Abstract
In Japan, nationwide epidemiological surveys on carbapenem-resistant Enterobacterales (CREs), including comprehensive information, are scarce, with most data available only through public reports. This study analyzed data on the Enterobacterales family collected from nationwide testing centers between January 2016 and December 2022, focusing on isolates that met the criteria for CRE in Japan based on drug susceptibility. We investigated 5,323,875 Enterobacterales isolates of 12 different species; among 4696 (0.09%) CRE strains, the proportion of major CRE isolates was as follows: Escherichia coli, 31.3%; Klebsiella pneumoniae, 28.0%; Enterobacter cloacae, 18.5%; and Klebsiella aerogenes, 6.7%. Moreover, over a 7-year period, Providencia rettgeri, E. cloacae, K. aerogenes, and K. pneumoniae demonstrated relatively high CRE percentages of 0.6% (156/26,185), 0.47% (869/184,221), 0.28% (313/110,371), and 0.17% (1314/780,958), respectively. The number of CRE strains isolated from different samples was as follows: urine, 2390; respiratory specimens, 1254; stool, 425; blood, 252; others, 375. In the broader context, including colonization, the predominant isolates of CREs collected at nationwide testing centers are E. coli and K. pneumoniae. Furthermore, recently, attention has been directed toward less common CRE species, such as Klebsiella oxytoca and Providencia rettgeri, and thus, it might be necessary to continue monitoring these less common species.
1. Introduction
Carbapenem-resistant Enterobacterales (CREs) are multidrug-resistant organisms that cause healthcare-associated infections, posing a public health threat [1]. Outbreaks of CRE infections frequently occur in healthcare facilities, leading to an increased number of deaths, events of illnesses, and medical costs resulting from this type of antimicrobial resistance (AMR) [2].
Carbapenem resistance in Enterobacterales is primarily due to the production of carbapenemases (CPs), which break down carbapenem agents classified under classes A, B, and D according to the Ambler classification, or the species may be non-CP-producers [3]. The representative of Class A CPs is Klebsiella pneumoniae carbapenemase (KPC), while that of Class B is New Delhi metallo-β-lactamase (NDM) [4,5]. The possession of these CPs is a topic of great importance due to high mortality rates and horizontal transfer resulting from CREs [6,7]. Moreover, based on review reports from various regions around the world, K. pneumoniae is commonly reported as the predominant causative agent of both CRE colonization and infection, although in some cases, Enterobacter spp. may be predominant [1,8].
The World Health Organization (WHO) has developed an action plan to handle AMR in microbes and has described the accumulation of knowledge and evidence through research as one of the key pillars of AMR control [9]. The monitoring and investigation of the drug resistance of CREs are carried out worldwide, as well as investigations into the presence of resistance genes in regional CREs [1,10,11,12]. In Japan, two nationwide surveillance systems for antibiotic-resistant bacteria exist under the purview of the Ministry of Health, Labour, and Welfare (MHLW). The first is the National Epidemiological Surveillance of Infectious Diseases (NESID), which focuses on CRE infection cases identified based on the Infectious Disease Control Law. The second is Japan Nosocomial Infections Surveillance (JANIS), which focuses on hospitals actively participating in infection control programs linked with healthcare reimbursement and accounts for approximately one-quarter of medical institutions in Japan [13,14]. These public surveillance systems reported Klebsiella aerogenes and Enterobacter cloacae as the predominant CRE species in Japan [15], whereas previous research conducted in specific regions of Japan has reported that CREs identified in colonization cases primarily consisted of Escherichia coli and K. pneumoniae [16], suggesting a difference in CRE species between infection and colonization cases. The overall landscape of isolated and cultured CREs in Japan, including colonization, has not been elucidated.
Various healthcare institutions, particularly those lacking their own testing facilities, outsource bacterial tests. Testing companies receive and handle specimens, including those related to infection or colonization. However, thus far, a comprehensive overview of the overall spectrum of bacterial species isolated and cultured by these testing companies has not been reported. Moreover, it has not been determined which bacterial species exhibit a high prevalence of CREs among those tested at testing centers.
In this study, by analyzing nationwide data, including colonization cases from a domestic testing center, we conducted an epidemiological investigation of Enterobacterales species that met the criteria for CRE in Japan. Such a nationwide study, which includes data collected from a private center, has not been previously reported.
2. Materials and Methods
2.1. Bacterial Database
Bacterial data were obtained from a major domestic laboratory (BML Inc., Tokyo, Japan). BML offers bacterial testing services in all prefectures across Japan. All bacterial data were categorized based on bacterial species, material type, and drug susceptibility. Samples from which the data were obtained from various places were submitted for bacterial cultivation and identification between January 2016 and December 2022. Enterobacterales in the samples were identified and subjected to antimicrobial susceptibility testing using the Microscan WalkAway® system (Beckman Coulter, Brea, CA, USA) with accompanying panels of the Microscan Neg® series (Neg Combo EN 4J and Neg MIC EN 2J) (Beckman Coulter, Brea, CA, USA). The bacterial solution used for this test was prepared using the prompt inoculation method [17], according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [18]. CRE was defined based on the Infectious Disease Control Law in Japan—Enterobacterales isolates with a minimum inhibitory concentration (MIC) of ≥2 µg/mL for meropenem, ≥2 µg/mL for imipenem, and ≥64 µg/mL for cefmetazole.
