Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Nigeria
2.2. Ethiopia
2.3. Animal Study
2.4. DNA Extraction and PCR Amplification
2.5. Phylogenetic Analysis
3. Results
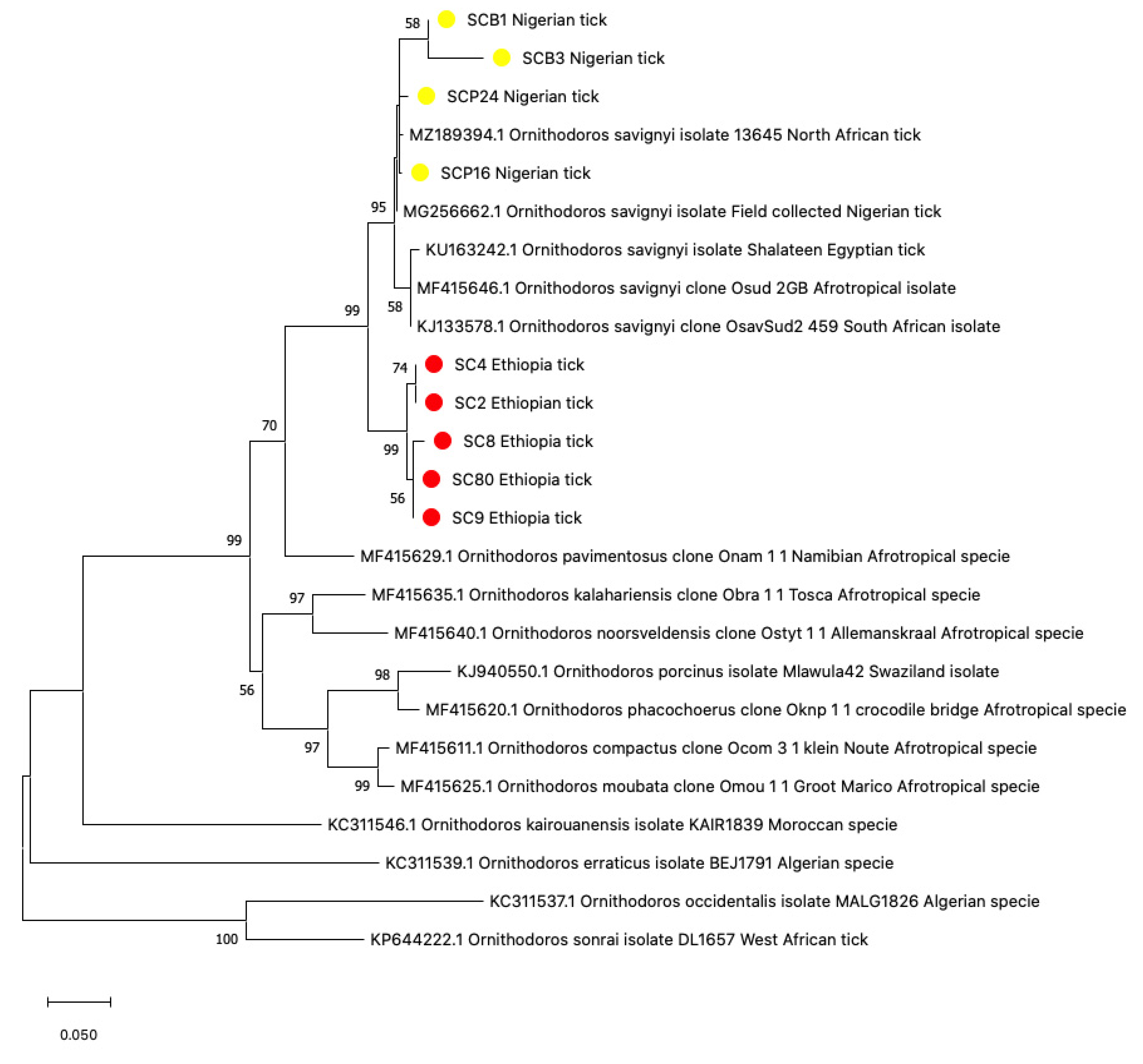
3.1. Tick Identification
3.2. Sequence Analysis for Tick Identification
3.3. Polymerase Chain Reactions for Borrelia Infections
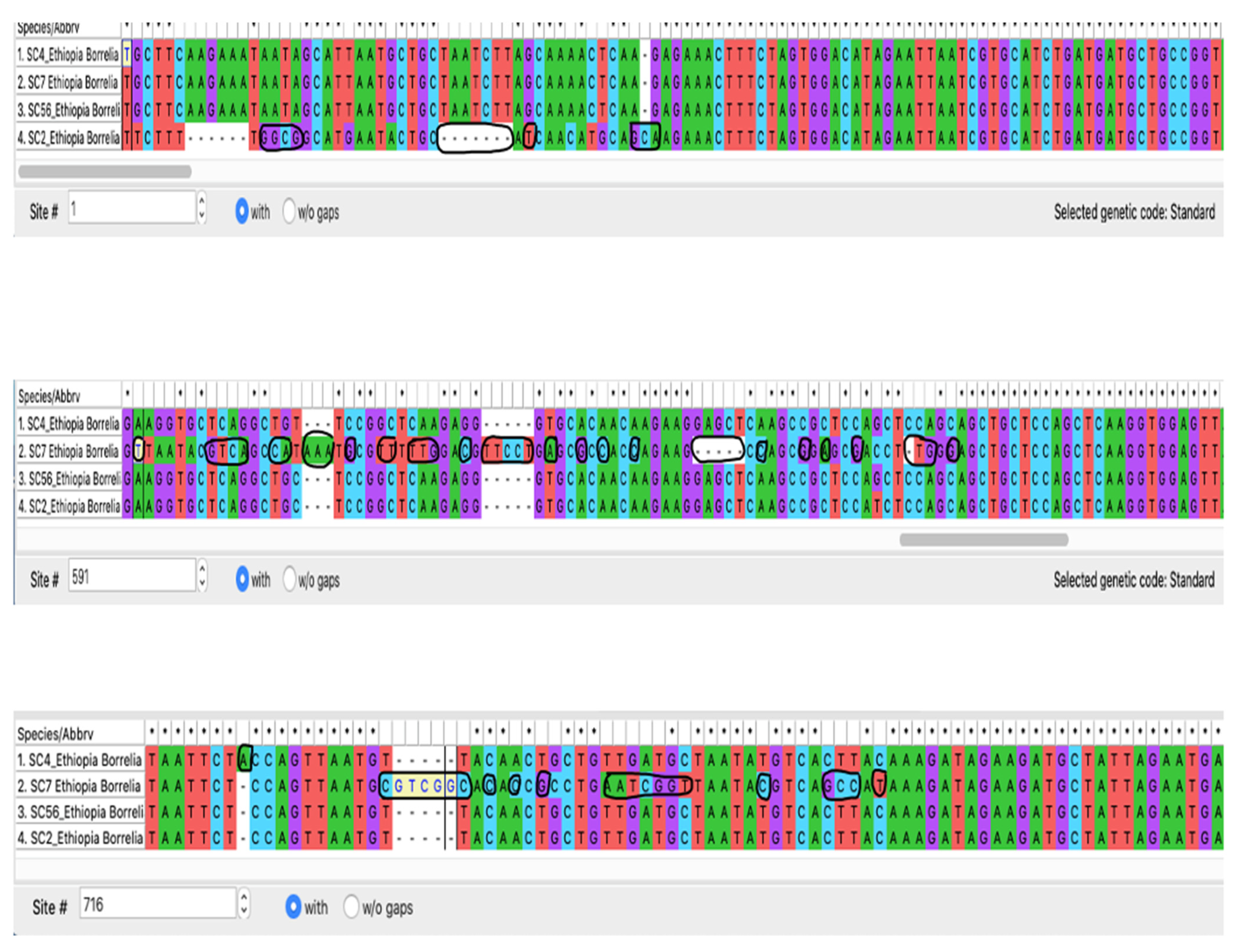
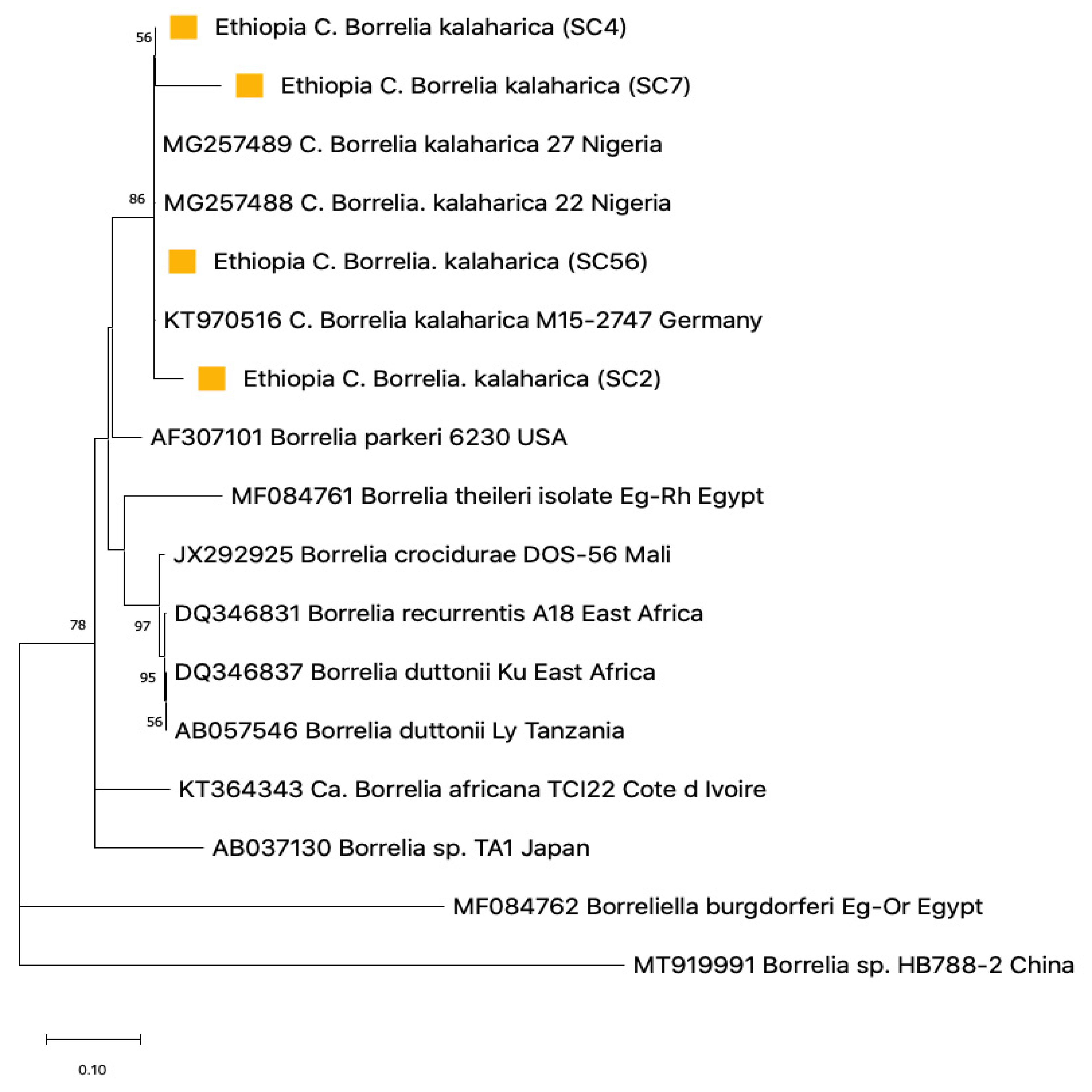
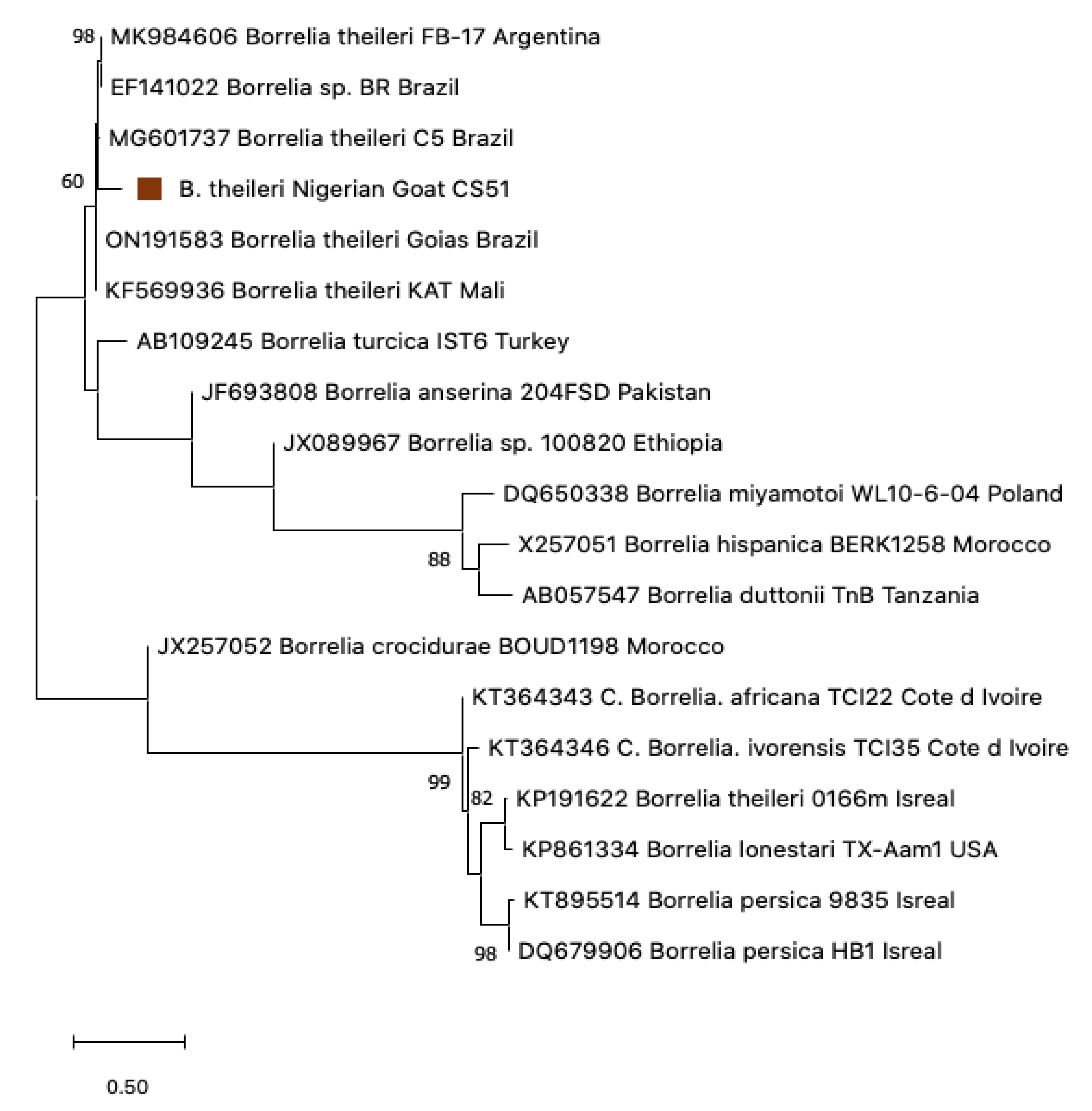
3.4. Sequence Analysis for Borrelia
3.5. Phylogenetic Construction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diatta, G.; Duplantier, J.-M.; Granjon, L.; Bâ, K.; Chauvancy, G.; Ndiaye, M.; Trape, J.-F. Borrelia infection in small mammals in West Africa and its relationship with tick occurrence inside burrows. Acta Trop. 2015, 152, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.S.; Khalil, G.M.; Hoogstraal, H.; Aboul-Nasr, A.E. Borrelia crocidurae localization and transmission in Ornithodoros erraticus and O. savignyi. Parasitology 1984, 88, 403–413. [Google Scholar] [CrossRef]
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E.; Borchardt, S.M. Tick-Borne Relapsing Fever. Infect. Dis. Clin. North Am. 2008, 22, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Goutier, S.; Ferquel, E.; Pinel, C.; Bosseray, A.; Hoen, B.; Couetdic, G.; Bourahoui, A.; Lapostolle, C.; Pelloux, H.; Garnier, M.; et al. Borrelia crocidurae Meningoencephalitis, West Africa. Emerg. Infect. Dis. 2013, 19, 301–304. [Google Scholar] [CrossRef]
