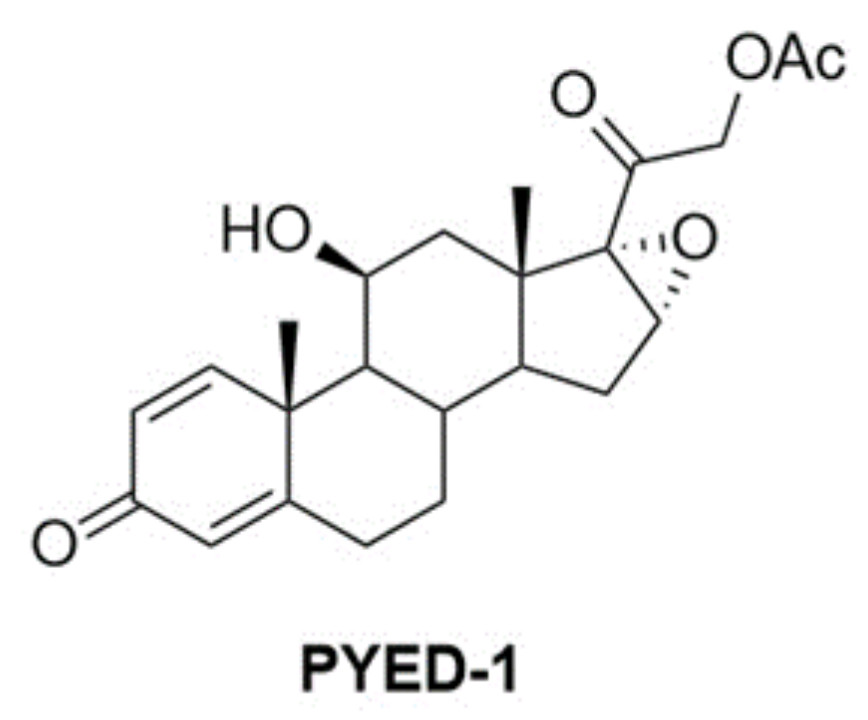
PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Bacterial Strains and Growth Conditions
2.3. Broth Microdilution Method for Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4. Checkerboard