Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed-Treated Hybrids/Plant Material
2.2. Pathogen Origin, Growth Media, Infection
2.3. Disease Documentation
2.4. Statistical Analyses
3. Results
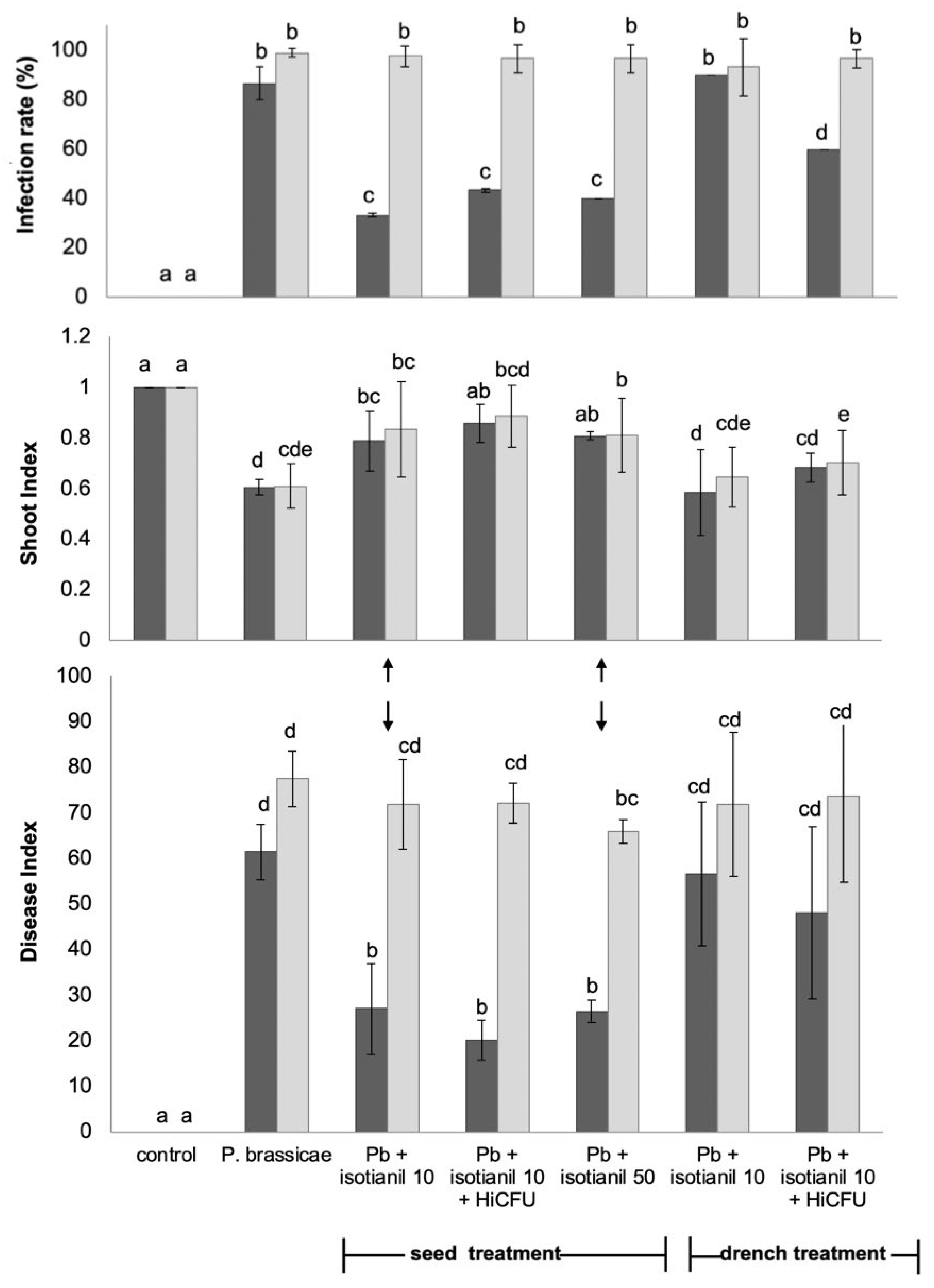
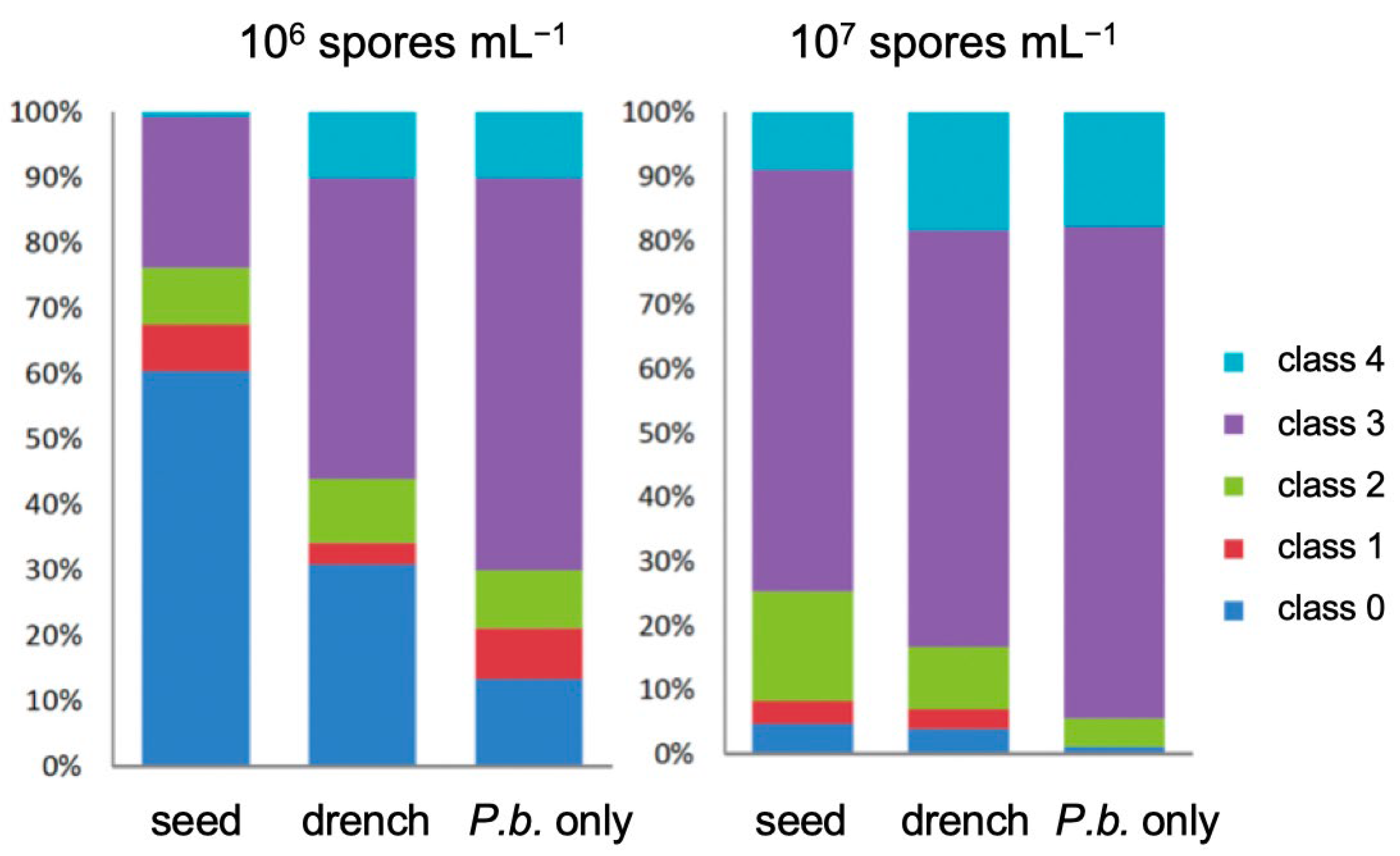
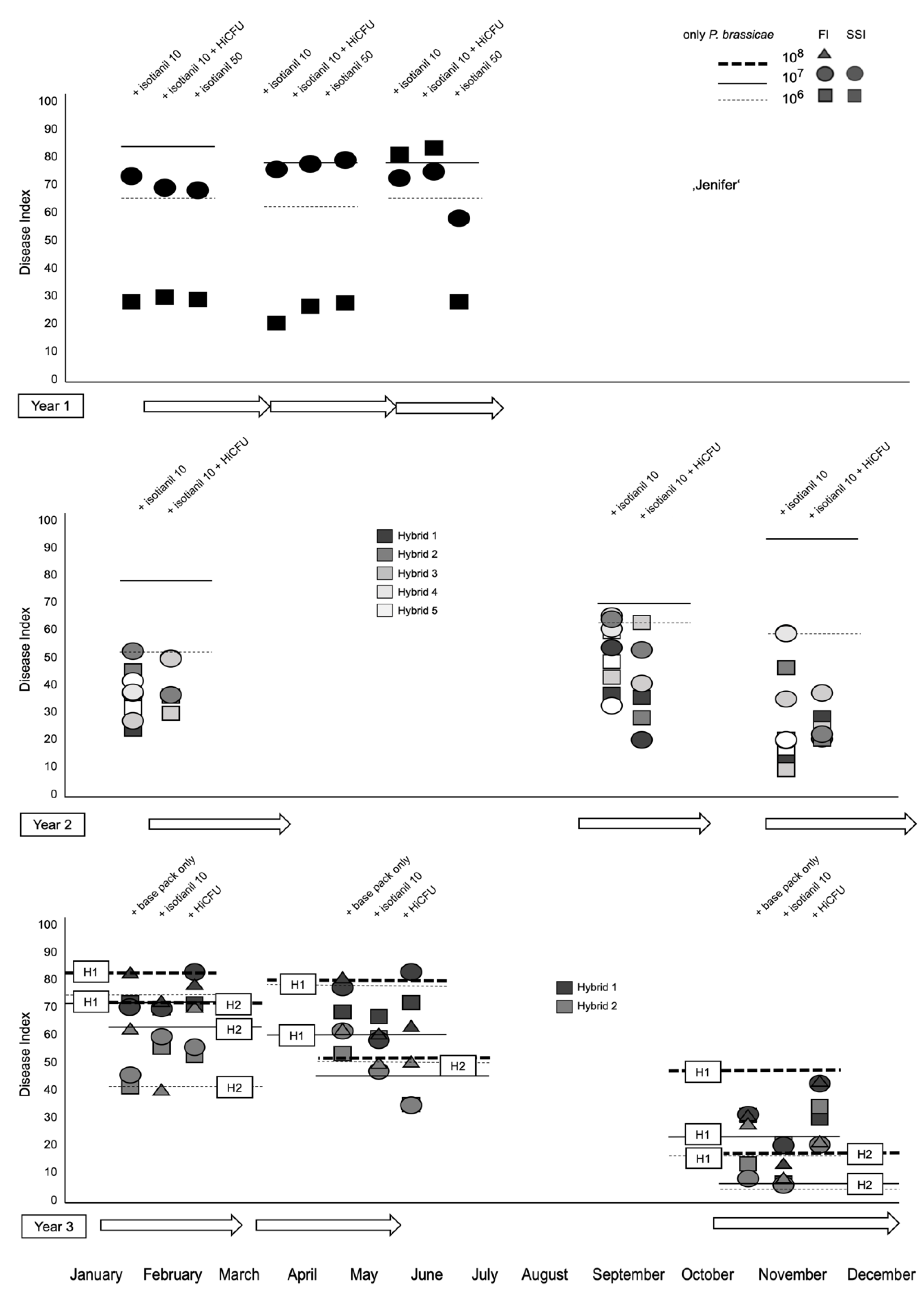
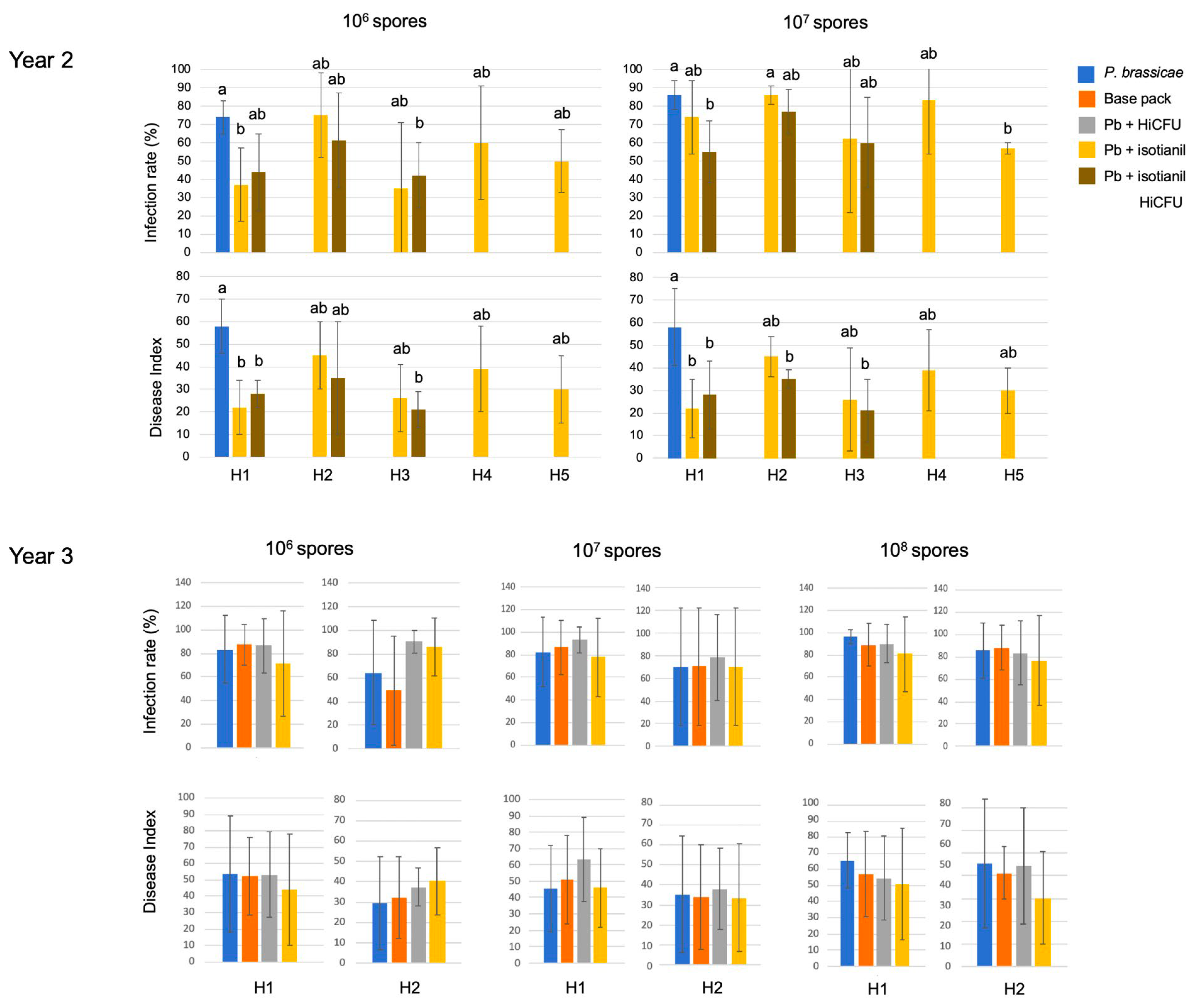
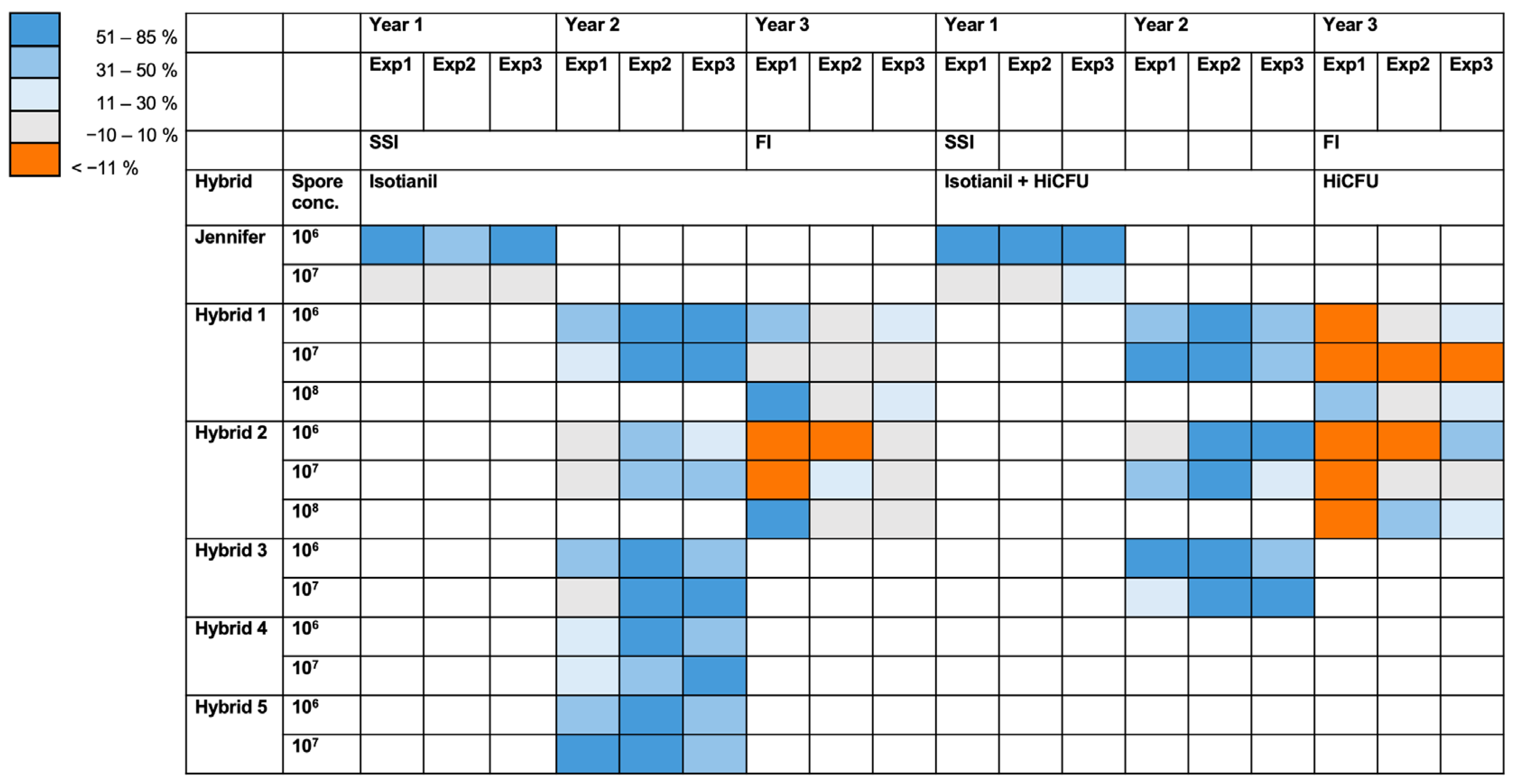
3.1. Product Performances Following Different Application Techniques
3.2. The Effect of Isotianil Is Primarily Dependent on Hybrid, P. brassicae Isolate, Spore Concentration, and the Season
3.3. Shoot Growth Is Not Consistently Affected
4. Discussion
4.1. Infection-Related Changes in Response to Host Plants