2.2. Analysis of CRE Data
All Enterobacterales species were listed, and species with more than 20 CRE isolates were selected for analysis. Among Enterobacterales species, pathogenic E. coli, Yersinia, Salmonella, and Shigella species were excluded from the study because these species were most likely submitted for testing by employees who primarily handle food products. Individual and hospital identifications were not performed, and duplicate samples were collected.
The CRE percentage was calculated as follows: (CRE isolates count/total bacterial isolates count) × 100 percent (%). The specimens associated with the detected CRE isolates were also investigated. The samples were categorized into three groups: urological samples, mainly including urine; respiratory tract samples, including sputum, nasal discharge, and throat swabs; and stool samples. All other samples were grouped under “Others”, which encompassed pus, ear discharge, vaginal secretions, wound site fluids from bedsores, drain fluids, and others.
2.3. Statistical Analysis
The CRE percentage was analyzed using an analysis of variance (ANOVA), and the Tukey–Kramer HSD test was used for the pairwise comparisons of mean values. The analyses were performed using JMP Pro 16 (SAS Institute, Cary, NC, USA). Results with p < 0.05 were considered statistically significant.
3. Results
3.1. Overview of Enterobacterales Species in This Study
In total, 5,323,875 Enterobacterales strains were isolated between 2016 and 2022. The analysis included 12 bacterial species, listed in descending order based on the number of isolates: E. coli (57.2%) > K. pneumoniae (14.7%) > Proteus mirabilis (6.1%) > Serratia marcescens (4.0%) > Klebsiella oxytoca (3.9%) > Enterobacter cloacae (3.5%) > Morganella morganii (2.6%) > Citrobacter koseri (2.3%) > Citrobacter freundii (2.1%) > Klebsiella aerogenes (2.1%) > Providencia stuartii (1.3%) > Providencia rettgeri (0.5%). E. coli and K. pneumoniae accounted for more than 50% of the total bacterial population. Throughout the 7-year period, the proportion of each bacterial species remained relatively constant.
3.2. Epidemiological Trends of CRE Species in Japan
Out of 5,323,875 Enterobacterales isolates, there were a total of 4696 CRE isolates. The number of CRE isolates and CRE percentages per year in 12 bacterial species from 2016 to 2022, respectively, were as follows: 416 isolates (0.06%), 937 isolates (0.13%), 833 isolates (0.11%), 669 isolates (0.09%), 641 isolates (0.08%), 704 isolates (0.09%), and 496 isolates (0.06%). The proportion of each bacterial species for the 7-year period was as follows: E. coli, 1469 (31.3%); K. pneumoniae, 1314 (28.0%); E. cloacae, 869 (18.5%); K. aerogenes, 313 (6.7%); P. rettgeri, 156 (3.3%); S. marcescens, 134 (2.9%); C. koseri, 118 (2.5%); K. oxytoca, 114 (2.4%); M. morganii, 66 (1.4%); P. stuartii, 66 (1.4%); C. freundii, 49 (1.0%); and P. mirabilis, 28 (0.6%). Therefore, E. coli was the most prevalent species (Figure 1).
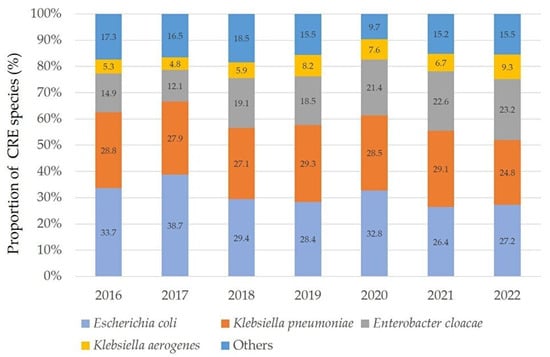
Figure 1.
Annual frequency of representative CRE species from 2016 to 2022. In 2019 and 2021, K. pneumoniae was the most prevalent, but in other years, E. coli was the most common. The combined total of carbapenem-resistant E. coli and K. pneumoniae accounted for over 50% annually. “Others” includes the following bacterial species: S. marcescens, K. oxytoca, P. stuartii, P. rettgeri, C. koseri, C. freundii, M. morganii, and P. mirabilis.
For the four representative CRE species with the highest number of isolates, the number of CRE isolates and CRE percentage are presented in Figure 2. In the regression analysis of CRE isolates, E. coli and K. pneumoniae showed a decreasing trend, while E. cloacae and K. aerogenes also exhibited an increasing trend. However, the p-values for statistical significance in the regression analysis were 0.372, 0.638, 0.221, and 0.194, respectively, indicating no statistical significance.