Assay
2.5. Time–Kill Assays
2.6. Biofilm Assay
2.7. Hemolysis Assay
2.8. Statistical Analysis
3. Results and Discussion
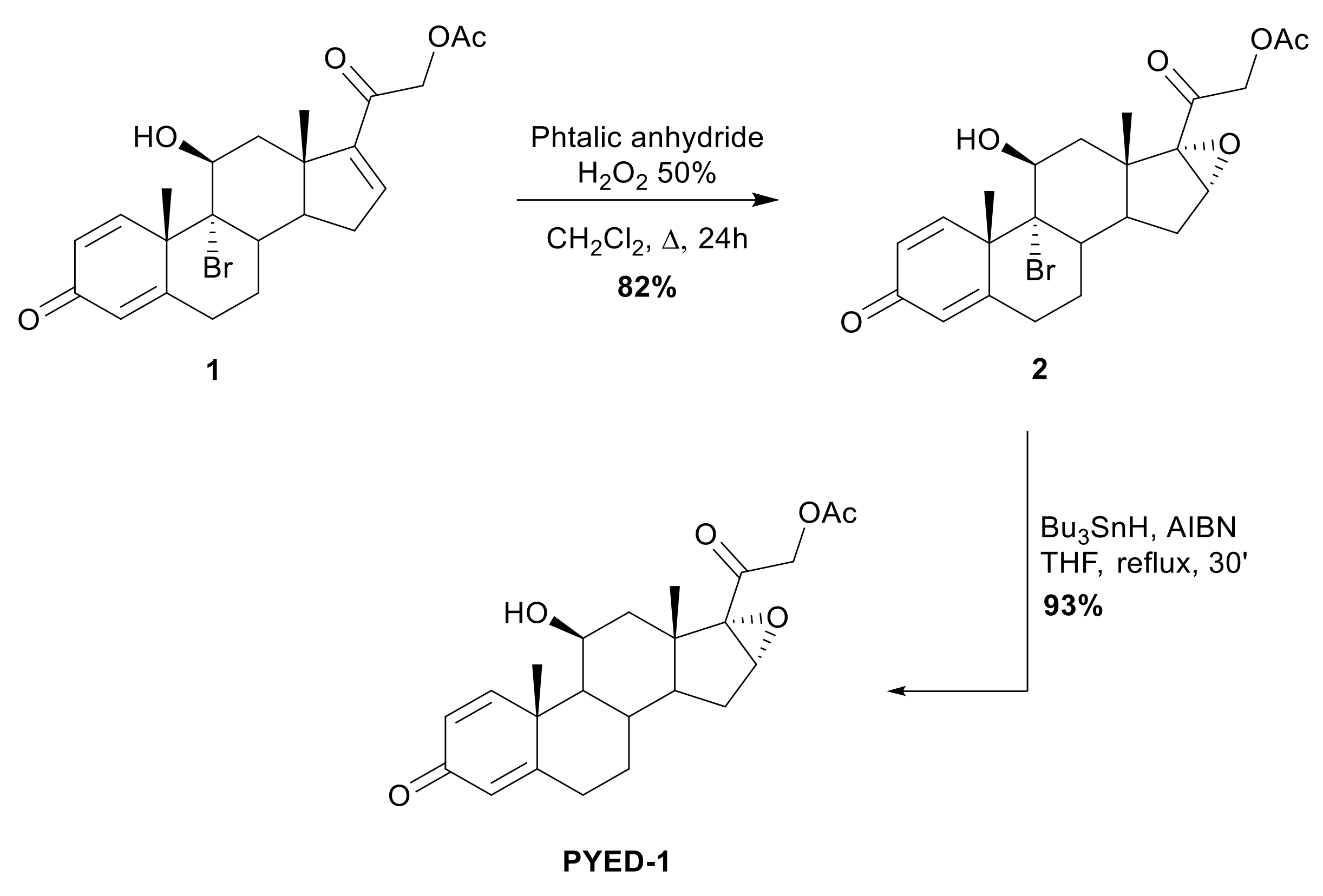
3.1. Chemistry
