Characterization of Equine Rhinitis B Virus Infection in Clinically Ill Horses in the United States during the Period 2012–2023
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Handling and Processing
2.2. Statistical Analysis
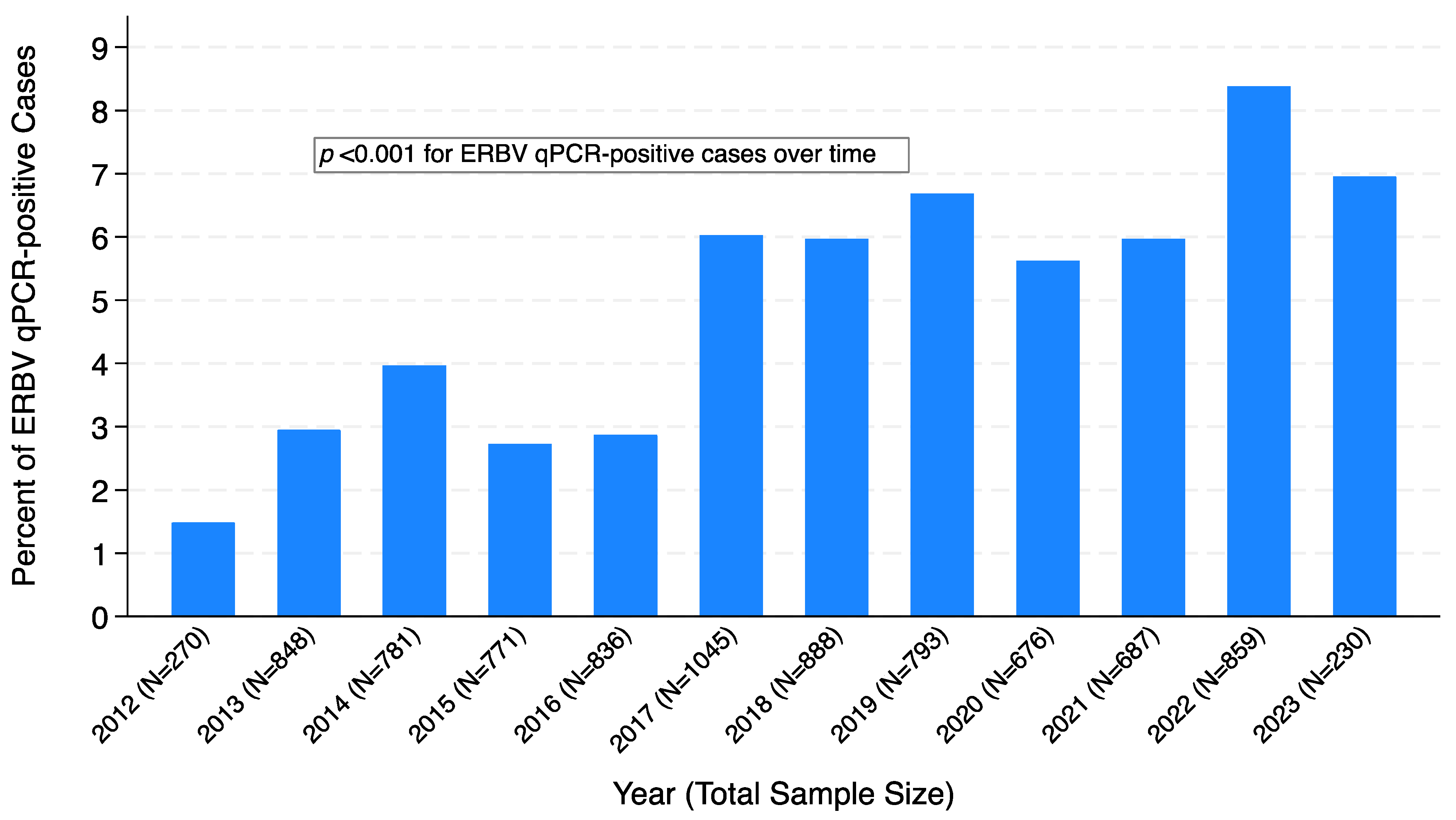
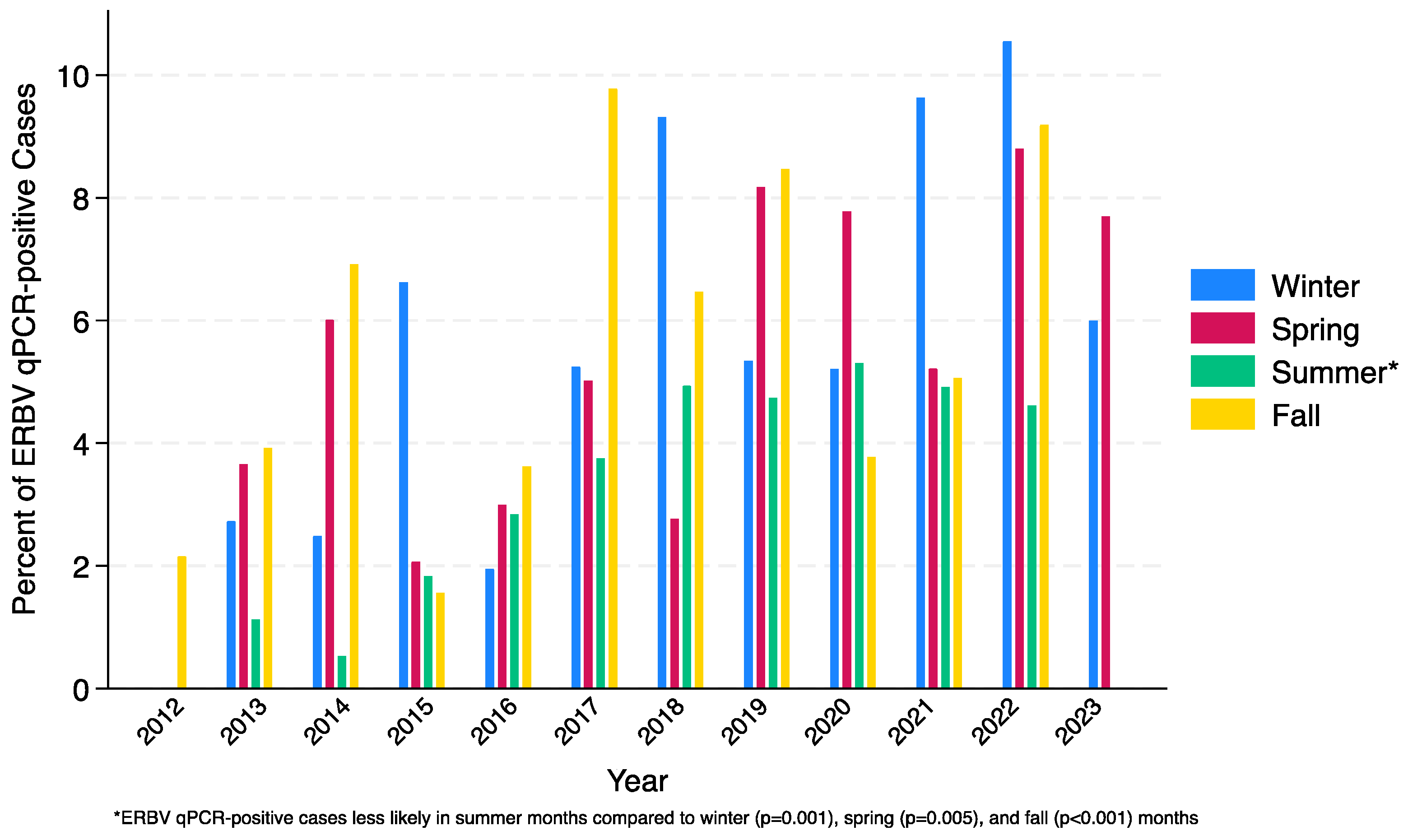
3. Results
3.1. ERBV qPCR-Negative vs. ERBV qPCR-Positive
3.2. ERBV qPCR-Negative vs. ERBV qPCR-Positive Sole Pathogen vs. ERBV qPCR-Positive Coinfection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| State | Number of Submissions (N, % of Total) |
|---|---|
| AL | 28 (0.54%) |
| AR | 6 (0.12%) |
| AZ | 164 (3.17%) |
| CA | 189 (3.65%) |
| CO | 166 (3.21%) |
| CT | 1 (0.02%) |
| FL | 225 (4.35%) |
| GA | 61 (1.18%) |
| IA | 51 (0.99%) |
| ID | 11 (0.21%) |
| IL | 3 (0.06%) |
| IN | 6 (0.12%) |
| KS | 20 (0.39%) |
| KY | 92 (1.78%) |
| KZ | 3 (0.06%) |
| LA | 2 (0.04%) |
| MD | 81 (1.57%) |
| MI | 77 (1.49%) |
| MN | 85 (1.64%) |
| MO | 188 (3.63%) |
| MP | 2 (0.04%) |
| MS | 1 (0.02%) |
| MT | 22 (0.43%) |
| NC | 67 (1.3%) |
| NE | 87 (1.68%) |
| NJ | 8 (0.15%) |
| NV | 5 (0.1%) |
| NY | 262 (5.06%) |
| OH | 251 (4.85%) |
| OK | 43 (0.83%) |
| OR | 198 (3.83%) |
| PA | 315 (6.09%) |
| RI | 124 (2.4%) |
| SC | 237 (4.58%) |
| SD | 11 (0.21%) |
| TN | 553 (10.69%) |
| TX | 1045 (20.2%) |
| UT | 1 (0.02%) |
| VA | 133 (2.57%) |
| VT | 1 (0.02%) |
| WA | 213 (4.12%) |
| WI | 100 (1.93%) |
| WV | 6 (0.12%) |
| WY | 29 (0.56%) |
References
- Hofer, B.; Steck, F.; Gerber, H.; Löhrer, J.; Nicolet, J.; Paccaud, M.F. An investigation of the etiology of viral respiratory disease in a remount depot. In Equine Infectious Diseases III, Proceedings of the Third International Conference of Equine Infectious Diseases, Paris, France, 17–21 July 1972; Bryans, J.T., Gerber, H., Eds.; S Karger AG: Basel, Switzerland, 1973; pp. 527–545. [Google Scholar]