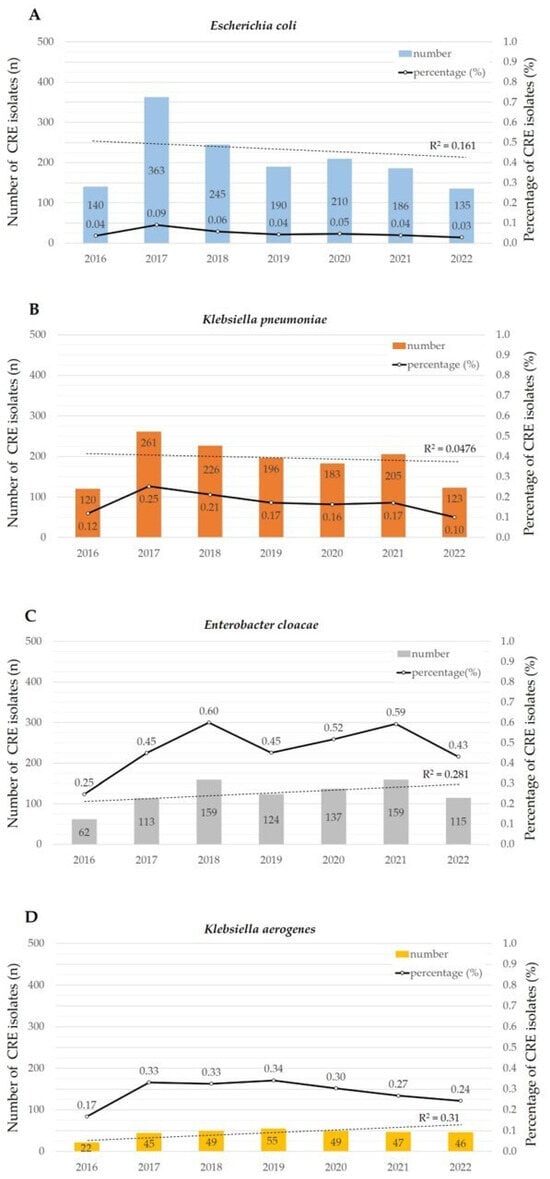
Figure 2.
Temporal trends of four representative CRE species. The bar graph represents the number of CRE isolates, while the line graph illustrates the percentage of CRE isolates, The dashed line represents the regression analysis of isolated CRE counts (A–D). In 2017, both E. coli and K. pneumoniae shown a decreasing trend, as indicated in the regression analysis. E. cloacae and K. aerogenes showed significance was obtained in ANOVA. E. cloacae exhibited a significantly higher CRE percentage than C. freundii, E. coli, M. morganii, K. oxytoca, S. marcescens, P. stuartii, C. koseri, and K. pneumoniae for seven years. The CRE percentage of K. aerogenes was significantly higher than that of P. mirabilis.
The CRE percentage for the 7 years was as follows: P. rettgeri, 0.6%; E. cloacae, 0.47%; K. aerogenes, 0.28%; K. pneumoniae, 0.17%; C. koseri, 0.1%; P. stuartii, 0.1%; S. marcescens, 0.06%; K. oxytoca, 0.06%; E. coli, 0.05%; M. morganii, 0.05%; C. freundii, 0.04%; and P. mirabilis, 0.01%. Notably, the number of CRE isolates and CRE percentage of P. rettgeri detected over the three years from 2017 to 2019 were, respectively, 36 isolates (1.02%), 36 isolates (1.09%), and 37 isolates (1.05%). No other species besides P. rettgeri had a CRE percentage exceeding 1%.
In a comparison of the CRE percentage using the Tukey–Kramer HSD test, P. rettgeri showed a significantly higher CRE percentage than P. mirabilis, C. freundii, E. coli, M. morganii, K. oxytoca, S. marcescens, C. koseri, P. stuartii, K. pneumoniae (p < 0.0001), and K. aerogenes (p = 0.0004). Moreover, E. cloacae exhibited a significantly higher CRE percentage than C. freundii, E. coli, M. morganii, K. oxytoca, S. marcescens, P. stuartii, C. koseri (p < 0.0001), and K. pneumoniae (p = 0.0037). The CRE percentage of K. aerogenes was significantly higher than that of P. mirabilis (p = 0.0125), but no statistically significant differences were observed between other bacterial species.
3.3. Relationship between the Submitted Samples and CRE Species
Among the total submitted samples with CRE isolates, the proportion of urine and urogenital samples (2390 specimens) was the highest, followed by respiratory and throat specimens (1254 specimens), stool (424 specimens), blood (252 specimens), and others (375 specimens). Of the 12 bacterial species, 10 were primarily isolated from urine and urogenital samples. The ranking of stool, blood, and other samples varied according to bacterial species. P. mirabilis and E. cloacae were mainly isolated from the respiratory tract and throat. Details of the sample proportions of representative CREs are provided in Figure 3. For each CRE species, the proportion of blood samples suggesting evident infections was as follows: K. oxytoca; 12.3% (14/114); S. marcescens 9.7% (13/134); K. aerogenes 8.3% (26/313); C. freundii 8.2% (4/49); E. cloacae, 7.7% (67/869); K. pneumoniae, 4.7% (62/1313); M. morganii, 4.5% (3/66); E. coli, 4.0% (59/1469); P. mirabilis, 3.6% (1/28); C. koseri, 1.7% (2/118); P. rettgeri, 0.6% (1/156); and P. stuartii, 0% (0/66). Pairwise comparisons using the Tukey–Kramer HSD test indicated that the proportion of K. oxytoca was significantly higher than that of P. stuartii (p < 0.05), but no significant differences were observed in the proportions of other species.