3.2. Antibacterial Activity of PYED-1
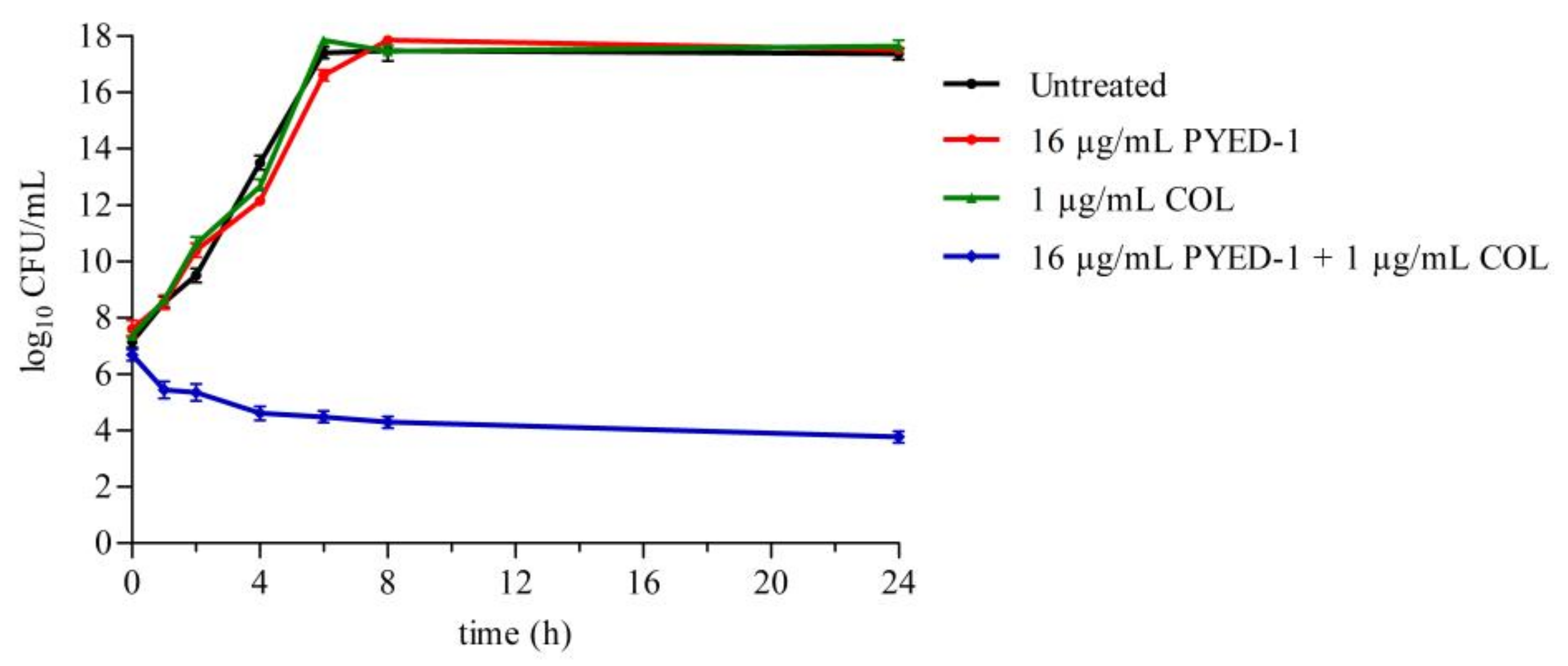
3.3. PYED-1 Displays Excellent Synergistic Activity with Colistin
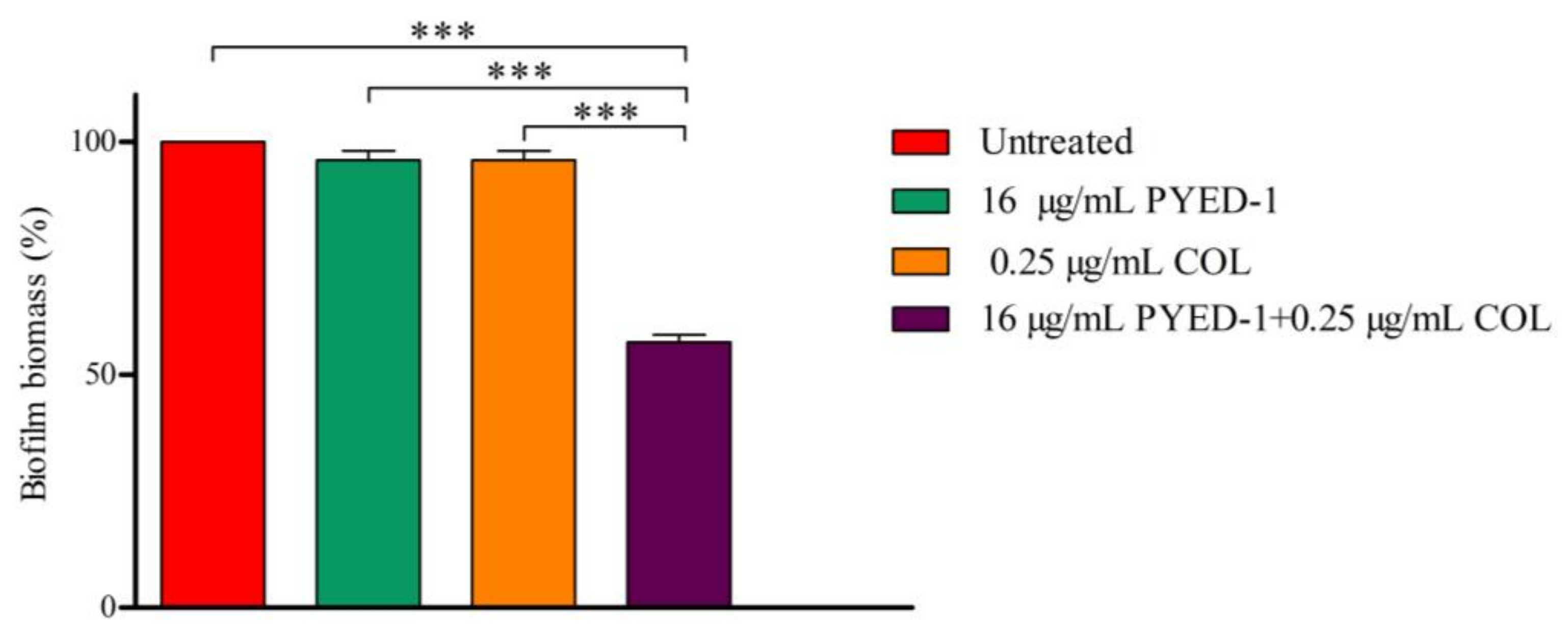
3.4. Effects of PYED-1 on the Formation of A. baumannii Biofilm