- Hasin, T.; Davidovitch, N.; Cohen, R.; Dagan, T.; Romem, A.; Orr, N.; Klement, E.; Lubezky, N.; Kayouf, R.; Sela, T.; et al. Postexposure Treatment with Doxycycline for the Prevention of Tick-Borne Relapsing Fever. N. Engl. J. Med. 2006, 355, 148–155. [Google Scholar] [CrossRef]
- Sharma, S.P.; Amanfu, W.; Losho, T.C. Bovine borreliosis in Botswana. Onderstepoort J. Vet. Res. 2000, 67, 221–223. [Google Scholar]
- Sakharoff, M.N. Spirochaeta anserina et la septicémie des oies. Annales de l’Institut Pasteur (Paris). Ann. Inst. Pasteur. 1891, 5, 564–566. [Google Scholar]
- Chauhan, H.V.S.; Roy, S. Poultry Diseases: Diagnosis and Treatment, 2nd ed.; New Age International: New Dehli, India, 1996. [Google Scholar]
- Simuunza, M.; Weir, W.; Courcier, E.; Tait, A.; Shiels, B. Epidemiological analysis of tick-borne diseases in Zambia. Vet. Parasitol. 2011, 175, 331–342. [Google Scholar] [CrossRef]
- Nambota, A.; Samui, K.; Sugimoto, C.; Kakuta, T.; Onuma, M. Theileriosis in Zambia: Etiology, epidemiology and control measures. Jpn. J. Vet. Res. 1994, 42, 1–18. [Google Scholar] [CrossRef]
