Ticks (Acari: Ixodida) are hematophagous ectoparasitic arachnids that feed on the blood of vertebrate hosts, posing significant concern due to their unrivaled capacity to transmit various pathogens, which surpasses those of all other known arthropod vectors [1]. These pathogens include protozoa (e.g., Babesia spp. and Theileria spp.), bacteria (e.g., Rickettsia spp. and Borrelia spp.), viruses (e.g., Crimean–Congo hemorrhagic fever and tick-borne encephalitis), and helminths (e.g., Cercopithifilaria) [2,3,4].
Recent research published by Ostfeld and Keesing [5] investigates the effectiveness of various methods for reducing tick populations, specifically host-seeking nymphal blacklegged ticks. Additionally, it seeks to understand the potential impact of these tick reduction methods on human exposure to tick-borne diseases (TBDs), particularly Lyme disease. The research investigates whether reducing tick populations through acaricidal treatments and other means actually leads to a reduction in human encounters with ticks and a decrease in the incidence of TBDs in affected populations. The main conclusion of the research is that while reducing tick populations holds promise for tick control, its direct impact on reducing human encounters with ticks and TBDs is not well-established and may be influenced by various factors that require further investigation.
Environmental factors play significant roles in tick populations and pathogen prevalence [6]. Variables like temperature, humidity, and vegetation affect tick abundance and distribution [6]. Moreover, the presence of suitable hosts influences tick populations and their ability to acquire and transmit pathogens [7,8]. Changes in land use and habitat can disrupt ecological dynamics, potentially affecting TBDs prevalence in a region [9,10]. Thus, environmental factors create a complex interplay that shapes the relationship between ticks and the pathogens they carry, impacting tick-borne pathogen (TBP) transmission. The study by Ostfeld and Keesing [5] suggests that these factors result in the emergence of nonlinear dynamics between tick abundance and human exposure to TBDs. Reducing tick populations does not proportionally decrease TBDs incidence, and the mechanisms behind these nonlinearities remain poorly understood.
Modern sequencing techniques have transformed our ability to study bacterial pathogens and symbionts, opening new avenues for exploring the complex tick microbiome [11,12,13]. The tick microbiome plays a crucial role in tick physiology, host–pathogen interactions, and potentially influences pathogen transmission and disease ecology. The significance of tick microbiome for pathogen transmission cannot be overstated [12]. Could the tick microbiome and factors influencing it contribute to the nonlinear dynamics between tick abundance and human exposure to TBDs?
The tick microbiome comprises various microorganisms that can either compete with or facilitate pathogen growth and colonization [14,15,16]. For example, one study found that an increase in the proportion and quantity of the maternally inherited symbiont Rickettsia belli in the microbiota negatively correlates with the infection levels of the TBP Anaplasma marginale in Dermacentor andersoni ticks [17]. Regarding Borrelia, tick microbiome composition and diversity influence Borrelia acquisition in ticks [18,19]. For instance, the abundance of Pseudomonas, Bacillus, or Enterobacteriacea negatively correlates with Borrelia burgdorferi abundance in nymphal Ixodes scapularis ticks [20]. However, this relationship is not always hostile. An earlier study showed that a reduction in Francisella-like endosymbionts was linked to lower infection levels of Francisella novicida in Dermacentor andersoni ticks, indicating a positive pathogen–endosymbiont relationship [17]. Therefore, various factors affecting the tick microbiome can indirectly shape the disease ecology and dynamics.
Biotic factors significantly impact tick microbial communities (Figure 1). Nymph and adult ticks exhibit distinct bacterial profiles influenced by factors like tick species and geographic location [12]. Tick species are a primary influencer, with different species having unique adaptations that affect gut microbial communities [21,22,23,24]. Life stage also plays a role, with larvae, nymphs, and adults displaying different feeding behaviors and microbiome compositions [22,25,26]. Development and molting introduce changes to gut microbiome composition and diversity. Geographical locations strongly influence tick microbiome due to diverse wildlife hosts, carrying distinct microorganisms in their blood and/or skin [27,28,29,30]. Regional variations in pathogen prevalence indirectly affect tick microbiome dynamics [31,32].
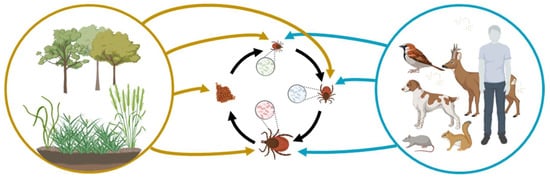
Figure 1.
Interplay between abiotic and biotic factors in shaping the tick microbiome and disease ecology. Blue arrows indicate microorganisms obtained through tick bites. Black arrows represent maternally inherited tick symbionts acquired through transovarial and transstadial transmission. Yellow arrows signify microorganisms acquired from the environment including soil and plants (amended from a previous publication [12]). The figure was generated using BioRender.com.