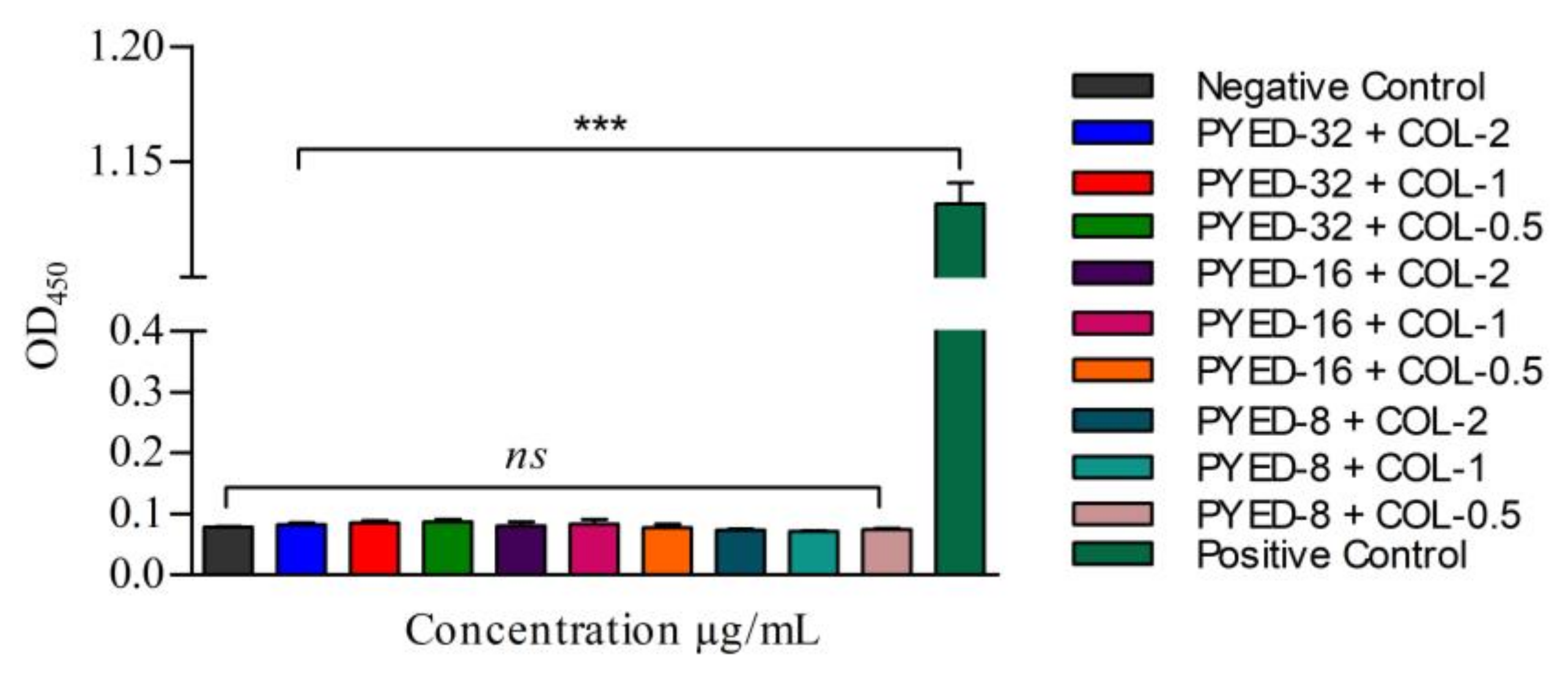
3.5. Safety Evaluation of the Combined Use of PYED-1 with Colistin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 29 September 2023).