- Mumford, J.A.; Thomson, G.R. Studies on picornaviruses isolated from the respiratory tract of horses. In Proceedings of the Fourth International Conference on Equine Infectious Diseases; Veterinary Publications: Princeton, NJ, USA, 1978; pp. 419–429. [Google Scholar]
- Carmen, S.; Rosendal, S.; Huber, L.; Gyles, C.; McKee, S.; Willoughby, R.A.; Dubovi, E.; Thorsen, J.; Lein, D. Infectious agents in acute respiratory disease in horses in Ontario. J. Vet. Diagn. Investig. 1997, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dunowska, M.; Wilks, C.R.; Studdert, M.J.; Meers, J. Viruses associated with outbreaks of equine respiratory disease in New Zealand. N. Z. Vet. J. 2002, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Dynon, K.; Black, W.D.; Ficorilli, N.; Hartley, C.A.; Studdert, M.J. Detection of viruses in nasal swab samples from horses with acute, febrile, respiratory disease using virus isolation, polymerase chain reaction and serology. Aust. Vet. J. 2007, 85, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendez, A.; Viel, L.; Hewson, J.; Doig, P.; Carman, S.; Chambers, T.; Tiwari, A.; Dewey, C. Surveillance of equine respiratory viruses in Ontario. Can. J. Vet. Res. 2010, 74, 271–278. [Google Scholar] [PubMed]
- Pusterla, N.; Mapes, S.; Wademan, C.; White, A.; Hodzic, E. Investigation of the role of lesser characterized respiratory viruses associated with upper respiratory tract infections in horses. Vet. Rec. 2013, 172, 315. [Google Scholar] [CrossRef] [PubMed]