- Abanda, B.; Paguem, A.; Abdoulmoumini, M.; Kingsley, M.T.; Renz, A.; Eisenbarth, A. Molecular identification and prevalence of tick-borne pathogens in zebu and taurine cattle in North Cameroon. Parasites Vectors 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Squarre, D.; Nakamura, Y.; Lau, A.C.C.; Moonga, L.C.; Kawai, N.; Ohnuma, A.; Hayashida, K.; Nakao, R.; Yamagishi, J.; et al. Evidence of Borrelia theileri in Wild and Domestic Animals in the Kafue Ecosystem of Zambia. Microorganisms 2021, 9, 2405. [Google Scholar] [CrossRef] [PubMed]
- Assoku, R.K.G. Study of the incidence of blood-borne parasites of livestock in southern Ghana. Bull. Anim. Health Prod. Afr. 1979, 27, 29–39. [Google Scholar]
- Trees, A. The transmission ofBorrelia theileri byBoophilus annulatus (say, 1821). Trop. Anim. Health Prod. 1978, 10, 93–94. [Google Scholar] [CrossRef]
- Barre, N.; Morel, P.C. [Ticks (Acarina, Ixodoidea) of the Mascarene Islands and tick-borne diseases [Babesiosis; theileriosis; borreliosis; heartwater]]. J. Anim. Husb. Vet. Med. Trop. Ctries. 1983, 36, 371–377. [Google Scholar]
- Scott, J.; Wright, D.; Cutler, S.J. Typing African relapsing fever spirochetes. Emerg. Infect. Dis. 2005, 11, 1722–1729. [Google Scholar] [CrossRef]
- Jongen, V.H.W.M.; van Roosmalen, J.; Tiems, J.; Van Holten, J.; Wetsteyn, J.C.F.M. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet. Et Gynecol. Scand. 1997, 76, 834–838. [Google Scholar] [CrossRef]