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Fahy, S.; O’Connor, J.A.; Lucey, B.; Sleator, R.D. Hospital Reservoirs of Multidrug Resistant Acinetobacter Species—The Elephant in the Room! Br. J. Biomed. Sci. 2023, 80, 11098. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Minarini, L.A.D.R.; de Andrade, L.N.; De Gregorio, E.; Grosso, F.; Naas, T.; Zarrilli, R.; Camargo, I.L.B.C. Editorial: Antimicrobial Resistance as a Global Public Health Problem: How Can We Address It? Front. Public Health 2020, 8, 612844. [Google Scholar] [CrossRef]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, S.; Davarpanah, M.A.; Roudgari, A.; Bahador, A.; Karbasizade, V.; Kargar Jahromi, Z. Molecular evaluation of colistin-resistant gene expression changes in Acinetobacter baumannii with real-time polymerase chain reaction. Infect. Drug Resist. 2017, 10, 455–462. [Google Scholar] [CrossRef]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, ARBA-0009-2017. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Madec, J.Y.; Laxminarayan, R. Colistin: From the shadows to a One Health approach for addressing antimicrobial resistance. Int. J. Antimicrob. Agents 2023, 61, 106713. [Google Scholar] [CrossRef] [PubMed]
- Binsker, U.; Käsbohrer, A.; Hammerl, J.A. Global colistin use: A review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol. Rev. 2022, 46, fuab049. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Hassan, B.; Farzana, R.; Ali, Q.; Sands, K.; Mathias, J.; Afegbua, S.; Haque, M.N.; Walsh, T.R.; Mohsin, M. International manufacturing and trade in colistin, its implications in colistin resistance and One Health global policies: A microbiological, economic, and anthropological study. Lancet Microbe 2023, 4, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Mehdinejadiani, K.; Gholizadeh, P.; Nasiri, M.J.; Mohtavinejad, N.; Dadashi, M.; Karimaei, S.; Safari, H.; Azimi, T. Global prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: A systematic review and meta-analysis. Microb. Pathog. 2020, 139, 103887. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D.; et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter baumannii Clinical Isolates. mBio 2019, 10, e01083-19. [Google Scholar] [CrossRef]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef]
- Wareham, D.W.; Gordon, N.C.; Hornsey, M. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 2011, 66, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.C.; Png, K.; Wareham, D.W. Potent synergy and sustained bactericidal activity of a vancomycin/colistin combination versus multi-drug resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 5316–5322. [Google Scholar] [CrossRef]
- Esposito, A.; De Gregorio, E.; De Fenza, M.; D’Alonzo, D.; Satawani, A.; Guaragna, A. Expeditious synthesis and preliminary antimicrobial activity of deflazacort and its precursors. RSC Adv. 2019, 9, 21519–21524. [Google Scholar] [CrossRef] [PubMed]
- Vollaro, A.; Esposito, A.; Antonaki, E.; Iula, V.D.; D’Alonzo, D.; Guaragna, A.; De Gregorio, E. Steroid derivatives as potential antimicrobial agents against Staphylococcus aureus planktonic cells. Microorganisms 2020, 8, 468. [Google Scholar] [CrossRef]
- Esposito, A.; Vollaro, A.; Esposito, E.P.; D’Alonzo, D.; Guaragna, A.; Zarrilli, R.; De Gregorio, E. Antibacterial and antivirulence activity of glucocorticoid PYED-1 against Stenotrophomonas maltophilia. Antibiotics 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Vollaro, A.; Esposito, A.; Esposito, E.P.; Zarrilli, R.; Guaragna, A.; De Gregorio, E. PYED-1 inhibits biofilm formation and disrupts the preformed biofilm of Staphylococcus aureus. Antibiotics 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Del Franco, M.; Andini, R.; Bernardo, M.; Giannouli, M.; Zarrilli, R. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2015, 82, 222–226. [Google Scholar] [CrossRef]
- Di Nocera, P.P.; De Gregorio, E.; Rocco, F. GTAG- and CGTC-tagged palindromic DNA repeats in prokaryotes. BMC Genom. 2013, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, S.; Poulou, A.; Dafopoulou, K.; Chabane, Y.N.; Kristo, I.; Makris, D.; Hardouin, J.; Cosette, P.; Tsakris, A.; Dé, E. Growth retardation, reduced invasiveness, and impaired colistin-mediated cell death associated with colistin resistance development in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 828–832. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters v.12.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 29 September 2023).