4.2. The Environment Can Influence Disease Severity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Qin, L.; Zhou, Z.; Hendriks, W.G.H.M.; Liu, S.; Wei, Y. Refining the life cycle of Plasmodiophora brassicae. Phytopathology 2020, 110, 1704–1712. [Google Scholar] [CrossRef]
- Amponsah, J.; Tegg, R.S.; Thangavel, T.; Wilson, C.R. Moments of weaknesses—Exploiting vulnerabilities between germination and encystment in the Phytomyxea. Biol. Rev. 2021, 96, 1603–1615. [Google Scholar] [CrossRef]
- Wallenhammar, A.C. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 1996, 45, 710–719. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Peng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and pathotype classification of Plasmodiophora brassicae populations collected from clubroot resistant canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Fredua-Agyeman, R.; Hwang, S.F.; Zhang, H.; Falak, I.; Huang, X.; Strelkov, S.E. Clubroot resistance derived from the European Brassica napus cv. ‘Tosca’ is not effective against virulent Plasmodiophora brassicae isolates from Alberta, Canada. Sci. Rep. 2021, 11, 14472. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Krohne, I.; Koopmann, B. Greenhouse Evaluation of Clubroot Resistant-Brassica napus cv. Mendel and Its Efficacy Concerning Virulence and Soil Inoculum Levels of Plasmodiophora brassicae. Pathogens 2021, 10, 151. