SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants
Abstract
1. Introduction
2. SARS-CoV-2-Specific T Cells in Healthy Individuals
2.1. Cellular Immunity during and after SARS-CoV-2 Infection
2.2. Cellular Immunity Following SARS-CoV-2 Vaccination
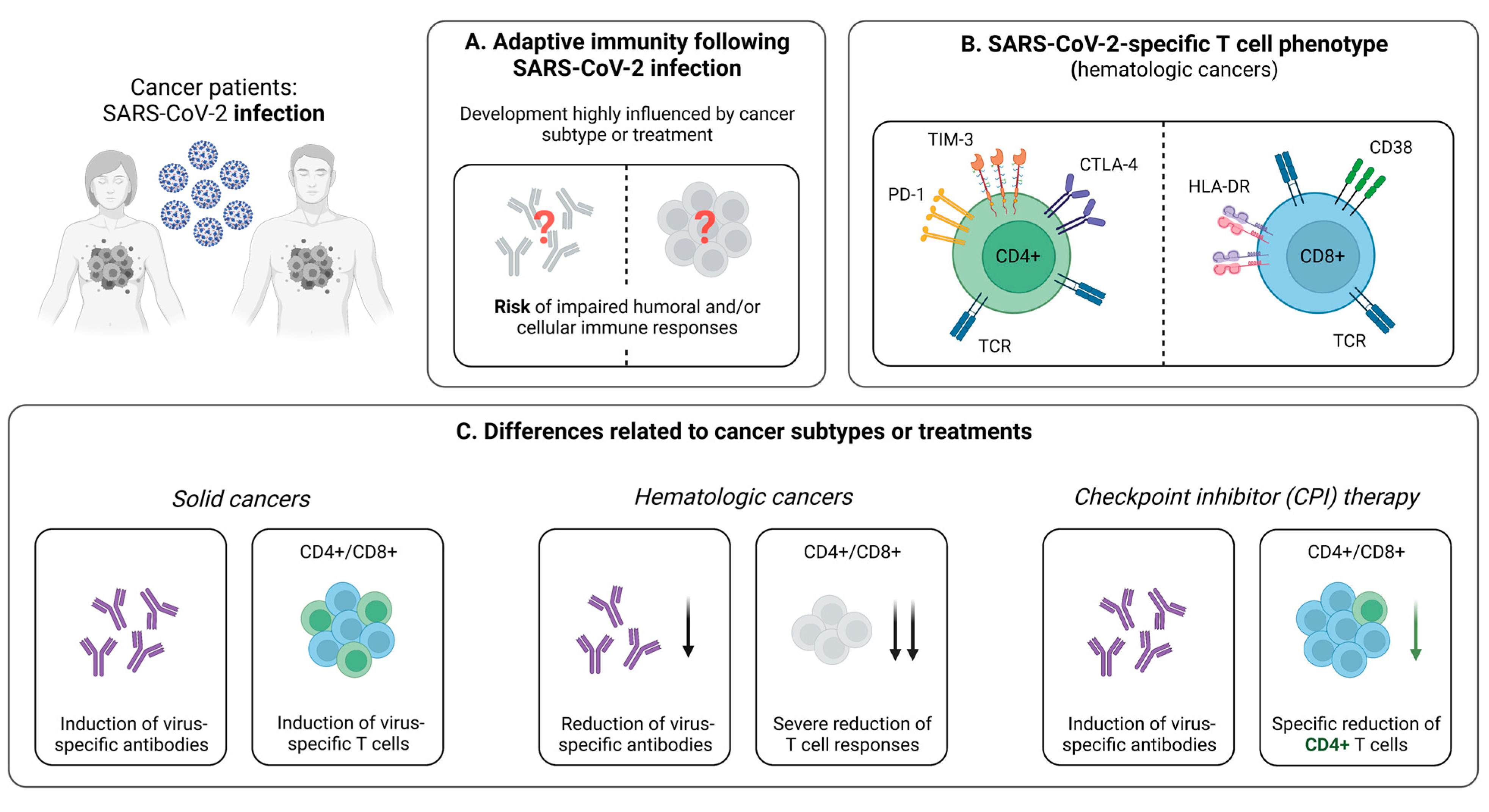
3. SARS-CoV-2-Specific T Cells in Cancer Patients
3.1. Cellular Immunity during and after SARS-CoV-2 Infection
3.1.1. Reduced Virus-Specific T Cell Responses Following SARS-CoV-2 Infection in Cancer Patients
3.1.2. Influence of Cancer Treatment on T Cell Immunity
3.1.3. Prolonged SARS-CoV-2 Infection in Cancer Patients
3.2. Cellular Immunity Following SARS-CoV-2 Vaccination
3.2.1. Impaired Vaccine-Induced T Cell Immunity in Cancer Patients
3.2.2. SARS-CoV-2-Specific T Cell Immunity upon Booster Vaccination