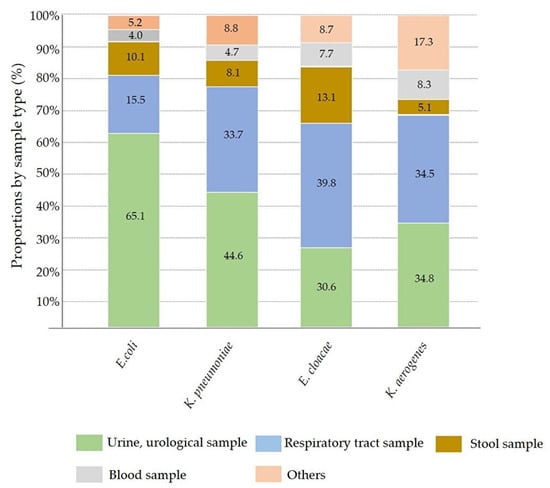
Figure 3.
The proportion of submitted samples containing representative CRE isolates. E. coli, the most common CRE isolate, was found in approximately 65% of urinary samples. Unlike E. coli, K. pneumoniae, E. cloacae, and K. aerogenes showed a higher frequencies in upper respiratory and sputum samples.
4. Discussion
In this study, we examined the prevalence and distribution of 12 CRE species, which were identified and tested for antimicrobial susceptibility from 2016 to 2022 at a nationwide test center. We aimed to focus on the detection of CRE isolates in specimens submitted for testing, rather than targeting infectious agents.
The NESID and JANIS primarily monitor CRE infections and have consistently reported a high prevalence of K. aerogenes in cases of CRE infection [15,19]. However, in the present study, K. aerogenes was the least prevalent among the four major bacterial isolates, exhibiting a lower CRE isolate count and CRE percentage. This study reflects the results of isolating and culturing clinical specimens, including a substantial number of colonization cases; therefore, this different approach may have contributed to the differences between the public reports and the results of this study. Moreover, variations are observed even in the predominant specimen types; while the JANIS reports primarily involve respiratory specimens, our study predominantly involved urine specimens, with a lower frequency of blood specimens indicating overt infections [14].
In the following sections, we discuss each bacterial species that was included in the study.
4.1. Escherichia coli and Klebsiella pneumoniae
In recent years, reports have consistently identified K. pneumoniae as the predominant CRE isolate in major countries worldwide [1,12,20]. While there has been growing concern over the increase in carbapenem-resistant E. coli in Europe, it is important to note that E. coli is not currently considered the primary CRE isolate [21]. Even within Japan, the frequency of E. coli as a causative agent of CRE infections is not particularly high. According to domestic reports, infections caused by carbapenem-resistant E. coli account for only 6.5–10% of all CRE infections, making up a relatively low proportion [15]. However, when considering colonization, different possibilities have been suggested. In a previous report, the CRE percentage in long-term care hospitals was significantly higher (14.9%) than that in acute care hospitals (3.6%), and E. coli was the most predominant CRE isolate [16]. In our study as well, E. coli comprised 1469 carbapenem-resistant isolates and was the most common CRE bacterial species. There are fewer reported cases of CRE infections; however, when considering both colonization and isolations in laboratory settings, E. coli is possibly the most prevalent in terms of the total number of occurrences. In addition, although our study did not specifically investigate CP-Enterobacterales, CPs are common among E. coli that exhibit carbapenem resistance [16,22]. As E. coli is rarely the causative agent of CRE infections, the carbapenem-resistant E. coli isolates identified in this study are suggested to represent colonization to some extent. One of the reasons for E. coli being the most prevalent species in this study is speculated to be the distribution of facilities targeted by testing companies. Namely, it could reflect the test results from small- and medium-sized hospitals, long-term care facilities, and clinics without their own testing facilities. JANIS investigates not only infection cases but also colonization, unlike NESID. However, a notable absence of participation of small-scale hospitals with fewer than 200 beds, constituting over 50% of the inpatient beds, is observed [14].
The above conclusions for E. coli can also be applied to K. pneumonia; K. pneumoniae is the second most frequently observed isolate of CRE in localized regional surveillance for CRE colonization within Japan, following E. coli [16]. These findings align with the results of the present study. In our study, the CRE percentage for K. pneumoniae was 0.17%, more than three times higher than that for E. coli, and the number of CRE isolates was the second highest and close to that of E. coli, although a decreasing trend was observed for K. pneumoniae, similar to that seen for E. coli. Similar to E. coli, carbapenem-resistant K. pneumoniae is likely to be a CP species [16]. K. pneumoniae is one of the most studied bacteria for carbapenemase production, and KPC and NDM are the most prevalent CPs in neighboring countries [23,24]. In Japan, the predominant type of carbapenemase is the IMP type [15,25].