- Bernadino, P.; James, K.; Barnum, S.; Corbin, R.; Wademan, C.; Pusterla, N. What have we learned from 7 years of equine rhinitis B virus qPCR testing in nasal secretions from horses with respiratory signs. Vet. Rec. 2021, 188, e26. [Google Scholar] [CrossRef] [PubMed]
- Black, W.D.; Harley, C.A.; Ficorilli, N.P.; Studdert, M.J. Sequence variation divides Equine rhinitis B virus into three distinct phylogenetic groups that correlate with serotype and acid stability. J. Gen. Virol. 2005, 86, 2323–2332. [Google Scholar] [CrossRef]
- Kriegshauser, G.; Deutz, A.; Kuechler, E.; Skern, T.; Lussy, H.; Nowotny, N. Prevalence of neutralizing antibodies to Equine rhinitis A and B virus in horses and man. Vet. Microbiol. 2005, 106, 293–296. [Google Scholar] [CrossRef]
- Horsington, J.; Lynch, S.E.; Gilkerson, J.R.; Studdert, M.J.; Hartley, C.A. Equine picornaviruses: Well known but poorly understood. Vet. Microbiol. 2013, 167, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.E.; Barnett, D.C.; James, K.; Craig, B.; Bain, F.; Gaughan, E.; Schneider, C.; Vaala, W.; Barnum, S.M.; Pusterla, N. Voluntary surveillance program for equine influenza virus in the United States during 2008–2021. Pathogens 2023, 12, 192. [Google Scholar] [CrossRef]
- Pusterla, N.; Kass, P.; Mapes, S.; Wademan, C.; Akana, N.; Barnett, C.; MacKenzie, C.; Vaala, W. Voluntary Surveillance Program for Equine Influenza Virus in the United States from 2010 to 2013. J. Vet. Intern. Med. 2015, 29, 417–422. [Google Scholar] [CrossRef]
- Pusterla, N.; James, K.; Barnum, S.; Bain, F.; Barnett, D.C.; Chappell, D.; Gaughan, E.; Craig, B.; Schneider, C.; Vaala, W. Frequency of detection and prevalence factors associated with common respiratory pathogens in equids with acute onset of fever and/or respiratory signs (2008–2021). Pathogens 2022, 11, 759. [Google Scholar] [CrossRef]