3.2.3. Discordance between the Vaccine-Induced SARS-CoV-2-Specific Humoral and Cellular Immune Response
3.2.4. Influence of Cancer Treatment on the Vaccine-Induced Cellular Immune Response
3.2.5. Breakthrough Infections in Vaccinated Cancer Patients
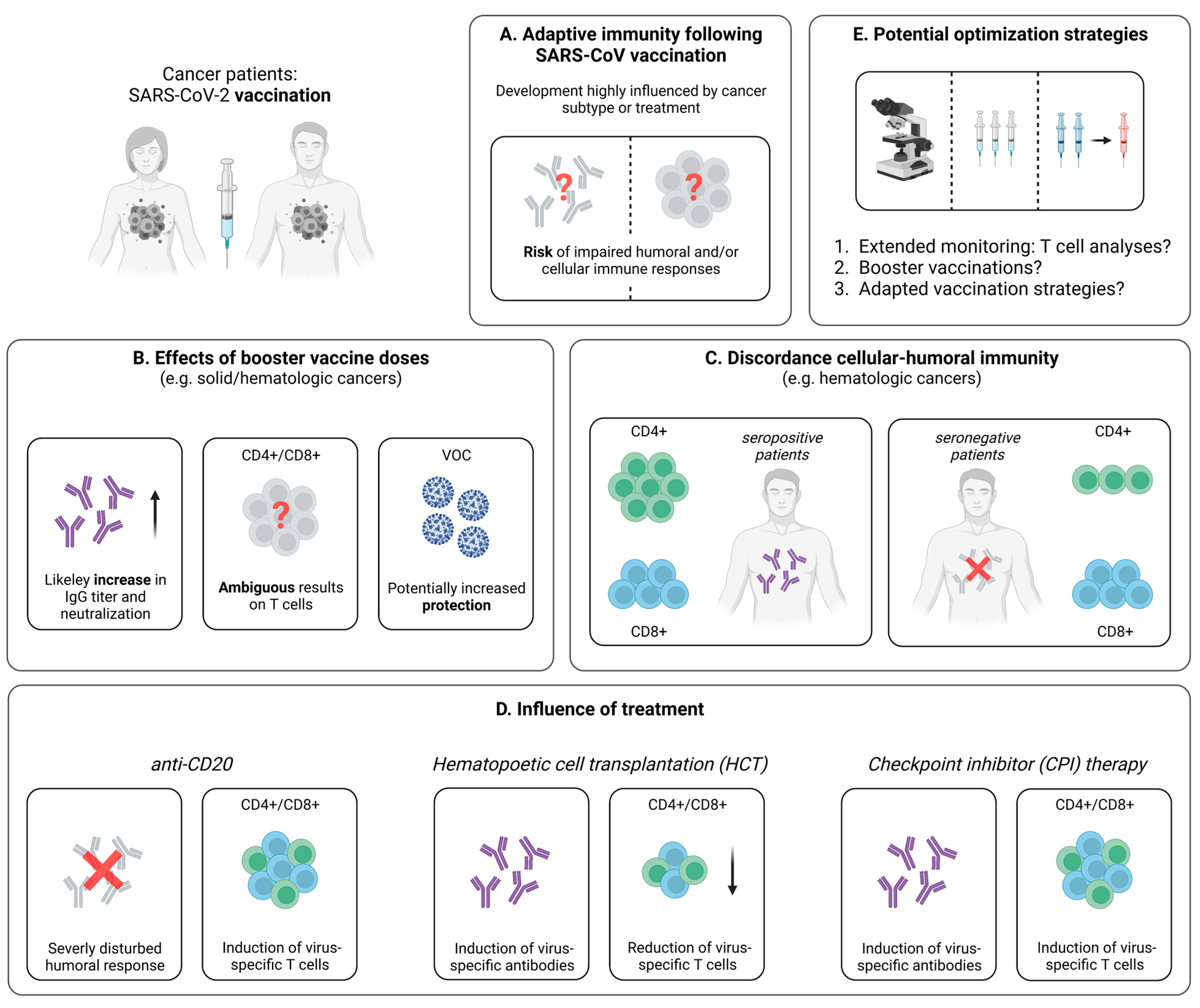
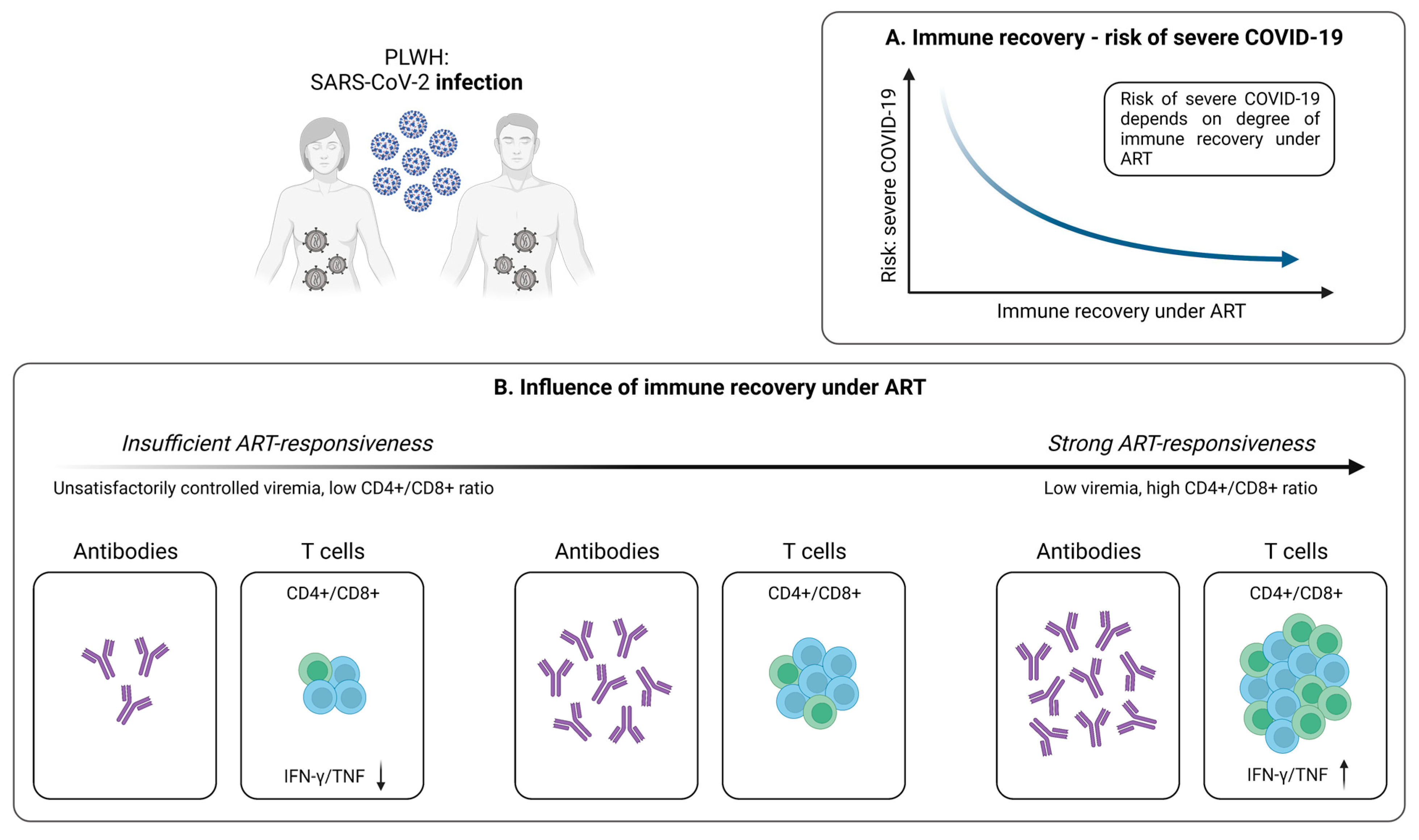
4. SARS-CoV-2-Specific T Cells in People Living with HIV (PLWH)
4.1. Cellular Immunity during and after SARS-CoV-2 Infection
4.1.1. SARS-CoV-2-Specific T Cells in PLWH
4.1.2. Antiretroviral Therapy Is Associated with Stronger SARS-CoV-2-Specific T Cell Responses
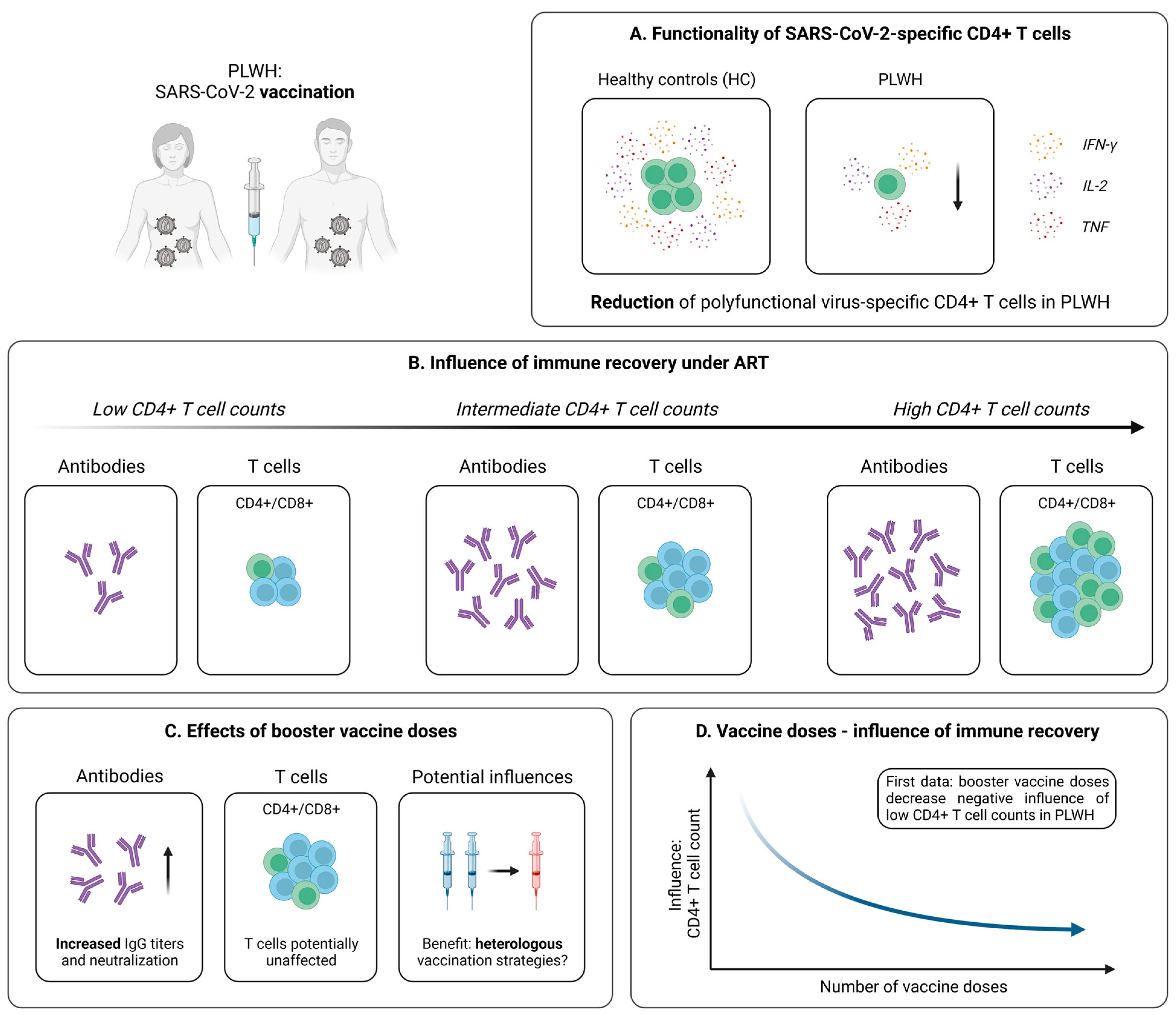
4.2. Cellular Immunity Following SARS-CoV-2 Vaccination
4.2.1. Robust Vaccine-Induced T Cell Immunity in PLWH
4.2.2. Influence of Immune Recovery under ART on the Vaccination Outcome
4.2.3. Optimization of SARS-CoV-2 Immunization Strategies in PLWH