- De Gregorio, E.; Esposito, A.; Vollaro, A.; De Fenza, M.; D’Alonzo, D.; Migliaccio, A.; Iula, V.D.; Zarrilli, R.; Guaragna, A. N-Nonyloxypentyl-l-Deoxynojirimycin Inhibits Growth, Biofilm Formation and Virulence Factors Expression of Staphylococcus aureus. Antibiotics 2020, 9, 362. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; The Lippincott Williams & Wilkins Co.: Philadelphia, PA, USA, 2005; pp. 365–440. [Google Scholar]
- Xu, M.; Yao, Z.; Zhao, Y.; Shi, S.; Sun, Y.; Feng, L.; Zhou, C.; Zhang, X.; Cao, J.; Zhou, T. Naringenin restores colistin activation against colistin-resistant gram-negative bacteria in vitro and in vivo. Front. Microbiol. 2022, 13, 916587. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, J.; Zhang, X.; Liu, Y.; Huang, Z.; Yuan, L.; Zhang, Y.; Cao, J.; Chen, L.; Liu, Y.; et al. Plumbagin resurrect colistin susceptible against colistin-resistant Pseudomonas aeruginosa in vitro and in vivo. Front. Microbiol. 2022, 13, 1020652. [Google Scholar] [CrossRef]
- Migliaccio, A.; Esposito, E.P.; Bagattini, M.; Berisio, R.; Triassi, M.; De Gregorio, E.; Zarrilli, R. Inhibition of AdeB, AceI, and AmvA Efflux Pumps Restores Chlorhexidine and Benzalkonium Susceptibility in Acinetobacter baumannii ATCC 19606. Front. Microbiol. 2022, 12, 790263. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Li, M.; Sun, X.; Wang, Y.; Yang, L.; Jiao, H.; Li, G. Synergistic Activity of Capsaicin and Colistin Against Colistin-Resistant Acinetobacter baumannii: In Vitro/Vivo Efficacy and Mode of Action. Front. Pharmacol. 2021, 12, 744494. [Google Scholar] [CrossRef] [PubMed]
- Collalto, D.; Fortuna, A.; Visca, P.; Imperi, F.; Rampioni, G.; Leoni, L. Synergistic Activity of Colistin in Combination with Clofoctol against Colistin Resistant Gram-Negative Pathogens. Microbiol. Spectr. 2023, 11, 0427522. [Google Scholar] [CrossRef] [PubMed]
- Sannio, F.; Brizzi, A.; Del Prete, R.; Avigliano, M.; Simone, T.; Pagli, C.; Ferraro, T.; De Luca, F.; Paolino, M.; Corelli, F.; et al. Optimization of Pyrazole Compounds as Antibiotic Adjuvants Active against Colistin- and Carbapenem-Resistant Acinetobacter baumannii. Antibiotics 2022, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.L.; Worthington, R.J.; Hittle, L.E.; Zurawski, D.V.; Ernst, R.K.; Melander, C. Small molecule downregulation of PmrAB reverses lipid A modification and breaks colistin resistance. ACS Chem. Biol. 2014, 9, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; Principato, S.; Colavecchio, O.L.; Pallecchi, L.; Rossolini, G.M. Synergistic activity of colistin in combination with resveratrol against colistin-resistant gram-negative pathogens. Front. Microbiol. 2018, 9, 1808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Kong, L.C.; Liu, J.; Ma, H.X. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the Mechanisms of Acinetobacter Baumannii Virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Huggins, W.M.; Barker, W.T.; Baker, J.T.; Hahn, N.A.; Melander, R.J.; Melander, C.; Meridianin, D. Analogues Display Antibiofilm Activity against MRSA and Increase Colistin Efficacy in Gram-Negative Bacteria. ACS Med. Chem. Lett. 2018, 9, 702–707. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Zhang, P.; Xu, M.; Yuan, W.; Bian, L.; Liu, Y.; Xia, J.; Leung, S.S.Y. Phage-Derived Depolymerase as an Antibiotic Adjuvant Against Multidrug-Resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 845500. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Feng, L.; Xu, M.; Wen, H.; Yao, Z.; Shi, S.; Wu, Q.; Zhou, C.; Cao, J.; et al. In vitro and in vivo synergistic effect of chrysin in combination with colistin against Acinetobacter baumannii. Front Microbiol. 2022, 13, 961498. [Google Scholar] [CrossRef] [PubMed]
| A. baumannii Clinical Isolates | PYED-1 | Colistin | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| 249 | 128 | 1024 | 256 | 512 |
| 347 | 128 | 1024 | 64 | 64 |
| 4451 | 64 | 1024 | 256 | 256 |
| 4452 | 64 | 1024 | 256 | 256 |
| 7120 | 256 | 1024 | 4 | 4 |
| 30831 | 128 | 1024 | 128 | 128 |
| 60520 | 128 | 1024 | 128 | 128 |
| 60794 | 256 | 1024 | 256 | 256 |
| 62258 | 128 | 1024 | 4 | 4 |
| 62790 | 128 | 1024 | 4 | 8 |
| average | 140.8 | 1024 | 135.6 | 161.6 |
| A. baumannii | MIC a | MIC c | FIC Index | |
|---|---|---|---|---|
| Clinical Isolate | Combination | |||
| 249 | PYED-1/colistin | 128/256 | 16/1 | 0.129 |
| 4451 | PYED-1/colistin | 64/256 | 8/1 | 0.129 |
| 30831 | PYED-1/colistin | 128/128 | 16/0.5 | 0.123 |
| 60794 | PYED-1/colistin | 256/256 | 16/1 | 0.066 |
| A. baumannii | MBC a | MBC c | FIC Index | |
|---|---|---|---|---|
| Clinical Isolate | Combination | |||
| 249 | PYED-1/colistin | 1024/512 | 16/2 | 0.018 |
| 4451 | PYED-1/colistin | 1024/256 | 8/1 | 0.012 |
| 30831 | PYED-1/colistin | 1024/128 | 16/0.5 | 0.006 |
| 60794 | PYED-1/colistin | 1024/256 | 16/2 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, M.; Esposito, A.; Iula, V.D.; Guaragna, A.; De Gregorio, E. PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii. Pathogens 2023, 12, 1323. https://doi.org/10.3390/pathogens12111323
Stabile M, Esposito A, Iula VD, Guaragna A, De Gregorio E. PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii. Pathogens. 2023; 12(11):1323. https://doi.org/10.3390/pathogens12111323
Chicago/Turabian StyleStabile, Maria, Anna Esposito, Vita Dora Iula, Annalisa Guaragna, and Eliana De Gregorio. 2023. "PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii" Pathogens 12, no. 11: 1323. https://doi.org/10.3390/pathogens12111323
APA StyleStabile, M., Esposito, A., Iula, V. D., Guaragna, A., & De Gregorio, E. (2023). PYED-1 Overcomes Colistin Resistance in Acinetobacter baumannii. Pathogens, 12(11), 1323. https://doi.org/10.3390/pathogens12111323