- Jaramillo-Morales, C.; James, K.; Barnum, S.; Vaala, W.; Chappell, D.E.; Schneider, C.; Craig, B.; Bain, F.; Barnett, D.C.; Gaughan, E.; et al. Voluntary Biosurveillance of Streptococcus equi subsp. equi in Nasal Secretions of 9409 Equids with Upper Airway Infection in the USA. Vet. Sci. 2023, 10, 78. [Google Scholar] [CrossRef]
- Mapes, S.; Leutenegger, C.M.; Pusterla, N. Nucleic acid extraction methods for detection of ehv-1 from blood and nasopharyngeal secretions. Vet. Rec. 2008, 162, 857–859. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort (N = 8684) | ERBV Negative (n = 8243) | ERBV Positive (n = 441) | p-Value * | |
|---|---|---|---|---|
| Age (in years) | <0.001 | |||
| <1 | 1369 (15.8%) | 1203 (14.6%) | 166 (37.6%) | <0.0001 |
| 1–4 | 2161 (24.9%) | 2043 (24.8%) | 118 (26.8%) | NS |
| 5–9 | 1809 (20.8%) | 1754 (21.3%) | 55 (12.5%) | <0.0001 |
| 10–14 | 1320 (15.2%) | 1287 (15.6%) | 33 (7.5%) | <0.0001 |
| 15–19 | 814 (9.4%) | 797 (9.7%) | 17 (3.9%) | <0.0001 |
| 20+ | 591 (6.8%) | 569 (6.9%) | 22 (5.0%) | NS |
| Unknown | 620 (7.1%) | 590 (7.2%) | 30 (6.8%) | -- |
| Breed | <0.001 | |||
| Quarter horse | 3228 (37.2%) | 3049 (37.0%) | 179 (40.6%) | NS |
| Thoroughbred | 1344 (15.5%) | 1259 (15.3%) | 85 (19.3%) | NS |
| Warmblood | 866 (10.0%) | 830 (10.1%) | 36 (8.2%) | NS |
| Paint | 351 (4.0%) | 338 (4.1%) | 13 (2.9%) | NS |
| Arabian | 518 (6.0%) | 480 (5.8%) | 38 (8.6%) | NS |
| Draft | 241 (2.8%) | 230 (2.8%) | 11 (2.5%) | NS |
| Pony | 339 (3.9%) | 331 (4.0%) | 8 (1.8%) | 0.001 |