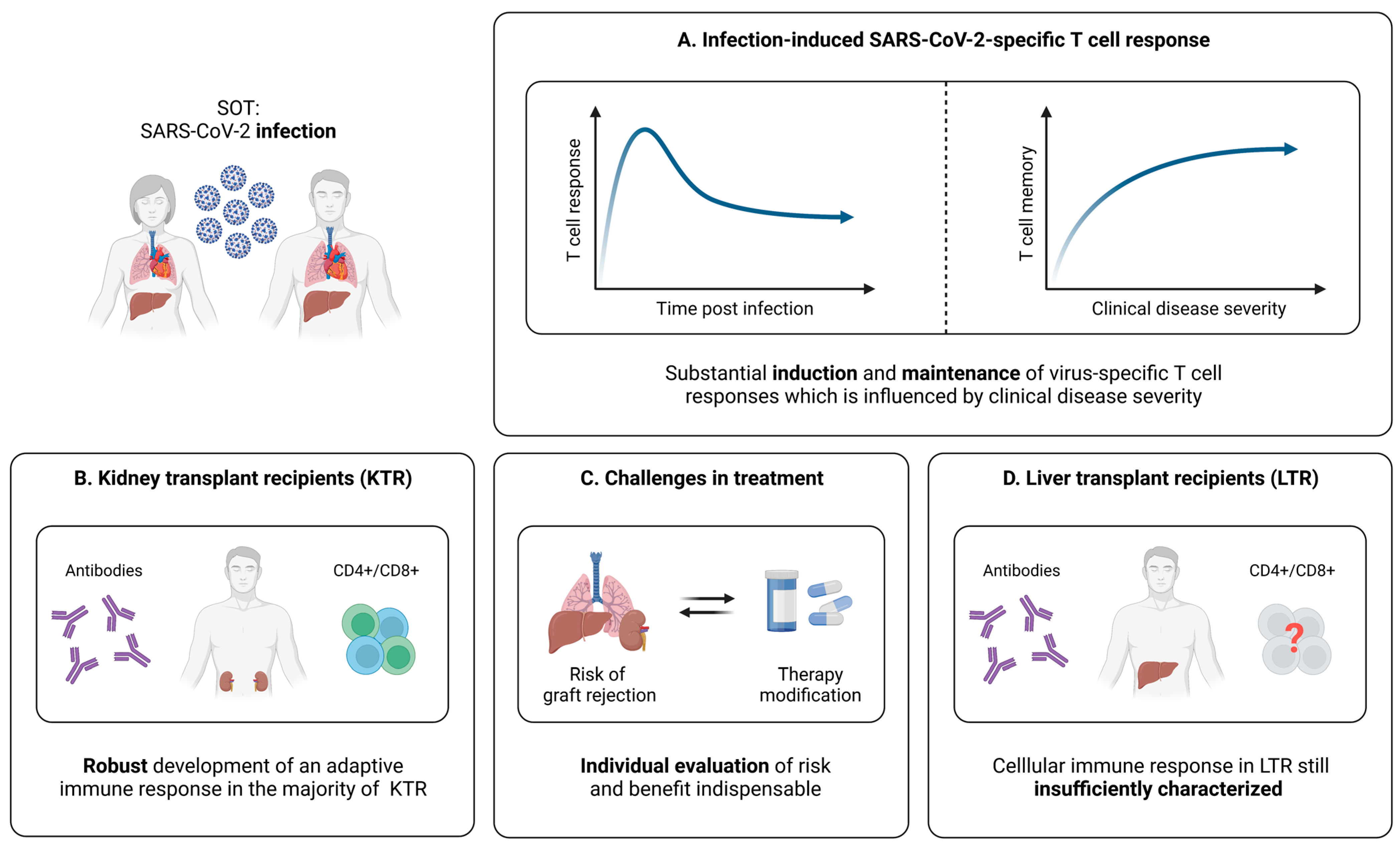
5. SARS-CoV-2-Specific T Cells in Solid Organ Transplant Recipients (SOT)
5.1. Cellular Immunity during and after SARS-CoV-2 Infection
5.1.1. SARS-CoV-2-Specific T Cells in SOT
5.1.2. Relation between SARS-CoV-2-Specific T Cell Responses and the Clinical Course of Infection in SOT
5.1.3. SARS-CoV-2-Specific T Cell Immunity in Kidney Transplant Recipients (KTR)
5.1.4. SARS-CoV-2-Specific T Cell Immunity in Liver Transplant Recipients (LTR)
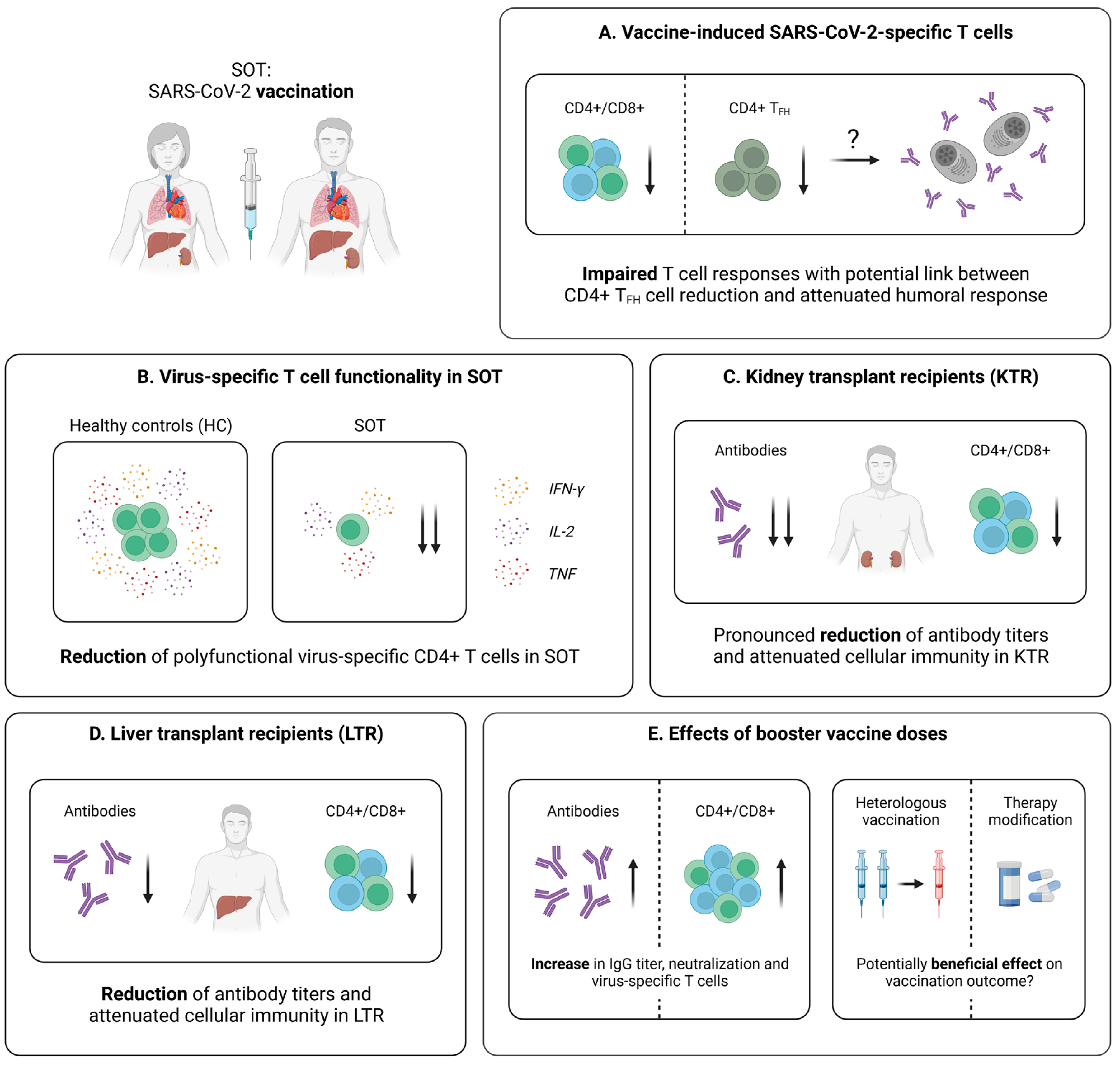
5.2. Cellular Immunity Following SARS-CoV-2 Vaccination
5.2.1. Vaccine-Induced SARS-CoV-2-Specific T Cell Immunity in SOT
5.2.2. Vaccine-Induced SARS-CoV-2-Specific T Cell Immunity in KTR
5.2.3. Vaccine-Induced SARS-CoV-2-Specific T Cell Immunity in LTR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 15 January 2023).