4.2. Enterobacter cloacae and Klebsiella aerogenes
Klebsiella aerogenes was originally classified under the Enterobacter genus and was reclassified under the Klebsiella genus in 2019 owing to its genetic similarities [26]. However, infections caused by K. aerogenes and E. cloacae exhibit similarities in patient profiles, occurrence, and prognoses [27]. According to nationwide surveys in Japan, the frequency of infections caused by carbapenem-resistant K. aerogenes and E. cloacae is the highest, unlike the global trend [15]. This finding is consistent with those of other domestic studies [28,29]. The CRE percentages obtained in this study were 0.28% (313/110,371) for K. aerogenes and 0.47% (869/184,221) for E. cloacae. Moreover, E. cloacae had a significantly higher CRE percentage than eight other bacterial species, namely, C. freundii, E. coli, M. morganii, K. oxytoca, S. marcescens, P. stuartii, C. koseri, and K. pneumoniae. No statistically significant difference was observed, but both E. cloacae and K. aerogenes show an increasing trend, warranting attention to future developments (Figure 2).
Regarding the mechanism of carbapenem resistance, CP K. aerogenes isolates are rare in Japan [30]. In particular, K. aerogenes is mostly non-CP, and its resistance to carbapenems is greatly influenced by the use of antibiotics [31]. Consequently, it is more likely to be detected in relatively large-scale acute-care hospitals [28,29]. Non-CP K. aerogenes isolates were characterized by resistance to imipenem and susceptibility to meropenem in a previous study [29]. Resistance mechanisms involve the overproduction of AmpC β-lactamase, the action of efflux pumps, and the downregulation of porins [32,33,34,35]. Similarly, E. cloacae produces AmpC β-lactamase, but unlike K. aerogenes, E. cloacae is considered a representative CP species in Japan [28,29].
4.3. Minor Carbapenem-Resistant Enterobacterales
Monitoring programs of CRE species have primarily focused on common carbapenem-resistant bacteria, such as E. coli, Enterobacter spp., and Klebsiella spp. However, by focusing solely on these bacterial species, there is a risk of overlooking infections caused by relatively rare CRE species [36]. In this study, among the 12 bacterial species, the less common carbapenem-resistant bacteria were S. marcescens, K. oxytoca, P. stuartii, P. rettgeri, C. koseri, C. freundii, M. morganii, and P. mirabilis. These less common species have attracted attention in recent years in the context of CRE surveillance, although consolidated data on such rare species are scarce domestically. Providencia spp., Proteus spp., and M. morganii are intestinal bacteria that exhibit intrinsic resistance to imipenem, and their resistance to other carbapenems needs to be confirmed [37]. In Japan, considering intrinsic resistance to imipenem among previous species such as M. morganii, imipenem and cefmetazole resistance was set as a criterion for CRE, which may not necessarily align with definitions used in overseas settings. Moreover, because carbapenemase-coding genes can be transferred to other bacterial species through horizontal transfer via plasmids, CP is more important in less common carbapenem-resistant species [38,39,40].
The proportion of Serratia. marcescens as a causative agent of CRE infections in Japan ranges from 3.4% to 5% [15]. The CRE percentage for S. marcescens in the present study was 0.06% over 7 years. There is no indication of an increase in the number of isolates or CRE percentage for carbapenem-resistant S. marcescens in this study. A previous, relatively large-scale study that included 28 university hospitals in Japan reported that carbapenem-resistant S. marcescens isolates were non-CP, suggesting that many carbapenem-resistance mechanisms in S. marcescens are non-carbapenemase-related [28].
In this study, the total number of Klebsiella oxytoca isolates was nearly equivalent to that of S. marcescens. The number of CRE isolates was low, with 114 isolates showing a low CRE percentage. There have been no nationwide epidemiological survey reports of carbapenem-resistant K. oxytoca in Japan. The first report on CP K. oxytoca was published in 2015, and it has recently gained attention in the country [41]. A notable characteristic of K. oxytoca isolates obtained in our study is the high proportion of blood culture specimens (12.3%). This finding suggests that they cause apparent infections with potentially poor prognoses. However, detailed information is lacking owing to the limited number of domestic reports. Similar to S. marcescens, there is no indication of an increase in the number of isolates or CRE percentage for K. oxytoca.
Providencia stuartii and P. rettgeri are the primary causative agents of urinary tract infections and food poisoning [42,43]. These have previously been reported in patients with long-term indwelling urinary catheters [44]. In the present study, P. stuartii and P. rettgeri were the only Providencia species detected. Although the total number of P. rettgeri isolates was 26,185, which was the lowest among the 12 species, the CRE percentage was the highest at 0.6%. A notable feature of P. rettgeri is its high CRE percentage, which was significantly higher than that of 10 other bacterial species. The CRE percentage of P. rettgeri exceeded 1% from 2017 to 2019. Due to the low number of isolates of P. rettgeri, it cannot be ruled out that specific facilities or regional outbreaks are being observed because the relative number of CRE isolates is low. Although there is no evidence to suggest that monitoring programs have focused on carbapenem-resistant P. rettgeri in Japan during the study period, there have been reports of IMP-type metallo-β-lactamase-producing P. rettgeri in Japan during the study period, indicating a possible correlation [45].