| Other | 1797 (20.7%) | 1726 (20.9%) | 71 (16.1%) | NS |
| Sex | 0.64 | |||
| Mare | 3078 (35.4%) | 2919 (35.4%) | 159 (36.1%) | |
| Gelding/Stallion | 4487 (51.7%) | 4266 (51.8%) | 221 (50.1%) | |
| Unknown | 1119 (12.9%) | 1058 (12.8%) | 61 (13.8%) | |
| Use | <0.001 | |||
| Competition | 3436 (39.6%) | 3222 (39.1%) | 214 (48.5%) | 0.0001 |
| Pleasure | 3217 (37.0%) | 3101 (37.6%) | 116 (26.3%) | <0.0001 |
| Breeding | 407 (4.7%) | 385 (4.7%) | 22 (5.0%) | NS |
| Other | 549 (6.3%) | 526 (6.4%) | 23 (5.2%) | NS |
| Unknown | 1075 (12.4%) | 1009 (12.2%) | 66 (15.0%) | -- |
| Total Cohort (N = 8684) | ERBV Negative (n = 8243) | ERBV Positive (n = 441) | p-Value * | |
|---|---|---|---|---|
| Presence, any severity | ||||
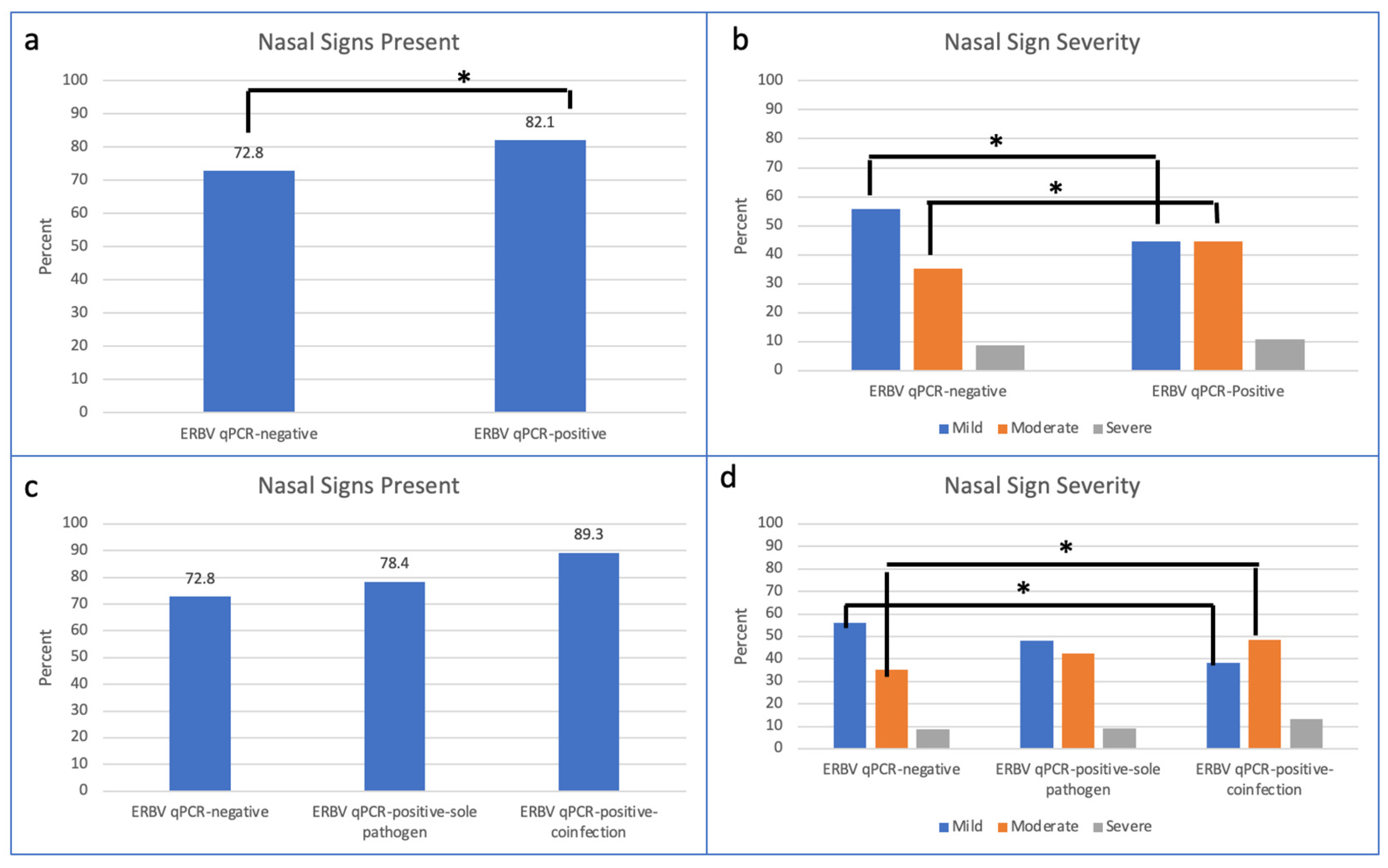
| Nasal discharge | 6366 (73.3%) | 6004 (72.8%) | 362 (82.1%) | <0.001 |
| Ocular signs | 2115 (24.4%) | 1982 (24.0%) | 133 (30.2%) | 0.014 |
| Cough | 4149 (47.8%) | 3909 (47.4%) | 240 (54.4%) | 0.006 |
| Limb edema | 870 (10.0%) | 834 (10.1%) | 36 (8.2%) | 0.22 |
| Anorexia | 5232 (60.2%) | 4956 (60.1%) | 276 (62.6%) | 0.57 |
| Lethargy | 6331 (72.9%) | 6006 (72.9%) | 325 (73.7%) | 0.40 |
| Fever (>101.5) | 7376 (84.9%) | 6988 (84.8%) | 388 (88.0%) | 0.44 |
| Severity of signs | ||||
| Nasal discharge | <0.001 | |||
| Mild | 3515 (55.2%) | 3354 (55.9%) | 161 (44.5%) | <0.0001 |
| Moderate | 2281 (35.8%) | 2119 (35.3%) | 162 (44.7%) | 0.0003 |
| Severe | 570 (8.9%) | 531 (8.8%) | 39 (10.8%) | NS |
| Ocular symptoms | 0.014 | |||
| Mild | 1688 (79.8%) | 1593 (80.4%) | 95 (71.4%) | 0.0123 |
| Moderate | 392 (18.5%) | 355 (17.9%) | 37 (27.8%) | 0.0044 |
| Severe | 35 (1.65%) | 34 (1.7%) | 1 (0.75%) | NS |