- CDC Cases, Data, and Surveillance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html (accessed on 16 January 2023).
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Gargiulo, C.I.; Malcangi, G.; Ciocia, A.M.; Patano, A.; Azzollini, D.; Piras, F.; Barile, G.; Settanni, V.; Mancini, A.; et al. Diagnosis of SARS-CoV-2 during the Pandemic by Multiplex RT-RPCR HCoV Test: Future Perspectives. Pathogens 2022, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from Pandemic Response to Control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Landini, N.; Sambataro, G.; Nardi, C.; Tofani, L.; Bruni, C.; Bellando-Randone, S.; Blagojevic, J.; Melchiorre, D.; Hughes, M.; et al. The Role of Chest CT in Deciphering Interstitial Lung Involvement: Systemic Sclerosis versus COVID-19. Rheumatology 2022, 61, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.; Mirza, A.F.; Sari, M.I. The Association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 195. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A Guide to Immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and Stable Mobilization of CD8+ T Cells by SARS-CoV-2 MRNA Vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid Induction of Antigen-Specific CD4+ T Cells Is Associated with Coordinated Humoral and Cellular Immunity to SARS-CoV-2 MRNA Vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and Strong Memory CD4+ and CD8+ T Cells Induced by SARS-CoV-2 in UK Convalescent Individuals Following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Sagar; Daul, F.; Salvat Lago, M.; Decker, A.; et al. Characterization of Pre-Existing and Induced SARS-CoV-2-Specific CD8+ T Cells. Nat. Med. 2021, 27, 78–85. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for Antibody as a Protective Correlate for COVID-19 Vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Mescia, F.; Turner, L.; Hanson, A.L.; Kotagiri, P.; Dunmore, B.J.; Ruffieux, H.; De Sa, A.; Huhn, O.; Morgan, M.D.; et al. Longitudinal Analysis Reveals That Delayed Bystander CD8+ T Cell Activation and Early Immune Pathology Distinguish Severe COVID-19 from Mild Disease. Immunity 2021, 54, 1257–1275.e8. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-Specific T Cells Cross-Recognize the Omicron Variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef]
- Lang-Meli, J.; Luxenburger, H.; Wild, K.; Karl, V.; Oberhardt, V.; Salimi Alizei, E.; Graeser, A.; Reinscheid, M.; Roehlen, N.; Reeg, D.B.; et al. SARS-CoV-2-Specific T-Cell Epitope Repertoire in Convalescent and MRNA-Vaccinated Individuals. Nat. Microbiol. 2022, 7, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.R.; Ke, H.; Coherd, C.D.; Wang, Y.; Mashima, K.; Kastrunes, G.M.; Huang, C.-Y.; Marasco, W.A. Analysis of a SARS-CoV-2 Convalescent Cohort Identified a Common Strategy for Escape of Vaccine-Induced Anti-RBD Antibodies by Beta and Omicron Variants. EBioMedicine 2022, 80, 104025. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef]
- Fendler, A.; Au, L.; Shepherd, S.T.C.; Byrne, F.; Cerrone, M.; Boos, L.A.; Rzeniewicz, K.; Gordon, W.; Shum, B.; Gerard, C.L.; et al. Functional Antibody and T Cell Immunity Following SARS-CoV-2 Infection, Including by Variants of Concern, in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T Cells Contribute to Survival in Patients with COVID-19 and Hematologic Cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Nkosi, T.; Chasara, C.; Papadopoulos, A.O.; Nguni, T.L.; Karim, F.; Moosa, M.-Y.S.; Gazy, I.; Jambo, K.; COMMIT-KZN-Team; Hanekom, W.; et al. Unsuppressed HIV Infection Impairs T Cell Responses to SARS-CoV-2 Infection and Abrogates T Cell Cross-Recognition. eLife 2022, 11, e78374. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; Demissie, E.G.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; et al. Effect of Immunosuppression on the Immunogenicity of MRNA Vaccines to SARS-CoV-2: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 1572–1585. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Molino Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Embi, P.J. Effectiveness of 2-Dose Vaccination with MRNA COVID-19 Vaccines Against COVID-19–Associated Hospitalizations Among Immunocompromised Adults—Nine States, January–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Rahav, G.; Lustig, Y.; Lavee, J.; Benjamini, O.; Magen, H.; Hod, T.; Shem-Tov, N.; Shmueli, E.S.; Merkel, D.; Ben-Ari, Z.; et al. BNT162b2 MRNA COVID-19 Vaccination in Immunocompromised Patients: A Prospective Cohort Study. eClinicalMedicine 2021, 41, 101158. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl Huber, S.; van Beek, J.; de Jonge, J.; Luytjes, W.; van Baarle, D. T Cell Responses to Viral Infections—Opportunities for Peptide Vaccination. Front. Immunol. 2014, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; van Bockel, D.; Kent, S.J.; Kelleher, A.D.; Zaunders, J.J.; Munier, C.M.L. Cytotoxic CD4 T Cells—Friend or Foe during Viral Infection? Front. Immunol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-Specific T Cells in Infection and Vaccination. Cell. Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Notarbartolo, S.; Ranzani, V.; Bandera, A.; Gruarin, P.; Bevilacqua, V.; Putignano, A.R.; Gobbini, A.; Galeota, E.; Manara, C.; Bombaci, M.; et al. Integrated Longitudinal Immunophenotypic, Transcriptional and Repertoire Analyses Delineate Immune Responses in COVID-19 Patients. Sci. Immunol. 2021, 6, eabg5021. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset That Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e4. [Google Scholar] [CrossRef] [PubMed]
- Lafon, E.; Diem, G.; Witting, C.; Zaderer, V.; Bellmann-Weiler, R.M.; Reindl, M.; Bauer, A.; Griesmacher, A.; Fux, V.; Hoermann, G.; et al. Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients. Front. Immunol. 2021, 12, 684014. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates with Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by COVID-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T Cell and Antibody Responses Induced by a Single Dose of ChAdOx1 NCoV-19 (AZD1222) Vaccine in a Phase 1/2 Clinical Trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. MRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, M.; Luxenburger, H.; Karl, V.; Graeser, A.; Giese, S.; Ciminski, K.; Reeg, D.B.; Oberhardt, V.; Roehlen, N.; Lang-Meli, J.; et al. COVID-19 MRNA Booster Vaccine Induces Transient CD8+ T Effector Cell Responses While Conserving the Memory Pool for Subsequent Reactivation. Nat. Commun. 2022, 13, 4631. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A.; Padilla, M.; Hoyland, W.; McGlinchey, K.; Fields, P.A.; Bibi, S.; Faust, S.N.; McDermott, A.B.; Lambe, T.; Pollard, A.J.; et al. AZD1222/ChAdOx1 NCoV-19 Vaccination Induces a Polyfunctional Spike Protein-Specific TH1 Response with a Diverse TCR Repertoire. Sci. Transl. Med. 2021, 13, eabj7211. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 Vaccination Induces Immunological T Cell Memory Able to Cross-Recognize Variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in Patients with Thoracic Malignancies (TERAVOLT): First Results of an International, Registry-Based, Cohort Study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 Mortality in Patients with Cancer on Chemotherapy or Other Anticancer Treatments: A Prospective Cohort Study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G.; et al. Clinical Impact of COVID-19 on Patients with Cancer (CCC19): A Cohort Study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Crolley, V.E.; Hanna, D.; Joharatnam-Hogan, N.; Chopra, N.; Bamac, E.; Desai, M.; Lam, Y.