In the present study, there were 120,754 and 113,090 Citrobacter. koseri and C. freundii isolates. Although the number of isolates was nearly the same, the number of carbapenem-resistant C. koseri isolates was high (118 isolates) compared to 49 carbapenem-resistant C. freundii isolates, and the CRE percentage was also higher for C. koseri. In previous domestic reports on infectious diseases, C. freundii has been more frequently observed, suggesting that C. koseri has a higher colonization percentage than infection percentage [15,28]. C. koseri and C. freundii typically have low isolate numbers and CRE percentages each year, suggesting that they are well controlled.
Morganella morganii is a chromosomal AmpC β-lactamase-producing bacterium that exhibits intrinsic resistance to imipenem [37]. However, the number of M. morganii isolates meeting the criteria for CRE in Japan is limited. According to the NESID, there were no reports of carbapenem-resistant M. morganii from 2017 to 2020. In a relatively large-scale study involving 28 university hospitals, 10 cases of non-CP carbapenem-resistant M. morganii infection were recorded [28]. Carbapenem-resistant M. morganii also has a low number of isolates and a low CRE rate, suggesting that it is well controlled.
Proteus mirabilis can cause various human infections, including wound, eye, digestive system, and urinary tract infections. It also causes catheter-associated urinary tract infections (CAUTIs) in the catheterized urinary tract [46]. Although there is a possibility that P. mirabilis produces carbapenemases, it is more likely that its carbapenem resistance is due to non-CP mechanisms [47]. In this study, carbapenem-resistant P. mirabilis accounted for the lowest number of isolates (28 isolates) over a span of 7 years, and the CRE percentage was less than 0.01%, indicating that resistance to carbapenems is not a major concern.
This study has several limitations. First, the distribution of bacterial isolates might be biased even within the same country due to variations in population density, average age, and other factors. Nevertheless, this is the first epidemiologic study utilizing a nationwide database including CRE infection and colonization in the extensive scope of testing in Japan. Second, individual identification of bacterial isolates was not conducted, suggesting that our database may include multiple redundant isolates from the same individual. However, carbapenem-resistant E. coli and K. pneumoniae, due to their much higher numbers, are unlikely to be affected, unlike the minor CRE species, by duplication. Third, as we screened for CRE based on Japanese criteria, they do not correspond to CREs defined in other countries’ guidelines (e.g., Clinical and Laboratory Standards Institute (CLSI) or European Committee on Anti-microbial Susceptibility Testing (EUCAST) [9,48]). Fourth, because we did not conduct genetic tests or genome analyses, the mechanisms underlying antibiotic resistance (e.g., carbapenemases, extended-spectrum β-lactamases, and AmpC β-lactamases) were not investigated.
5. Conclusions
We investigated the number and CRE percentage of Enterobacterales species in samples, including colonization, collected at the nationwide testing center from 2016 to 2022. We found that E. coli was the predominant CRE species, followed by K. pneumoniae. These two species together accounted for over 50% of the total CRE isolates in our study. Small and medium-sized hospitals, long-term care facilities, and clinics without their own testing facilities may rely on testing companies. In specimens requested from these healthcare facilities, carbapenem-resistant E. coli and K. pneumoniae could be frequently encountered. In addition, carbapenem resistance in E. coli and K. pneumoniae is likely to be due to CP. Considering the frequent movement of patients between healthcare facilities, CP-CREs raise concerns about horizontal gene transfer to other bacterial species. More attention should be paid to the potential spread of CREs in these healthcare institutions, and monitoring colonization will also be necessary to prevent the proliferation of CRE species.
Author Contributions
Conceptualization, K.T. and H.K.; data curation and interpretation, K.T. and H.K; inspection enforcement, R.S. and M.O.; supervision, R.S., M.O. and H.K.; visualization, K.T.; writing—original draft, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) Grant Number 21K08504.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Tohoku University Graduate School of Medicine (IRB Numbers: 2018-1-445 and 2022-1-865).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets created and analyzed during the current study are not publicly available due to contain patient information and confidentiality obligations of the company. The dataset is owned by BML Inc., Tokyo, Japan.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215 (Suppl. 1), S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 8 April 2023).
- Suh, B.; Bae, I.K.; Kim, J.; Jeong, S.H.; Yong, D.; Lee, K. Outbreak of meropenem-resistant Serratia marcescens comediated by chromosomal AmpC beta-lactamase overproduction and outer membrane protein loss. Antimicrob. Agents Chemother. 2010, 54, 5057–5061. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla (NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Suda, K.J.; Fitzpatrick, M.A.; Bartle, B.; Pfeiffer, C.D.; Jones, M.; Rubin, M.A.; Perencevich, E.; Evans, M.; Evans, C.T.; et al. Risk Factors Associated with Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Positive Cultures in a Cohort of US Veterans. Clin. Infect. Dis. 2021, 73, 1370–1378. [Google Scholar] [CrossRef]
- Lutgring, J.D.; Limbago, B.M. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J. Clin. Microbiol. 2016, 54, 2427. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Mathema, B.; Larson, E.L. Carbapenem-Resistant Enterobacteriaceae in the Community: A Scoping Review. Int. J. Antimicrob. Agents 2017, 50, 127–134. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/193736 (accessed on 31 July 2023).