| ERBV-Negative (n = 8243) | ERBV-Sole Pathogen (n = 291) | ERBV-Coinfection (n = 150) | p-Value: ERBV-Negative vs. ERBV-Sole Pathogen * | p-Value: ERBV-Negative vs. ERBV-Coinfection * | p-Value: ERBV-Sole Pathogen vs. ERBV-Coinfection * | |
|---|---|---|---|---|---|---|
| Age (in years) | <0.001 | <0.001 | 0.26 | |||
| <1 | 1203 (14.6%) | 111 (38.1%) | 55 (36.7%) | <0.0001 | <0.0001 | |
| 1–4 | 2043 (24.8%) | 80 (27.5%) | 38 (25.3%) | NS | NS | |
| 5–9 | 1754 (21.3%) | 30 (10.3%) | 25 (16.7%) | <0.0001 | NS | |
| 10–14 | 1287 (15.6%) | 26 (8.9%) | 7 (4.7%) | 0.002 | 0.0002 | |
| 15–19 | 797 (9.7%) | 12 (4.1%) | 5 (3.3%) | 0.001 | 0.0079 | |
| 20+ | 569 (6.9%) | 13 (4.5%) | 9 (6.0%) | NS | NS | |
| Unknown | 590 (7.2%) | 19 (6.5%) | 11 (7.3%) | -- | NS | |
| Breed | 0.028 | 0.065 | 0.75 | |||
| Quarter horse | 3049 (37.0%) | 114 (39.2%) | 65 (43.3%) | NS | ||
| Thoroughbred | 1259 (15.3%) | 60 (20.6%) | 25 (16.7%) | NS | ||
| Warmblood | 830 (10.1%) | 26 (8.9%) | 10 (6.7%) | NS | ||
| Paint | 338 (4.1%) | 9 (3.1%) | 4 (2.7%) | NS | ||
| Arabian | 480 (5.8%) | 22 (7.6%) | 16 (10.7%) | NS | ||
| Draft | 230 (2.8%) | 8 (2.7%) | 3 (2.0%) | NS | ||
| Pony | 331 (4.0%) | 4 (1.4%) | 4 (2.7%) | NS | ||
| Other | 1726 (20.9%) | 48 (16.5%) | 23 (15.3%) | -- | ||
| Sex | 0.96 | 0.37 | 0.45 | |||
| Mare | 2919 (35.4%) | 102 (35.1%) | 57 (38.0%) | NS | ||
| Gelding/Stallion | 4266 (51.8%) | 150 (51.5%) | 71 (47.3%) | NS | ||
| Unknown | 1058 (12.8%) | 39 (13.4%) | 22 (14.7%) | -- | ||
| Use | <0.001 | 0.053 | 0.76 | |||
| Competition | 3222 (39.1%) | 142 (48.8%) | 72 (48.0%) | 0.0009 | ||
| Pleasure | 3101 (37.6%) | 73 (25.1%) | 43 (28.7%) | <0.0001 | ||
| Breeding | 385 (4.7%) | 14 (4.8%) | 8 (5.3%) | NS | ||