-C.; Dipro, S.; Kanani, R.; Benson, J.; et al. COVID-19 in Cancer Patients on Systemic Anti-Cancer Therapies: Outcomes from the CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London) Cohort Study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920971147. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 Prevalence and Mortality in Patients with Cancer and the Effect of Primary Tumour Subtype and Patient Demographics: A Prospective Cohort Study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Atilla, E.; Sahin, D.; Atilla, P.A.; Dolapci, I.; Tekeli, A.; Bozdag, S.C.; Yuksel, M.K.; Toprak, S.K.; Ilhan, O.; Arslan, O.; et al. Upper Respiratory Viral Infections in Patients with Haematological Malignancies after Allogeneic Haematopoietic Stem Cell Transplantation: A Retrospective Study. Antivir. Ther. 2018, 23, 523–527. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Ghosh, S.; Bodey, G.P.; Rohatgi, N.; Safdar, A.; Keating, M.J.; Champlin, R.E.; Aguilera, E.A.; Tarrand, J.J.; Raad, I.I. Respiratory Viral Infections in Adults with Hematologic Malignancies and Human Stem Cell Transplantation Recipients: A Retrospective Study at a Major Cancer Center. Medicine 2006, 85, 278–287. [Google Scholar] [CrossRef]
- García-Suárez, J.; de la Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; Sánchez-Godoy, P.; et al. Impact of Hematologic Malignancy and Type of Cancer Therapy on COVID-19 Severity and Mortality: Lessons from a Large Population-Based Registry Study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Bilich, T.; Roerden, M.; Maringer, Y.; Nelde, A.; Heitmann, J.S.; Dubbelaar, M.L.; Peter, A.; Hörber, S.; Bauer, J.; Rieth, J.; et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021, 11, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.-Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of Clinical Factors and Recent Anticancer Therapy with COVID-19 Severity among Patients with Cancer: A Report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Yatim, N.; Boussier, J.; Tetu, P.; Smith, N.; Bruel, T.; Charbit, B.; Barnabei, L.; Corneau, A.; Da Meda, L.; Allayous, C.; et al. Immune Checkpoint Inhibitors Increase T Cell Immunity during SARS-CoV-2 Infection. Sci. Adv. 2021, 7, eabg4081. [Google Scholar] [CrossRef] [PubMed]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-Cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef]
- Channappanavar, R.; Twardy, B.S.; Suvas, S. Blocking of PDL-1 Interaction Enhances Primary and Secondary CD8 T Cell Response to Herpes Simplex Virus-1 Infection. PLoS ONE 2012, 7, e39757. [Google Scholar] [CrossRef]
- Crotty, S. Follicular Helper CD4 T Cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Williams, M.A.; Tyznik, A.J.; Bevan, M.J. Interleukin-2 Signals during Priming Are Required for Secondary Expansion of CD8+ Memory T Cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef]
- de Goër de Herve, M.-G.; Abdoh, M.; Jaafoura, S.; Durali, D.; Taoufik, Y. Follicular CD4 T Cells Tutor CD8 Early Memory Precursors: An Initiatory Journey to the Frontier of B Cell Territory. iScience 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Lyudovyk, O.; Kim, J.Y.; Qualls, D.; Hwee, M.A.; Lin, Y.-H.; Boutemine, S.R.; Elhanati, Y.; Solovyov, A.; Douglas, M.; Chen, E.; et al. Impaired Humoral Immunity Is Associated with Prolonged COVID-19 despite Robust CD8 T Cell Responses. Cancer Cell 2022, 40, 738–753.e5. [Google Scholar] [CrossRef] [PubMed]
- Weigang, S.; Fuchs, J.; Zimmer, G.; Schnepf, D.; Kern, L.; Beer, J.; Luxenburger, H.; Ankerhold, J.; Falcone, V.; Kemming, J.; et al. Within-Host Evolution of SARS-CoV-2 in an Immunosuppressed COVID-19 Patient as a Source of Immune Escape Variants. Nat. Commun. 2021, 12, 6405. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.M.; Gadkari, M.; Howe, K.N.; Sun, J.; Kardava, L.; Kumar, P.; Kumari, S.; Hu, Z.; Fraser, I.D.C.; Moir, S.; et al. Immune Regulation by Glucocorticoids Can Be Linked to Cell Type-Dependent Transcriptional Responses. J. Exp. Med. 2019, 216, 384–406. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune Responses to Two and Three Doses of the BNT162b2 MRNA Vaccine in Adults with Solid Tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef]
- Scurr, M.J.; Zelek, W.M.; Lippiatt, G.; Somerville, M.; Burnell, S.E.A.; Capitani, L.; Davies, K.; Lawton, H.; Tozer, T.; Rees, T.; et al. Whole Blood-Based Measurement of SARS-CoV-2-Specific T Cells Reveals Asymptomatic Infection and Vaccine Immunogenicity in Healthy Subjects and Patients with Solid-Organ Cancers. Immunology 2022, 165, 250–259. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Greenberger, L.M.; Saltzman, L.A.; Senefeld, J.W.; Johnson, P.W.; DeGennaro, L.J.; Nichols, G.L. Antibody Response to SARS-CoV-2 Vaccines in Patients with Hematologic Malignancies. Cancer Cell 2021, 39, 1031–1033. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion Rates Following COVID-19 Vaccination among Patients with Cancer. Cancer Cell 2021, 39, 1081–1090.e2. [Google Scholar] [CrossRef]
- McKenzie, D.R.; Muñoz-Ruiz, M.; Monin, L.; Alaguthurai, T.; Lechmere, T.; Abdul-Jawad, S.; Graham, C.; Pollock, E.; Graham, R.; Sychowska, K.; et al. Humoral and Cellular Immunity to Delayed Second Dose of SARS-CoV-2 BNT162b2 MRNA Vaccination in Patients with Cancer. Cancer Cell 2021, 39, 1445–1447. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Wood, H.; Ierullo, M.; Majchrzak-Kita, B.; Manguiat, K.; Robinson, A.; Kulasingam, V.; Humar, A.; Kumar, D. Delayed-Interval BNT162b2 MRNA COVID-19 Vaccination Enhances Humoral Immunity and Induces Robust T Cell Responses. Nat. Immunol. 2022, 23, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Al Hajji, Y.; Taylor, H.; Starkey, T.; Lee, L.Y.W.; Tilby, M. Antibody Response to a Third Booster Dose of SARS-CoV-2 Vaccination in Adults with Haematological and Solid Cancer: A Systematic Review. Br. J. Cancer 2022, 127, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Schmitt, A.M.; Tippu, Z.; Farag, S.; Rogiers, A.; Harvey, R.; et al. Immune Responses Following Third COVID-19 Vaccination Are Reduced in Patients with Hematological Malignancies Compared to Patients with Solid Cancer. Cancer Cell 2022, 40, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Storti, P.; Marchica, V.; Vescovini, R.; Franceschi, V.; Russo, L.; Notarfranchi, L.; Raimondi, V.; Toscani, D.; Burroughs Garcia, J.; Costa, F.; et al. Immune Response to SARS-CoV-2 MRNA Vaccination and Booster Dose in Patients with Multiple Myeloma and Monoclonal Gammopathies: Impact of Omicron Variant on the Humoral Response. Oncoimmunology 2022, 11, 2120275. [Google Scholar] [CrossRef] [PubMed]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graça, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and Cellular Responses after a Third Dose of SARS-CoV-2 BNT162b2 Vaccine in Patients with Lymphoid Malignancies. Nat. Commun. 2022, 13, 864. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wu, M.; Harvey, R.; Wilkinson, K.A.; Schmitt, A.M.; Tippu, Z.; Shum, B.; Farag, S.; et al. Functional Immune Responses against SARS-CoV-2 Variants of Concern after Fourth COVID-19 Vaccine Dose or Infection in Patients with Blood Cancer. Cell Rep. Med. 2022, 3, 100781. [Google Scholar] [CrossRef]
- Atanackovic, D.; Kreitman, R.J.; Cohen, J.; Hardy, N.M.; Omili, D.; Iraguha, T.; Burbelo, P.D.; Gebru, E.; Fan, X.; Baddley, J.; et al. T Cell Responses against SARS-CoV-2 and Its Omicron Variant in a Patient with B Cell Lymphoma after Multiple Doses of a COVID-19 MRNA Vaccine. J. Immunother. Cancer 2022, 10, e004953. [Google Scholar] [CrossRef]