Notably, ticks spend a substantial portion of their life cycle off-host in the environment [33], where they can acquire bacteria from the surrounding soil and plants [34]. Interestingly, changes in B. burgdorferi prevalence in ticks have been associated with factors shaping soil microbial diversity. Forest fragmentation, impacting both host and soil microbial diversity by creating new ecotone habitats [35], has been linked to higher B. burgdorferi prevalence in ticks [36,37]. However, the influence of land use and soil properties on the tick microbiome is an area that requires further investigation. During their off-host stages, ticks acquire bacteria, primarily from the surrounding soil and plants, through direct contact. This acquisition process involves the proteolytic activity of surrounding bacteria and fungi, which can penetrate the chitin shell of ticks via enzymatic processes [38]. Soil properties, including texture and microbial communities, likely shape the tick microbiome, impacting TBPs in ticks.
The tick microbiome has been shown to contain soil-associated bacteria. Microbiome diversity has been studied in various hard tick species, such as Ixodes scapularis and Dermacentor variabilis, using next-generation sequencing techniques [34]. Soil-associated bacteria often comprise the most diverse component of the tick microbiome [39], primarily influenced by the tick’s geographic location [40]. However, the specific impact of soil bacteria composition on the tick microbiome community, including TBPs, remains unknown.
In conclusion, the intricate world of the tick microbiome emerges as a pivotal player in the complex and nonlinear dynamics between tick abundance and human exposure to TBDs delineated by Ostfeld and Keesing [5]. As highlighted by recent research, tick microbiome wields significant influence over the physiology, behavior, and ecology of ticks, acting as key mediators in the transmission of various TBPs. The interplay between the diverse microorganisms within ticks, acquired from the environment and influenced by factors ranging from tick species to geographical locations, presents a dynamic landscape. These microorganisms can either inhibit or facilitate the transmission of TBDs, introducing a layer of complexity to the straightforward relationship between reducing tick populations and decreasing the incidence of TBDs. Understanding the nuanced role of the tick microbiome in shaping disease ecology is thus paramount, not only in comprehending the nonlinearities observed, but also for developing more targeted and effective strategies in the realm of tick control and the prevention of TBDs. Future research endeavors aiming to unravel the intricate relationships within the tick microbiome hold the promise of providing valuable insights into the broader dynamics of TBDs and refining our strategies to mitigate their impact on public health.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cabezas-Cruz, A.; Estrada-Peña, A.; de la Fuente, J. The Good, the Bad and the Tick. Front. Cell Dev. Biol. 2019, 7, 79. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Does Experimental Reduction of Blacklegged Tick (Ixodes scapularis) Abundance Reduce Lyme Disease Incidence? Pathogens 2023, 12, 714. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; de la Fuente, J. Impact of Climate Trends on Tick-Borne Pathogen Transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, X.; White, A.; Gortázar, C.; Ruiz-Fons, F. The Impact of Host Abundance on the Epidemiology of Tick-Borne Infection. Bull. Math. Biol. 2023, 85, 30. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Léger, E.; Dietrich, M. Host specialization in ticks and transmission of tick-borne diseases: A review. Front. Cell. Infect. Microbiol. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of Land Use Changes and Habitat Fragmentation on the Eco-epidemiology of Tick-Borne Diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef]
- Mateos-Hernández, L.; Obregón, D.; Wu-Chuang, A.; Maye, J.; Bornères, J.; Versillé, N.; de la Fuente, J.; Díaz-Sánchez, S.; Bermúdez-Humarán, L.G.; Torres-Maravilla, E.; et al. Anti-Microbiota Vaccines Modulate the Tick Microbiome in a Taxon-Specific Manner. Front. Immunol. 2021, 12, 704621. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Fikrig, E. Tick microbiome: The force within. Trends Parasitol. 2015, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Hayes, S.F.; Mavros, A. Nonpathogenic Rickettsiae in Dermacentor andersoni: A Limiting Factor for the Distribution of Rickettsia rickettsii. 1980. Available online: https://www.semanticscholar.org/paper/Nonpathogenic-rickettsiae-in-Dermacentor-andersoni%3A-Burgdorfer-Hayes/5fb5e67e3cc542f31e4f679d62a4b7a1365278e5 (accessed on 13 September 2023).