- Livorsi, D.J.; Chorazy, M.L.; Schweizer, M.L.; Balkenend, E.C.; Blevins, A.E.; Nair, R.; Samore, M.H.; Nelson, R.E.; Khader, K.; Perenceivich, E.N. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob. Resist. Infect. Control 2018, 7, 55. [Google Scholar] [CrossRef]
- Wielders, C.C.H.; Schouls, L.M.; Woudt, S.H.S.; Notermans, D.W.; Hendrickx, A.P.A.; Bakker, J.; Kuijper, E.J.; Schoffelen, A.F.; de Greeff, S.C.; Infectious Diseases Surveillance Information System-Antimicrobial Resistance (ISIS-AR) Study Group; et al. Epidemiology of carbapenem-resistant and carbapenemase-producing Enterobacterales in the Netherlands 2017–2019. Antimicrob. Resist. Infect. Control 2022, 11, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 2018, 62, e01882-17. [Google Scholar] [CrossRef]
- Suzuki, S. A view on 20 years of antimicrobial resistance in Japan by two national surveillance systems: The national epidemiological surveillance of infectious diseases and Japan nosocomial infections surveillance. Antibiotics 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, A.; Suzuki, S. Japan nosocomial infections surveillance (JANIS): A model of sustainable national antimicrobial resistance surveillance based on hospital diagnostic microbiology laboratories. BMC Health Serv. Res. 2018, 20, 799. [Google Scholar] [CrossRef] [PubMed]
- Kamio, K.; Espinoza, J.L. The predominance of Klebsiella aerogenes among carbapenem-resistant Enterobacteriaceae infections in Japan. Pathogens 2022, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Asada, R.; Kawahara, R.; Hagiya, H.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Yukawa, S.; Yamamoto, K.; Takayama, Y.; et al. Prevalence of, and risk factors for, carriage of CRE among hospitalized patients in Japan. J. Hosp. Infect. 2017, 97, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Flejzor, B.; Bokkenheuser, V.D. Performance of the prompt system in identification and antimicrobial susceptibility testing of clinical isolates. J. Clin. Microbiol. 1985, 21, 267–268. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100-S30; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Imai, S.; Inoue, N.; Naga, H. Economic and clinical burden from carbapenem-resistant bacterial infections and factors contributing: A retrospective study using electronic medical records in Japan. BMC Infect. Dis. 2022, 29, 581. [Google Scholar] [CrossRef]
- van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Increase in Cases of Carbapenem-Resistant E. coli Carrying the blaNDM-5 Gene, EU/EEA Countries; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023. Available online: https://www.ecdc.europa.eu/en/news-events/increase-cases-carbapenem-resistant-e-coli-carrying-blandm-5-gene-eueea-countries (accessed on 1 September 2023).
- Li, F.; Ye, K.; Li, X.; Ye, L.; Guo, L.; Wang, L.; Yang, J. Genetic characterization of carbapenem-resistant Escherichia coli from China, 2015–2017. BMC Microbiol. 2021, 21, 248. [Google Scholar] [CrossRef]
- Helio, S.S.; Rodrigo, E.M.; Cecilia, G.C.; John, H.K.; Mariana, C. Changing epidemiology of carbapenemases among carbapenem-resistant Enterobacterales from United States Hospitals and the activity of aztreonam-avibactam against contemporary Enterobacterales (2019–2021). Open Forum Infect. Dis. 2023, 31, ofad046. [Google Scholar] [CrossRef]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Yonggui, Z.; Yan, G.; Rong, Z.; Fupin, H. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front. Cell. Infect. Microbiol. 2020, 10, 32719751. [Google Scholar] [CrossRef]
- Yonekawa, S.; Mizuno, T.; Nakano, R.; Nakano, A.; Suzuki, Y.; Asada, T.; Ishii, A.; Kakuta, N.; Tsubaki, K.; Mizuno, S.; et al. Molecular and epidemiological characteristics of carbapenemase-producing Klebsiella pneumoniae clinical isolates in Japan. mSphere 2020, 5, e00490-20. [Google Scholar] [CrossRef] [PubMed]
- Wesevich, A.; Sutton, G.; Ruffin, F.; Park, L.P.; Fouts, D.E.; Fowler, V.G., Jr.; Thaden, J.T. Newly Named Klebsiella aerogenes (formerly Enterobacter aerogenes) Is Associated with Poor Clinical Outcomes Relative to Other Enterobacter Species in Patients with Bloodstream Infection. J. Clin. Microbiol. 2020, 58, e00582-20. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Marín, R.; Lepe, J.A.; Gasch-Blasi, O.; Rodríguez-Martínez, J.M.; Calvo-Montes, J.; Lara-Contreras, R.; Martín-Gandul, C.; Tubau-Quintano, F.; Cano-García, M.E.; Rodríguez-López, F.; et al. Clinical characteristics and outcome of bacteraemia caused by Enterobacter cloacae and Klebsiella aerogenes: More similarities than differences. J. Glob. Antimicrob. Resist. 