- Rousseau, B.; Loulergue, P.; Mir, O.; Krivine, A.; Kotti, S.; Viel, E.; Simon, T.; de Gramont, A.; Goldwasser, F.; Launay, O.; et al. Immunogenicity and Safety of the Influenza A H1N1v 2009 Vaccine in Cancer Patients Treated with Cytotoxic Chemotherapy and/or Targeted Therapy: The VACANCE Study. Ann. Oncol. 2012, 23, 450–457. [Google Scholar] [CrossRef]
- Mairhofer, M.; Kausche, L.; Kaltenbrunner, S.; Ghanem, R.; Stegemann, M.; Klein, K.; Pammer, M.; Rauscher, I.; Salzer, H.J.F.; Doppler, S.; et al. Humoral and Cellular Immune Responses in SARS-CoV-2 MRNA-Vaccinated Patients with Cancer. Cancer Cell 2021, 39, 1171–1172. [Google Scholar] [CrossRef]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Di Martino, S.; Laquintana, V.; La Malfa, A.; et al. Fifth-Week Immunogenicity and Safety of Anti-SARS-CoV-2 BNT162b2 Vaccine in Patients with Multiple Myeloma and Myeloproliferative Malignancies on Active Treatment: Preliminary Data from a Single Institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef]
- Van Oekelen, O.; Gleason, C.R.; Agte, S.; Srivastava, K.; Beach, K.F.; Aleman, A.; Kappes, K.; PVI/Seronet Team; Mouhieddine, T.H.; Wang, B.; et al. Highly Variable SARS-CoV-2 Spike Antibody Responses to Two Doses of COVID-19 RNA Vaccination in Patients with Multiple Myeloma. Cancer Cell 2021, 39, 1028–1030. [Google Scholar] [CrossRef]
- Aleman, A.; Upadhyaya, B.; Tuballes, K.; Kappes, K.; Gleason, C.R.; Beach, K.; Agte, S.; Srivastava, K.; PVI/Seronet Study Group; Van Oekelen, O.; et al. Variable Cellular Responses to SARS-CoV-2 in Fully Vaccinated Patients with Multiple Myeloma. Cancer Cell 2021, 39, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Luetkens, T.; Omili, D.; Iraguha, T.; Lutfi, F.; Hardy, N.M.; Fan, X.; Avila, S.V.; Saharia, K.K.; Husson, J.S.; et al. Vaccine-Induced T-Cell Responses against SARS-CoV-2 and Its Omicron Variant in Patients with B Cell–Depleted Lymphoma after CART Therapy. Blood 2022, 140, 152–156. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 MRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Brydak, L.B.; Całbecka, M. Immunogenicity of Influenza Vaccine in Patients with Hemato-Oncological Disorders. Leuk. Lymphoma 1999, 32, 369–374. [Google Scholar] [CrossRef]
- Berglund, A.; Willén, L.; Grödeberg, L.; Skattum, L.; Hagberg, H.; Pauksens, K. The Response to Vaccination against Influenza A(H1N1) 2009, Seasonal Influenza and Streptococcus Pneumoniae in Adult Outpatients with Ongoing Treatment for Cancer with and without Rituximab. Acta Oncol. 2014, 53, 1212–1220. [Google Scholar] [CrossRef]
- Parrino, J.; McNeil, S.A.; Lawrence, S.J.; Kimby, E.; Pagnoni, M.F.; Stek, J.E.; Zhao, Y.; Chan, I.S.F.; Kaplan, S.S. Safety and Immunogenicity of Inactivated Varicella-Zoster Virus Vaccine in Adults with Hematologic Malignancies Receiving Treatment with Anti-CD20 Monoclonal Antibodies. Vaccine 2017, 35, 1764–1769. [Google Scholar] [CrossRef]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of Haemopoietic Stem Cell Transplant Recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-Based CART Therapy-A Single-Center Prospective Cohort Study. Transplant Cell Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Easdale, S.; Shea, R.; Ellis, L.; Bazin, J.; Davis, K.; Dallas, F.; Thistlethwayte, E.; Ethell, M.; Potter, M.; Arias, C.; et al. Serologic Responses Following a Single Dose of SARS-CoV-2 Vaccination in Allogeneic Stem Cell Transplantation Recipients. Transplant Cell Ther. 2021, 27, 880.e1–880.e4. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 MRNA Vaccine and Early Clinical Outcomes in Patients with Haematological Malignancies in Lithuania: A National Prospective Cohort Study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Saha, C.; Saunders, J.; Child, F.; Dillon, R.; Saglam, S.; Raj, K.; McLornan, D.; Avenoso, D.; et al. Repeated Vaccination against SARS-CoV-2 Elicits Robust Polyfunctional T Cell Response in Allogeneic Stem Cell Transplantation Recipients. Cancer Cell 2021, 39, 1448–1449. [Google Scholar] [CrossRef]
- Mair, M.J.; Mitterer, M.; Gattinger, P.; Berger, J.M.; Trutschnig, W.; Bathke, A.C.; Gansterer, M.; Berghoff, A.S.; Laengle, S.; Gottmann, L.; et al. Enhanced SARS-CoV-2 Breakthrough Infections in Patients with Hematologic and Solid Cancers Due to Omicron. Cancer Cell 2022, 40, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough Infections with SARS-CoV-2 Omicron despite MRNA Vaccine Booster Dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T Cell Responses to SARS-CoV-2 Spike Cross-Recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T Cell Reactivity to the SARS-CoV-2 Omicron Variant Is Preserved in Most but Not All Individuals. Cell 2022, 185, 1041–1051.e6. [Google Scholar] [CrossRef]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV Infection. Nat. Rev. Dis. Prim. 2015, 1, 15035. [Google Scholar] [CrossRef]
- Tesoriero, J.M.; Swain, C.-A.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef]
- Hoffmann, C.; Casado, J.L.; Härter, G.; Vizcarra, P.; Moreno, A.; Cattaneo, D.; Meraviglia, P.; Spinner, C.D.; Schabaz, F.; Grunwald, S.; et al. Immune Deficiency Is a Risk Factor for Severe COVID-19 in People Living with HIV. HIV Med. 2021, 22, 372–378. [Google Scholar] [CrossRef]
- Braunstein, S.L.; Lazar, R.; Wahnich, A.; Daskalakis, D.C.; Blackstock, O.J. Coronavirus Disease 2019 (COVID-19) Infection Among People With Human Immunodeficiency Virus in New York City: A Population-Level Analysis of Linked Surveillance Data. Clin. Infect. Dis. 2021, 72, e1021–e1029. [Google Scholar] [CrossRef] [PubMed]
- Adachi, E.; Saito, M.; Nagai, H.; Ikeuchi, K.; Koga, M.; Tsutsumi, T.; Yotsuyanagi, H. Transient Depletion of T Cells during COVID-19 and Seasonal Influenza in People Living with HIV. J. Med. Virol. 2022, 94, 1789–1791. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, Y.; Luo, Q.; Huang, Z.; Zhao, R.; Liu, S.; Le, A.; Li, J.; Wan, L. T-Cell Subset Counts in Peripheral Blood Can Be Used as Discriminatory Biomarkers for Diagnosis and Severity Prediction of Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 198–202. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Pérez-Elías, M.J.; Dronda, F.; Casado, J.L.; Moreno, A.; Royuela, A.; Pérez-Molina, J.A.; Sainz, T.; Navas, E.; Hermida, J.M.; et al. Increased Risk of Serious Non-AIDS-Related Events in HIV-Infected Subjects on Antiretroviral Therapy Associated with a Low CD4/CD8 Ratio. PLoS ONE 2014, 9, e85798. [Google Scholar] [CrossRef] [PubMed]
- Lohse, N.; Obel, N. Update of Survival for Persons With HIV Infection in Denmark. Ann. Intern. Med. 2016, 165, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Sharov, K.S. HIV/SARS-CoV-2 Co-Infection: T Cell Profile, Cytokine Dynamics and Role of Exhausted Lymphocytes. Int. J. Infect. Dis. 2021, 102, 163–169. [Google Scholar] [CrossRef]
- Alrubayyi, A.; Gea-Mallorquí, E.; Touizer, E.; Hameiri-Bowen, D.; Kopycinski, J.; Charlton, B.; Fisher-Pearson, N.; Muir, L.; Rosa, A.; Roustan, C.; et al. Characterization of Humoral and SARS-CoV-2 Specific T Cell Responses in People Living with HIV. Nat. Commun. 2021, 12, 5839. [Google Scholar] [CrossRef]
- Riou, C.; du Bruyn, E.; Stek, C.; Daroowala, R.; Goliath, R.T.; Abrahams, F.; Said-Hartley, Q.; Allwood, B.W.; Hsiao, N.-Y.; Wilkinson, K.A.; et al. Relationship of SARS-CoV-2–Specific CD4 Response to COVID-19 Severity and Impact of HIV-1 and Tuberculosis Coinfection. J. Clin. Investig. 2021, 131, e149125. [Google Scholar] [CrossRef]
- van den Berg, R.; van Hoogstraten, I.; van Agtmael, M. Non-Responsiveness to Hepatitis B Vaccination in HIV Seropositive Patients; Possible Causes and Solutions. AIDS Rev. 2009, 11, 157–164. [Google Scholar]