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jülke, S.; Geiß, K.; Richter, F.; Sola, I.; Rusak, G.; Mithöfer, A.; Keenan, S.; Bulman, S. A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae methylates salicylic acid. Mol. Plant Pathol. 2015, 16, 349–364. [Google Scholar] [CrossRef]
- Hossain, M.M.; Pérez-López, E.; Todd, C.D.; Wei, Y.; Bonham-Smith, P.C. Endomembrane-Targeting Plasmodiophora brassicae Effectors Modulate PAMP Triggered Immune Responses in Plants. Front. Microbiol. 2021, 12, 651279. [Google Scholar] [CrossRef]
- Wang, W.; Feng, B.; Zhou, J.M.; Tang, D. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020, 62, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.A.; van der Heijdt, W.H.; Wendehenne, D.; Pugin, A. Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Mol. Plant Microbe Interact. 2008, 21, 1609–1621. [Google Scholar] [CrossRef]
- Conrath, U. Priming of induced plant defense responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar] [CrossRef]
- FRAC Fungicide Resistance Action Committee 2023. Available online: https://www.frac.info/ (accessed on 20 March 2023).
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Toquin, V.; Sirven, C.; Assmann, L.; Sawada, H. Host Defense inducers. In Modern Crop Protection Compounds; Krämer, W., Schirmer, U., Jeschke, P., Witschel, M., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 908–928. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Portz, K.; Casanova, F.; Jordine, A.; Bohnert, S.; Mehl, A.; Portz, D.; Schaffrath, U. Wheat blast caused by Magnaporthe oryzae pathovar Triticum is efficiently controlled by the plant defence inducer Isotianil. J. Plant Dis. Protect. 2021, 128, 249–259. [Google Scholar] [CrossRef]
- Labourdette, G.; Chen, Y.-H.; Ceciliano, R.; Suan, G.; Pop, D. Use of Isotianil against Panama Disease, in German Patent Application PCT/EP20, World Intellectual Property Organization International Bureau: WO2019057661A1. WIPO (PCT). 2018. Available online: https://patentimages.storage.googleapis.com/f5/2d/37/b7a218786ef268/WO2019057661A1.pdf (accessed on 20 March 2023).