- Nordmann, T.; Feldt, T.; Bosselmann, M.; Tufa, T.B.; Lemma, G.; Holtfreter, M.; Häussinger, D. Outbreak of Louse-Borne Relapsing Fever among Urban Dwellers in Arsi Zone, Central Ethiopia, from July to November 2016. Am. J. Trop. Med. Hyg. 2018, 98, 1599–1602. [Google Scholar] [CrossRef]
- Cutler, S.; Abdissa, A.; Adamu, H.; Tolosa, T.; Gashaw, A. Borrelia in Ethiopian ticks. Ticks Tick-Borne Dis. 2011, 3, 14–17. [Google Scholar] [CrossRef]
- Kumsa, B.; Socolovschi, C.; Raoult, D.; Parola, P. New Borrelia species detected in ixodid ticks in Oromia, Ethiopia. Ticks Tick-borne Dis. 2015, 6, 401–407. [Google Scholar] [CrossRef]
- Trape, J.-F.; Diatta, G.; Arnathau, C.; Bitam, I.; Sarih, M.; Belghyti, D.; Bouattour, A.; Elguero, E.; Vial, L.; Mané, Y.; et al. The Epidemiology and Geographic Distribution of Relapsing Fever Borreliosis in West and North Africa, with a Review of the Ornithodoros erraticus Complex (Acari: Ixodida). PLoS ONE 2013, 8, e78473. [Google Scholar] [CrossRef]
- Ndiaye, E.H.I.; Diouf, F.S.; Ndiaye, M.; Bassene, H.; Raoult, D.; Sokhna, C.; Parola, P.; Diatta, G. Tick-borne relapsing fever Borreliosis, a major public health problem overlooked in Senegal. PLoS Neglected. Trop. Dis. 2021, 15, e0009184. [Google Scholar] [CrossRef]
- Leen, I.; Bruynseels, P.; Mukadi, B.K.; Van Oort, M.; Akker, M.V.D. A 13-year old girl with pancytopenia at the presentation of a Borrelia hispanica infection: A case report and review of the literature. J. Med. Case Rep. 2017, 11, 51. [Google Scholar] [CrossRef]