| Other | 526 (6.4%) | 17 (5.8%) | 6 (4.0%) | NS | ||
| Unknown | 1009 (12.2%) | 45 (15.5%) | 21 (14.0%) | -- |
| Total Cohort (N = 8684) * | ERBV-Negative (n = 8243) | ERBV-Coinfection (n = 150) | p-Value | |
|---|---|---|---|---|
| qPCR positive co-infections | ||||
| EHV-1 (blood) | 40 (0.5%) | 39 (0.5%) | 1 (0.7%) | 0.56 |
| EHV-1 (nasal) | 114 (1.3%) | 110 (1.3%) | 4 (1.0%) | 0.05 |
| EHV-4 | 939 (10.8%) | 891 (10.8%) | 48 (32.0%) | <0.001 |
| S. equi subspecies equi | 855 (9.9%) | 768 (9.3%) | 87 (58.0%) | <0.001 |
| ERAV | 14 (0.2%) | 13 (0.2%) | 1 (0.7%) | 0.24 |
| EIV | 936 (10.8%) | 911 (11.1%) | 25 (16.7%) | <0.001 |
| ERBV-Negative (n = 8243) | ERBV-Sole Pathogen (n = 291) | ERBV-Coinfection (n = 150) | p-Value: ERBV-Negative vs. ERBV-Sole pathogen * | p-Value: ERBV-Negative vs. ERBV-Coinfection * | p-Value: ERBV-Sole Pathogen vs. ERBV-Coinfection * | |
|---|---|---|---|---|---|---|
| Presence, any severity | ||||||
| Nasal discharge | 6004 (72.8%) | 228 (78.4%) | 134 (89.3%) | 0.11 | <0.001 | 0.01 |
| Ocular signs | 1982 (24.0%) | 86 (29.6%) | 47 (31.3%) | 0.09 | 0.12 | 0.93 |
| Cough | 3909 (47.4%) | 145 (49.8%) | 95 (63.3%) | 0.41 | <0.001 | 0.014 |
| Limb edema | 834 (10.1%) | 24 (8.2%) | 12 (8.0%) | 0.56 | 0.20 | 0.43 |
| Anorexia | 4956 (60.1%) | 179 (61.5%) | 97 (64.7%) | 0.83 | 0.53 | 0.77 |
| Lethargy | 6006 (72.9%) | 211 (72.5%) | 114 (76.0%) | 0.43 | 0.63 | 0.68 |
| Fever (>101.5) | 6988 (84.8%) | 46 (86.8%) | 342 (88.1%) | 0.80 | 0.09 | 0.18 |
| Severity of signs | ||||||
| Nasal discharge | 0.06 | <0.001 | 0.133 | |||
| Mild | 3355 (55.9%) | 110 (48.2%) | 51 (38.1%) | <0.0001 | ||
| Moderate | 2119 (35.3%) | 97 (42.5%) | 65 (48.5%) | 0.0008 | ||