- Fan, B.; Blom, J.; Klenk, H.-P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an “Operational Group B. amyloliquefaciens” within the B. subtilis Species Complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougeres, T.; Lobach, A.R.; Simon, R.; Pelerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2016, 83, 54–65. [Google Scholar] [CrossRef]
- Hofemeister, J.; Conrad, B.; Adler, B. Genetic analysis of the biosynthesis of non-ribosomal peptide- and polyketidelike antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol. Genet. Genom. 2004, 272, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Van Overbeek, L.S.; Saikkonen, K. Impact of Bacterial–Fungal Interactions on the Colonization of the Endosphere. Trends Plant Sci. 2016, 21, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- Park, K.S.; Zeng, M.S.; Jeun, Y.C.; Nam, K.W. Rhizobacteria-mediated induced systemic resistance against various plant pathogens and its mechanisms of action. Mitt. Biol. Bundesanst. Land-und Forstwirtsch. 2006, 408, 49–55. [Google Scholar]
- Alkooranee, J.T.; Aledan, T.R.; Ali, A.K.; Lu, G.; Zhang, X.; Wu, J.; Fu, C.; Li, M. Detecting the Hormonal Pathways in Oilseed Rape behind Induced Systemic Resistance by Trichoderma harzianum TH12 to Sclerotinia sclerotiorum. PLoS ONE 2017, 12, e0168850. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Goertz, S.; Moradtalab, N.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Drought-protective effects of nutrient seed treatments during early growth of oilseed rape. J. Plant Nutr. 2021, 45, 2945–2963. [Google Scholar] [CrossRef]
- Fähling, M.; Graf, H.; Siemens, J. Pathotype Separation of Plasmodiophora brassicae by the Host Plant. J. Phytopathol. 2003, 151, 425–430. [Google Scholar] [CrossRef]
- Fähling, M.; Graf, H.; Siemens, J. Characterization of a Single-Spore Isolate Population of Plasmodiophora brassicae Resulting from a Single Club. J. Phytopathol. 2004, 152, 438–444. [Google Scholar] [CrossRef]
- Buczacki, S.T.; Toxopeus, H.; Mattusch, P.; Johnston, T.D.; Dixon, G.R.; Hobolth, L.A. Study of Physiologic Specialization in Plasmodiophora brassicae: Proposals for Attempted Rationalization through an International Approach. Trans. Br. Mycol. Soc. 1975, 65, 295–303. [Google Scholar] [CrossRef]
- Siemens, J.; Nagel, M.; Ludwig-Müller, J.; Sacristán, M. The interaction of Plasmodiophora brassicae and Arabidopsis thaliana: Parameters for disease quantification and screening of mutant lines. J. Phytopathol. 2002, 150, 592–605. [Google Scholar] [CrossRef]
- Auer, S.; Ludwig-Müller, J. Biocontrol of clubroot disease: How successful are endophytic fungi and bacteria? In European Journal of Plant Pathology; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Peng, G.; McGregor, L.; Lahlali, R.; Gossen, B.D.; Hwang, S.F.; Adhikari, K.K.; Strelkov, S.E.; McDonald, M.R. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 2011, 60, 566–574. [Google Scholar] [CrossRef]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Lahlali, R.; Hwang, S.F.; Hynes, R.K.; Falk, C.K. Assessment of crop rotation, cultivar resistance and Bacillus subtilis biofungicide for control of clubroot on canola. Acta Hortic. 2013, 1005, 591–598. [Google Scholar] [CrossRef]
- Peng, G.; Lahlali, R.; Hwang, S.F.; Pageau, D.; Hynes, R.K.; McDonald, M.R.; Gossen, B.D.; Strelkov, S.E. Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophora brassicae) on canola. Can. J. Plant Pathol. 2013, 36 (Suppl. 1), 99–112. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; McGregor, L.; Gossen, B.D.; Hwang, S.F.; McDonald, M. Mechanisms of the biofungicide Serenade (Bacillus subtilis QST713) in suppressing clubroot. Biocontrol Sci. Technol. 2011, 546, 1351–1362. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; Gossen, B.D.; McGregor, L.; Yu, F.Q.; Hynes, R.K.; Hwang, S.F.; McDonald, M.R.; Boyetchko, S.M. Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef]
- Gossen, B.D.; Adhikari, K.K.C.; McDonald, M.R. Effects of temperature on infection and subsequent development of clubroot under controlled conditions. Plant Pathol. 2012, 61, 593–599. [Google Scholar] [CrossRef]