- Cutler, S.J.; Idris, J.M.; Ahmed, A.O.; Elelu, N. Ornithodoros savignyi, the Tick Vector of “Candidatus Borrelia kalaharica” in Nigeria. J. Clin. Microbiol. 2018, 56, e00532-18. [Google Scholar] [CrossRef] [PubMed]
- Musa, J.; Dung-Gwom, J.Y. Assessment of urban regeneration activities in the central area of Jos town, Nigeria. UPLanD J. Urban Plan. 2018, 3, 75–88. [Google Scholar] [CrossRef]
- Aliyu Mohammed, U.; Zafer Alibaba, H. Application of Bioclimatic Design Strategies to Solve Thermal Discomfort in Maiduguri Residences, Borno State Nigeria. Imp. J. Interdiscip. Res. IJIR 2018, 4, 227–233. [Google Scholar]
- Amaza, P.S.; Bila, Y.; Iheanacho, A.C. Identification of Factors that Influence Technical Efficiency of Food Crop Production in West Africa: Empirical Evidence from Borno State, Nigeria. J. Agric. Rural Dev. Trop. Subtrop. (JARTS) 2006, 107, 139–147. [Google Scholar]
- Abdulatife, M. Assessment of Pastoral Perceptions towards Range and Livestock Management Practices in Chifra District of Afar Regional State, Ethiopia. For. Res. 2015, 4, 2. [Google Scholar] [CrossRef]