- Avelino-Silva, V.I.; Miyaji, K.T.; Hunt, P.W.; Huang, Y.; Simoes, M.; Lima, S.B.; Freire, M.S.; Caiaffa-Filho, H.H.; Hong, M.A.; Costa, D.A.; et al. CD4/CD8 Ratio and KT Ratio Predict Yellow Fever Vaccine Immunogenicity in HIV-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0005219. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and Efficacy of the MRNA BNT162b2 Vaccine against SARS-CoV-2 in Five Groups of Immunocompromised Patients and Healthy Controls in a Prospective Open-Label Clinical Trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef] [PubMed]
- González de Aledo, M.; Cañizares, A.; Vázquez-Rodríguez, P.; Castro, Á.; Moldes, L.; López, S.; Míguez, E.; Bou, G.; Mena, Á. Safety and Immunogenicity of SARS-CoV-2 Vaccines in People with HIV. AIDS 2022, 36, 691–695. [Google Scholar] [CrossRef]
- Ruddy, J.A.; Boyarsky, B.J.; Bailey, J.R.; Karaba, A.H.; Garonzik-Wang, J.M.; Segev, D.L.; Durand, C.M.; Werbel, W.A. Safety and Antibody Response to Two-Dose SARS-CoV-2 Messenger RNA Vaccination in Persons with HIV. AIDS 2021, 35, 2399–2401. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Koen, A.L.; Izu, A.; Fairlie, L.; Cutland, C.L.; Baillie, V.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in People Living with and without HIV in South Africa: An Interim Analysis of a Randomised, Double-Blind, Placebo-Controlled, Phase 1B/2A Trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 MRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living With Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2022, 74, 1268–1270. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Wullimann, D.; Niessl, J.; Rivera-Ballesteros, O.; Chen, P.; Lange, J.; Cuapio, A.; Blennow, O.; Hansson, L.; et al. Immunodeficiency Syndromes Differentially Impact the Functional Profile of SARS-CoV-2-Specific T Cells Elicited by MRNA Vaccination. Immunity 2022, 55, 1732–1746.e5. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in HIV Infection: A Single-Arm Substudy of a Phase 2/3 Clinical Trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Aytenfisu, T.Y.; Johnston, T.S.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. Decay of Coronavirus Disease 2019 MRNA Vaccine-Induced Immunity in People with HIV. AIDS 2022, 36, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Ogbe, A.; Pace, M.; Bittaye, M.; Tipoe, T.; Adele, S.; Alagaratnam, J.; Aley, P.K.; Ansari, M.A.; Bara, A.; Broadhead, S.; et al. Durability of ChAdOx1 NCoV-19 Vaccination in People Living with HIV. JCI Insight 2022, 7, e157031. [Google Scholar] [CrossRef]
- Antinori, A.; Cicalini, S.; Meschi, S.; Bordoni, V.; Lorenzini, P.; Vergori, A.; Lanini, S.; De Pascale, L.; Matusali, G.; Mariotti, D.; et al. Humoral and Cellular Immune Response Elicited by MRNA Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in People Living With Human Immunodeficiency Virus Receiving Antiretroviral Therapy Based on Current CD4 T-Lymphocyte Count. Clin. Infect. Dis. 2022, 75, e552–e563. [Google Scholar] [CrossRef] [PubMed]
- Benet, S.; Blanch-Lombarte, O.; Ainsua-Enrich, E.; Pedreño-Lopez, N.; Muñoz-Basagoiti, J.; Raïch-Regué, D.; Perez-Zsolt, D.; Peña, R.; Jiménez, E.; de la Concepción, M.L.R.; et al. Limited Humoral and Specific T-Cell Responses After SARS-CoV-2 Vaccination in PWH With Poor Immune Reconstitution. J. Infect. Dis. 2022, 226, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Sisteré-Oró, M.; Andrade, N.; Wortmann, D.D.J.; Du, J.; Garcia-Giralt, N.; González-Cao, M.; Güerri-Fernández, R.; Meyerhans, A. Anti-SARS-CoV-2 Specific Immunity in HIV Immunological Non-Responders after MRNA-Based COVID-19 Vaccination. Front. Immunol. 2022, 13, 994173. [Google Scholar] [CrossRef]
- Ceravolo, A.; Orsi, A.; Parodi, V.; Ansaldi, F. Influenza Vaccination in HIV-Positive Subjects: Latest Evidence and Future Perspective. J. Prev. Med. Hyg. 2013, 54, 1–10. [Google Scholar]
- Lee, K.-Y.; Tsai, M.-S.; Kuo, K.-C.; Tsai, J.-C.; Sun, H.-Y.; Cheng, A.C.; Chang, S.-Y.; Lee, C.-H.; Hung, C.-C. Pneumococcal Vaccination among HIV-Infected Adult Patients in the Era of Combination Antiretroviral Therapy. Hum. Vaccines Immunother. 2014, 10, 3700–3710. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Urueña, J.M.; Davies, M.-A.; Sued, O.; Marcos, M.A.; Martínez, E.; Bertagnolio, S.; Alcamí, J.; Miro, J.M.; et al. Overview of SARS-CoV-2 Infection in Adults Living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef]
- Vergori, A.; Cozzi Lepri, A.; Cicalini, S.; Matusali, G.; Bordoni, V.; Lanini, S.; Meschi, S.; Iannazzo, R.; Mazzotta, V.; Colavita, F.; et al. Immunogenicity to COVID-19 MRNA Vaccine Third Dose in People Living with HIV. Nat. Commun. 2022, 13, 4922. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune Responses against SARS-CoV-2 Variants after Heterologous and Homologous ChAdOx1 NCoV-19/BNT162b2 Vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and Efficacy of Heterologous ChAdOx1-BNT162b2 Vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Favà, A.; Donadeu, L.; Sabé, N.; Pernin, V.; González-Costello, J.; Lladó, L.; Meneghini, M.; Charmetant, X.; García-Romero, E.; Cachero, A.; et al. SARS-CoV-2-Specific Serological and Functional T Cell Immune Responses during Acute and Early COVID-19 Convalescence in Solid Organ Transplant Patients. Am. J. Transplant. 2021, 21, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Justine Konik, M.; Meister, T.L.; Pfaender, S.; et al. The Magnitude and Functionality of SARS-CoV-2 Reactive Cellular and Humoral Immunity in Transplant Population Is Similar to the General Population Despite Immunosuppression. Transplantation 2021, 105, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Kamar, N.; Vergez, F.; Faguer, S.; Marion, O.; Beq, A.; Lathrache, Y.; Abravanel, F.; Izopet, J.; Treiner, E. Adaptive Lymphocyte Profile Analysis Discriminates Mild and Severe Forms of COVID-19 after Solid Organ Transplantation. Kidney Int. 2021, 100, 915–927. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Favà, A.; Donadeu, L.; Jouve, T.; Gonzalez-Costello, J.; Lladó, L.; Santana, C.; Toapanta, N.; Lopez, M.; Pernin, V.; Facundo, C.; et al. A Comprehensive Assessment of Long-Term SARS-CoV-2-Specific Adaptive Immune Memory in Convalescent COVID-19 Solid Organ Transplant Recipients. Kidney Int. 2022, 101, 1027–1038. [Google Scholar] [CrossRef]
- Candon, S.; Guerrot, D.; Drouot, L.; Lemoine, M.; Lebourg, L.; Hanoy, M.; Boyer, O.; Bertrand, D. T Cell and Antibody Responses to SARS-CoV-2: Experience from a French Transplantation and Hemodialysis Center during the COVID-19 Pandemic. Am. J. Transplant. 2021, 21, 854–863. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Drouot, L.; Lamulle, J.; Hanoy, M.; Edet, S.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; et al. SARS-CoV-2-Specific Humoral and Cellular Immunities in Kidney Transplant Recipients and Dialyzed Patients Recovered From Severe and Nonsevere COVID-19. Transplant. Direct 2021, 7, e792. [Google Scholar] [CrossRef]
- Charmetant, X.; Espi, M.; Benotmane, I.; Barateau, V.; Heibel, F.; Buron, F.; Gautier-Vargas, G.; Delafosse, M.; Perrin, P.; Koenig, A.; et al. Infection or a Third Dose of MRNA Vaccine Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Kidney Transplant Recipients. Sci. Transl. Med. 2022, 14, eabl6141. [Google Scholar] [CrossRef]
- Becchetti, C.; Broekhoven, A.G.C.; Dahlqvist, G.; Fraga, M.; Zambelli, M.F.; Ciccarelli, O.; Saouli, A.-C.; Trizzino, A.; Banz, V.; Dufour, J.-F.; et al. Humoral Response to SARS-CoV-2 Infection among Liver Transplant Recipients. Gut 2022, 71, 746–756. [Google Scholar] [CrossRef]
