vlase, A.-M., Crişan, G., Spînu, M., & Cozma, V. (2023). The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets. Pathogens, 12(2), 258. https://doi.org/10.3390/pathogens12020258