- Aspinwall, J.A.; Jarvis, S.M.; Noh, S.M.; Brayton, K.A. The Effect of Rickettsia bellii on Anaplasma marginale Infection in Dermacentor andersoni Cell Culture. Microorganisms 2023, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Lejal, E.; Chiquet, J.; Aubert, J.; Robin, S.; Estrada-Peña, A.; Rue, O.; Midoux, C.; Mariadassou, M.; Bailly, X.; Cougoul, A.; et al. Temporal patterns in Ixodes ricinus microbial communities: An insight into tick-borne microbe interactions. Microbiome 2021, 9, 153. [Google Scholar] [CrossRef]
- Gall, C.A.; Reif, K.E.; Scoles, G.A.; Mason, K.L.; Mousel, M.; Noh, S.M.; Brayton, K.A. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016, 10, 1846–1855. [Google Scholar] [CrossRef]
- Kurokawa, C.; Lynn, G.E.; Pedra, J.H.F.; Pal, U.; Narasimhan, S.; Fikrig, E. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol. 2020, 18, 587–600. [Google Scholar] [CrossRef]
- Sperling, J.L.H.; Fitzgerald, D.; Sperling, F.A.H.; Magor, K.E. Microbiome Composition and Borrelia Detection in Ixodes scapularis Ticks at the Northwestern Edge of Their Range. Trop. Med. Infect. Dis. 2020, 5, 173. [Google Scholar] [CrossRef]
- Landesman, W.J.; Mulder, K.; Fredericks, L.P.; Allan, B.F. Cross-kingdom analysis of nymphal-stage Ixodes scapularis microbial communities in relation to Borrelia burgdorferi infection and load. FEMS Microbiol. Ecol. 2019, 95, fiz167. [Google Scholar] [CrossRef]
- Chandra, S.; Šlapeta, J. Biotic Factors Influence Microbiota of Nymph Ticks from Vegetation in Sydney, Australia. Pathogens 2020, 9, 566. [Google Scholar] [CrossRef]
- Lau, A.C.C.; Mohamed, W.M.A.; Nakao, R.; Onuma, M.; Qiu, Y.; Nakajima, N.; Shimozuru, M.; Mohd-Azlan, J.; Moustafa, M.A.M.; Tsubota, T. The dynamics of the microbiome in Ixodidae are shaped by tick ontogeny and pathogens in Sarawak, Malaysian Borneo. Microb. Genom. 2023, 9, 000954. [Google Scholar] [CrossRef] [PubMed]
- Lalzar, I.; Harrus, S.; Mumcuoglu, K.Y.; Gottlieb, Y. Composition and Seasonal Variation of Rhipicephalus turanicus and Rhipicephalus sanguineus Bacterial Communities. Appl. Environ. Microbiol. 2012, 78, 4110–4116. [Google Scholar] [CrossRef]
- Duron, O.; Wilkes, T.E.; Hurst, G.D.D. Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol. Lett. 2010, 13, 1139–1148. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Clark, C.; Ocasio, K.; Gauthier, D.T.; Hynes, W.L. Factors affecting the microbiome of Ixodes scapularis and Amblyomma americanum. PLoS ONE 2020, 15, e0232398. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.; Tagliafierro, T.; Sameroff, S.; Cucura, D.M.; Oleynik, A.; Che, X.; Jain, K.; Lipkin, W.I. Microbiome analysis of Ixodes scapularis ticks from New York and Connecticut. Ticks Tick Borne Dis. 2019, 10, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Williams-Newkirk, A.J.; Rowe, L.A.; Mixson-Hayden, T.R.; Dasch, G.A. Characterization of the Bacterial Communities of Life Stages of Free Living Lone Star Ticks (Amblyomma americanum). PLoS ONE 2014, 9, e102130. [Google Scholar] [CrossRef]
- Zolnik, C.P.; Prill, R.J.; Falco, R.C.; Daniels, T.J.; Kolokotronis, S.O. Microbiome changes through ontogeny of a tick pathogen vector. Mol. Ecol. 2016, 25, 4963–4977. [Google Scholar] [CrossRef]
- Thapa, S.; Zhang, Y.; Allen, M.S. Bacterial microbiomes of Ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; DePonte, K.; Fish, D.; Fikrig, E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef]
- Swei, A.; Kwan, J.Y. Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 2017, 11, 813–816. [Google Scholar] [CrossRef]
- Wu-Chuang, A.; Mateos-Hernandez, L.; Maitre, A.; Rego, R.O.M.; Šíma, R.; Porcelli, S.; Rakotobe, S.; Foucault-Simonin, A.; Moutailler, S.; Palinauskas, V.; et al. Microbiota perturbation by anti-microbiota vaccine reduces the colonization of Borrelia afzelii in Ixodes ricinus. Microbiome 2023, 11, 151. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Wu-Chuang, A.; Hodžić, A.; Mateos-Hernández, L.; Estrada-Peña, A.; Obregon, D.; Cabezas-Cruz, A. Current debates and advances in tick microbiome research. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100036. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T.S. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Vaux, A.G.C.; Hansford, K.M.; Pietzsch, M.E.; Gillingham, E.L. Ticks in the ecotone: The impact of agri-environment field margins on the presence and intensity of Ixodes ricinus ticks (Acari: Ixodidae) in farmland in southern England. Med. Vet. Entomol. 2020, 34, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Influence of Meso- and Microscale Habitat Structure on Focal Distribution of Sympatric Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 285–294. [Google Scholar] [CrossRef]
- Habeeb, S.M.; Ashry, H.M.; Saad, M.M. Ovicidal effect of chitinase and protease enzymes produced by soil fungi on the camel tick Hyalomma dromedarii eggs (Acari: Ixodidae). J. Parasit. Dis. 2017, 41, 268–273. [Google Scholar] [CrossRef][Green Version]
- Andreotti, R.; Pérez de León, A.A.; Dowd, S.E.; Guerrero, F.D.; Bendele, K.G.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Aivelo, T.; Lemoine, M.; Tschirren, B. Elevational Changes in Bacterial Microbiota Structure and Diversity in an Arthropod-Disease Vector. Microb. Ecol. 2022, 84, 868–878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).