- Caballero-Marcos, A.; Salcedo, M.; Alonso-Fernández, R.; Rodríguez-Perálvarez, M.; Olmedo, M.; Graus Morales, J.; Cuervas-Mons, V.; Cachero, A.; Loinaz-Segurola, C.; Iñarrairaegui, M.; et al. Changes in Humoral Immune Response after SARS-CoV-2 Infection in Liver Transplant Recipients Compared to Immunocompetent Patients. Am. J. Transplant. 2021, 21, 2876–2884. [Google Scholar] [CrossRef]
- Caballero-Marcos, A.; Citores, M.J.; Alonso-Fernández, R.; Rodríguez-Perálvarez, M.; Valerio, M.; Graus Morales, J.; Cuervas-Mons, V.; Cachero, A.; Loinaz-Segurola, C.; Iñarrairaegui, M.; et al. Decreased Long-Term Severe Acute Respiratory Syndrome Coronavirus 2-Specific Humoral Immunity in Liver Transplantation Recipients 12 Months After Coronavirus Disease 2019. Liver Transpl. 2022, 28, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Olea, B.; Almendro-Vázquez, P.; Giménez, E.; Marcacuzco, A.; San Juan, R.; Justo, I.; Calvo-Pulido, J.; García-Sesma, Á.; Manrique, A.; et al. T Cell-Mediated Response to SARS-CoV-2 in Liver Transplant Recipients with Prior COVID-19. Am. J. Transplant. 2021, 21, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Yanis, A.; Haddadin, Z.; Spieker, A.J.; Waqfi, D.; Rankin, D.A.; Talj, R.; Thomas, L.; Birdwell, K.A.; Ezzell, L.; Blair, M.; et al. Humoral and Cellular Immune Responses to the SARS-CoV-2 BNT162b2 Vaccine among a Cohort of Solid Organ Transplant Recipients and Healthy Controls. Transpl. Infect. Dis. 2022, 24, e13772. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired Anti-SARS-CoV-2 Humoral and Cellular Immune Response Induced by Pfizer-BioNTech BNT162b2 MRNA Vaccine in Solid Organ Transplanted Patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular Immunity Predominates over Humoral Immunity after Homologous and Heterologous MRNA and Vector-Based COVID-19 Vaccine Regimens in Solid Organ Transplant Recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Marinelli, T.; Ierullo, M.; Ku, T.; Hall, V.G.; Majchrzak-Kita, B.; Kulasingam, V.; Humar, A.; Kumar, D. Severe Acute Respiratory Syndrome Coronavirus 2 Infection Induces Greater T-Cell Responses Compared to Vaccination in Solid Organ Transplant Recipients. J. Infect. Dis. 2021, 224, 1849–1860. [Google Scholar] [CrossRef]
- Almanzar, G.; Schwaiger, S.; Jenewein, B.; Keller, M.; Herndler-Brandstetter, D.; Würzner, R.; Schönitzer, D.; Grubeck-Loebenstein, B. Long-Term Cytomegalovirus Infection Leads to Significant Changes in the Composition of the CD8+ T-Cell Repertoire, Which May Be the Basis for an Imbalance in the Cytokine Production Profile in Elderly Persons. J. Virol. 2005, 79, 3675–3683. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of Immunological Markers in Predicting Responsiveness to Influenza Vaccination in Elderly Individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of MRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.-L.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary Antimetabolite Treatment Hold Boosts SARS-CoV-2 Vaccination-Specific Humoral and Cellular Immunity in Kidney Transplant Recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef] [PubMed]
- Harberts, A.; Schaub, G.M.; Ruether, D.F.; Duengelhoef, P.M.; Brehm, T.T.; Karsten, H.; Fathi, A.; Jahnke-Triankowski, J.; Fischer, L.; Addo, M.M.; et al. Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 MRNA Vaccination in Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2022, 20, 2558–2566.e5. [Google Scholar] [CrossRef]
- Davidov, Y.; Indenbaum, V.; Tsaraf, K.; Cohen-Ezra, O.; Likhter, M.; Ben Yakov, G.; Halperin, R.; Levy, I.; Mor, O.; Agmon-Levin, N.; et al. A Third Dose of the BNT162b2 MRNA Vaccine Significantly Improves Immune Responses among Liver Transplant Recipients. J. Hepatol. 2022, 77, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Vilain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-Cell Responses After 2 Injections of MRNA Vaccine in Kidney Transplant Recipients Treated With Belatacept. Transplantation 2021, 105, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired Humoral and Cellular Immunity after SARS-CoV-2 BNT162b2 (Tozinameran) Prime-Boost Vaccination in Kidney Transplant Recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Meunier, L.; Sanavio, M.; Dumortier, J.; Meszaros, M.; Faure, S.; Ursic Bedoya, J.; Echenne, M.; Boillot, O.; Debourdeau, A.; Pageaux, G.P. Mycophenolate Mofetil Decreases Humoral Responses to Three Doses of SARS-CoV-2 Vaccine in Liver Transplant Recipients. Liver Int. 2022, 42, 1872–1878. [Google Scholar] [CrossRef]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low Immunogenicity to SARS-CoV-2 Vaccination among Liver Transplant Recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Magicova, M.; Zahradka, I.; Fialova, M.; Neskudla, T.; Gurka, J.; Modos, I.; Hojny, M.; Raska, P.; Smejkal, P.; Striz, I.; et al. Determinants of Immune Response to Anti-SARS-CoV-2 MRNA Vaccines in Kidney Transplant Recipients: A Prospective Cohort Study. Transplantation 2022, 106, 842–852. [Google Scholar] [CrossRef]
- Allison, A.C.; Eugui, E.M. Mechanisms of Action of Mycophenolate Mofetil in Preventing Acute and Chronic Allograft Rejection. Transplantation 2005, 80, S181–S190. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.J.; Cook, J.M. The Inhibition of Nucleic Acid Synthesis by Mycophenolic Acid. Biochem. J. 1969, 113, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Robarts, P.; Chauhan, M. Analysis of Antibody Responses after COVID-19 Vaccination in Liver Transplant Recipients and Those with Chronic Liver Diseases. J. Hepatol. 2021, 75, 1434–1439. [Google Scholar] [CrossRef]
- Davidov, Y.; Tsaraf, K.; Cohen-Ezra, O.; Likhter, M.; Ben Yakov, G.; Levy, I.; Levin, E.G.; Lustig, Y.; Mor, O.; Rahav, G.; et al. Immunogenicity and Adverse Effects of the 2-Dose BNT162b2 Messenger RNA Vaccine Among Liver Transplantation Recipients. Liver Transpl. 2022, 28, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Toniutto, P.; Cussigh, A.; Cmet, S.; Bitetto, D.; Fornasiere, E.; Fumolo, E.; Fabris, M.; D’Aurizio, F.; Fabris, C.; Grillone, L.; et al. Immunogenicity and Safety of a Third Dose of Anti-SARS-CoV-2 BNT16b2 Vaccine in Liver Transplant Recipients. Liver Int. 2022, 43, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-Specific Humoral and T-Cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162–172.e9. [Google Scholar] [CrossRef] [PubMed]
- D’Offizi, G.; Agrati, C.; Visco-Comandini, U.; Castilletti, C.; Puro, V.; Piccolo, P.; Montalbano, M.; Meschi, S.; Tartaglia, E.; Sorace, C.; et al. Coordinated Cellular and Humoral Immune Responses after Two-Dose SARS-CoV2 MRNA Vaccination in Liver Transplant Recipients. Liver Int. 2022, 42, 180–186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeg, D.B.; Hofmann, M.; Neumann-Haefelin, C.; Thimme, R.; Luxenburger, H. SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants. Pathogens 2023, 12, 244. https://doi.org/10.3390/pathogens12020244
Reeg DB, Hofmann M, Neumann-Haefelin C, Thimme R, Luxenburger H. SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants. Pathogens. 2023; 12(2):244. https://doi.org/10.3390/pathogens12020244
Chicago/Turabian StyleReeg, David B., Maike Hofmann, Christoph Neumann-Haefelin, Robert Thimme, and Hendrik Luxenburger. 2023. "SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants" Pathogens 12, no. 2: 244. https://doi.org/10.3390/pathogens12020244
APA StyleReeg, D. B., Hofmann, M., Neumann-Haefelin, C., Thimme, R., & Luxenburger, H. (2023). SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants. Pathogens, 12(2), 244. https://doi.org/10.3390/pathogens12020244