2021, 25, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Matsumoto, A.; Tetsuka, N.; Morioka, H.; Iguchi, M.; Ishiguro, N.; Nagamori, T.; Takahashi, S.; Saito, N.; Tokuda, K.; et al. Clinical characteristics and treatment outcomes of carbapenem-resistant Enterobacterales infections in Japan. J. Glob. Antimicrob. Resist. 2022, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Kanamori, H.; Nakayama, A.; Chiba, M.; Takei, Y.; Seike, I.; Kitamura, C.; Baba, H.; Oshima, K.; Tokuda, K. Screening for metallo-beta-lactamases using non-carbapenem agents: Effective detection of MBL-producing Enterobacterales and differentiation of carbapenem-resistant Enterobacterales. Antibiotics 2023, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Yu, L.; Kawakami, S.; Yahara, K.; Hisatsune, J.; Yamamoto, M.; Yamamoto, K.; Shimono, N.; Kibe, Y.; Kiyosuke, M.; et al. Emergence of blaNDM-5-carrying Klebsiella aerogenes in Japan. Microbiol. Spectr. 2022, 29, e0222221. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Hussein, K.; Karram, M.; Saffuri, M.; Badaan, S.; Peleg, S.; de Kraker, M.E.; Aboelhega, W.; Warman, S.; Alon, T.; et al. Risk factors for acquisition of carbapenemase-producing versus non-carbapenemase-producing Enterobacterales: A case-control study. Clin. Microbiol. Infect. 2023, 29, 629–634. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Ma, D.Y.; Huang, H.Y.; Zou, H.; Wu, M.L.; Lin, Q.X.; Liu, B.; Huang, S.F. Carbapenem-resistant Klebsiella aerogenes clinical isolates from a teaching hospital in southwestern China: Detailed molecular epidemiology, resistance determinants, risk factors and clinical outcomes. Infect. Drug Resist. 2020, 13, 577–585. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front. Microbiol. 2019, 31, 44. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, Y.-K.; Lee, H.; Ko, K.S. High prevalence of non-clonal imipenem-nonsusceptible Enterobacter spp. isolates in Korea and their association with porin down-regulation. Diagn. Microbiol. Infect. Dis. 2017, 87, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shugart, A.; Mahon, G.; Huang, J.Y.; Karlsson, M.; Valley, A.; Lasure, M.; Gross, A.; Pattee, B.; Vaeth, E.; Brooks, R.; et al. Carbapenemase production among less-common Enterobacterales genera: 10 US sites, 2018. JAC Antimicrob. Resist. 2021, 3, dlab137. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE). November 2015 Update—CRE Toolkit 2015. Available online: https://www.cdc.gov/hai/pdfs/cre/cre-guidance-508.pdf (accessed on 10 August 2023).
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jean, S.S.; Lee, Y.L.; Lu, M.C.; Ko, W.C.; Liu, P.Y.; Hsueh, P.R. Carbapenem-Resistant Enterobacterales in Long-Term Care Facilities: A Global and Narrative Review. Front. Cell. Infect. Microbiol. 2021, 11, 601968. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.J.; Lin, M.Y.; Weinstein, R.A.; Trick, W.E. Spread of carbapenem-resistant Enterobacterales among Illinois healthcare facilities: The role of patient sharing. Clin. Infect. Dis. 2016, 63, 889–893. [Google Scholar] [CrossRef][Green Version]
- Hagiya, H.; Ogawa, H.; Takahashi, Y.; Yamamoto, A.; Otsuka, F. Klebsiella oxytoca-producing IMP-1 detected as the first strain of carbapenem-resistant Enterobacteriaceae in our hospital. Intern. Med. 2015, 54, 2939–2941. [Google Scholar] [CrossRef][Green Version]
- Rajni, E.; Jain, A.; Garg, V.K.; Sharma, R.; Vohra, R.; Jain, S.S. Providencia causing urinary tract infections: Are we reaching a dead end? Indian J. Crit. Care Med. 2022, 26, 446–451. [Google Scholar] [CrossRef]
- Simanjuntak, D.F.; Kusumawati, R.L.; Bader, O.; Lüder, C.G.K.; Zimmermann, O.; Groß, U. A comparative pilot study on Gram-negative bacteria contaminating the hands of children living in urban and rural areas of Indonesia versus Germany—A suitable monitoring strategy for diarrhea risk assessment? Front. Microbiol. 2023, 3, 5057–5061. [Google Scholar] [CrossRef]
- Warren, J.W. Providencia stuartii: A common cause of antibiotic-resistant bacteriuria in patients with long-term indwelling catheters. Rev. Infect. Dis. 1986, 8, 61–67. [Google Scholar] [CrossRef]
- Iwata, S.; Tada, T.; Hishinuma, T.; Tohya, M.; Oshiro, S.; Kuwahara-Arai, K.; Ogawa, M.; Shimojima, M.; Kirikae, T. Emergence of carbapenem-resistant Providencia rettgeri and Providencia stuartii producing IMP-type metallo-β-lactamase in Japan. Antimicrob. Agents Chemother. 2020, 64, e00382-20. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Wang, M.-C.; Hsueh, P.-R.; Liu, M.-C.; Hu, R.-M.; Wu, Y.-J.; Liaw, S.-J. Overexpression of an outer membrane protein associated with decreased susceptibility to carbapenems in Proteus mirabilis. PLoS ONE 2015, 10, e0120395. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Available online: http://www.eucast.org/resistance_mechanisms/ (accessed on 20 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).