- Sharma, K.; Gossen, B.D.; McDonald, M.R. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 2011, 60, 830–838. [Google Scholar] [CrossRef]
- Czajka, A.; Markiewicz, M.; Kowalska, B.; Smolińska, U. Reaction of clubroot-resistant genotypes of Brassica rapa, Brassica napus and Brassica oleracea to Polish Plasmodiophora brassicae pathotypes in laboratory tests. Eur. J. Plant Pathol. 2020, 158, 533–544. [Google Scholar] [CrossRef]
- Li, J.; Huang, T.; Lu, J.; Xu, X.; Zhang, W. Metabonomic profiling of clubroot-susceptible and clubroot-resistant radish and the assessment of disease-resistant metabolites. Front. Plant Sci. 2022, 13, 1037633. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.F.; McDonald, M.R. Interaction of pH and temperature affect infection and symptom development of Plasmodiophora brassicae in canola. Can. J. Plant Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Deora, A.; Peng, G.; McDonald, M.R. Effect of soil type, organic matter content, bulk density and saturation on clubroot severity and biofungicide efficacy. Plant Pathol. 2016, 65, 1238–1245. [Google Scholar] [CrossRef]
- Gravot, A.; Lemarié, S.; Richard, G.; Lime, T.; Lariagon, C.; Manzanares-Dauleux, M.J. Flooding affects the development of Plasmodiophora brassicae in Arabidopsis roots during the secondary phase of infection. Plant Pathol. 2016, 65, 1153–1160. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life Cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]

| Experimental Year | Experiment | Time Frame and Duration | No. of Plants/Treatment | Hybrid Name | Seed Source and Harvest Year | Seed Type | TKW (g) * | Germination |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 6.3.2019–24.4.2019 | 30 | Jenifer (4407) | Bayer AG, Oilseed Rape Centre, Monheim, 2018 | Conventional Hybrid 1 | 4.92 | 98 |
| 1b | 27.3.2019–21.5.2019 | |||||||
| 1c | 3.4.2019–4.6.2019 | |||||||
| 2 | 2a | 10.9.2020–30.10.2020 | 20 | Hybrid 1 Hybrid 2 Hybrid 3 Hybrid 4 Hybrid 5 | Bayer SAS Boissay France, 2020 | Conventional Hybrid Conventional Hybrid Conventional Hybrid HOLL Hybrid 2 Dwarf Hybrid 3 | 4.91 4.51 5.90 6.68 5.43 | 98 100 92 91 96 |
| 2b | 26.11.2020–19.1.2021 | |||||||
| 2c | 10.2.2021–8.4.2021 | |||||||
| 3 | 3a | 28.10.2021–6.1.2022 | 20 | Hybrid 1 Hybrid 2 | Bayer SAS Boissay France, 2020 | Conventional Hybrid Conventional Hybrid | 4.91 4.51 | 98 100 |
| 3b | 14.1.2022–17.3.2022 | |||||||
| 3c | 25.3.2022–17.5.2022 |
| Study Period | Treatments | Formulation | Dose Rates | Application | P. brassicae Inoculum |
|---|---|---|---|---|---|
| Year 1 |
| - | - | - | - |
| - | - | - | SSI | |
| * FS200 | 0.1 g/kg | ST | SSI | |
| FS200 and FS150 | 0.1 g/kg and 6.6 mL/kg | ST | SSI | |
| FS200 | 0.5 g/kg | ST | SSI | |
| FS200 | 0.5 g/ha | drench | SSI | |
| FS150 | 5 L/ha | drench | SSI | |
| Year 2 |
| - | - | - | - |
| - | - | - | SSI | |
| FS200 | 0.1 g/kg | ST | SSI | |
| FS200 and FS150 | 0.1 g/kg and 6.6 mL/kg | ST | SSI | |
| Year 3 |
| - | - | - | - |
| - | - | - | FI | |
| - | - | ST | FI | |
| and FS200 | and 0.1 g/kg | ST | FI | |
| and FS150 | and 6.6 mL/kg | ST | FI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klueken, A.M.; Mahfoud, Y.; Rößler, S.; Ludwig-Müller, J. Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids. Pathogens 2023, 12, 1339. https://doi.org/10.3390/pathogens12111339
Klueken AM, Mahfoud Y, Rößler S, Ludwig-Müller J. Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids. Pathogens. 2023; 12(11):1339. https://doi.org/10.3390/pathogens12111339
Chicago/Turabian StyleKlueken, A. Michael, Yamen Mahfoud, Sabine Rößler, and Jutta Ludwig-Müller. 2023. "Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids" Pathogens 12, no. 11: 1339. https://doi.org/10.3390/pathogens12111339
APA StyleKlueken, A. M., Mahfoud, Y., Rößler, S., & Ludwig-Müller, J. (2023). Testing Effects of Seed Treatments against Clubroot Disease in Various Oilseed Rape Hybrids. Pathogens, 12(11), 1339. https://doi.org/10.3390/pathogens12111339