| Severe | 531 (8.84%) | 21 (9.2%) | 18 (13.4%) | NS | ||
| Ocular signs | 0.06 | 0.11 | 0.36 | |||
| Mild | 1594 (80.4%) | 63 (73.3%) | 32 (68.1%) | |||
| Moderate | 355 (17.9%) | 23 (26.7%) | 14 (29.8%) | |||
| Severe | 34 (1.7%) | 0 (0.0%) | 1 (2.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, C.; James, K.; Craig, B.W.; Chappell, D.E.; Vaala, W.; van Harreveld, P.D.; Wright, C.A.; Barnum, S.; Pusterla, N. Characterization of Equine Rhinitis B Virus Infection in Clinically Ill Horses in the United States during the Period 2012–2023. Pathogens 2023, 12, 1324. https://doi.org/10.3390/pathogens12111324
Schneider C, James K, Craig BW, Chappell DE, Vaala W, van Harreveld PD, Wright CA, Barnum S, Pusterla N. Characterization of Equine Rhinitis B Virus Infection in Clinically Ill Horses in the United States during the Period 2012–2023. Pathogens. 2023; 12(11):1324. https://doi.org/10.3390/pathogens12111324
Chicago/Turabian StyleSchneider, Chrissie, Kaitlyn James, Bryant W. Craig, Duane E. Chappell, Wendy Vaala, Philip D. van Harreveld, Cara A. Wright, Samantha Barnum, and Nicola Pusterla. 2023. "Characterization of Equine Rhinitis B Virus Infection in Clinically Ill Horses in the United States during the Period 2012–2023" Pathogens 12, no. 11: 1324. https://doi.org/10.3390/pathogens12111324
APA StyleSchneider, C., James, K., Craig, B. W., Chappell, D. E., Vaala, W., van Harreveld, P. D., Wright, C. A., Barnum, S., & Pusterla, N. (2023). Characterization of Equine Rhinitis B Virus Infection in Clinically Ill Horses in the United States during the Period 2012–2023. Pathogens, 12(11), 1324. https://doi.org/10.3390/pathogens12111324