- Elelu, N.; Ola-Fadunsin, S.D.; Bankole, A.A.; Raji, M.A.; Ogo, N.I.; Cutler, S.J. Prevalence of tick infestation and molecular characterization of spotted fever Rickettsia massiliae in Rhipicephalus species parasitizing domestic small ruminants in north-central Nigeria. PLoS ONE 2022, 17, e0263843. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Ushijima, Y.; Aoki, Y.; Talbert, A. Detection of Borrelia duttonii, a Tick-Borne Relapsing Fever Agent in Central Tanzania, Within Ticks by Flagellin Gene-Based Nested Polymerase Chain Reaction. Vector-Borne Zoonotic Dis. 2001, 1, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Diatta, G.; Socolovschi, C.; Mediannikov, O.; Tall, A.; Bassene, H.; Trape, J.F.; Raoult, D. Tick-Borne Relapsing Fever Borreliosis, Rural Senegal. Emerg. Infect. Dis. 2011, 17, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Assous, M.; Wilamowski, A.; Bercovier, H.; Marva, E. Molecular Characterization of Tickborne Relapsing Fever Borrelia, Israel. Emerg. Infect. Dis. 2006, 12, 1740–1743. [Google Scholar] [CrossRef]
- Takano, A.; Goka, K.; Une, Y.; Shimada, Y.; Fujita, H.; Shiino, T.; Watanabe, H.; Kawabata, H. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ. Microbiol. 2010, 12, 134–146. [Google Scholar] [CrossRef]
- Zhai, B.; Niu, Q.; Yang, J.; Liu, Z.; Liu, J.; Yin, H.; Zeng, Q. Identification and molecular survey of Borrelia burgdorferi sensu lato in sika deer (Cervus nippon) from Jilin Province, north-eastern China. Acta Trop. 2017, 166, 54–57. [Google Scholar] [CrossRef]
- Parola, P.; Ryelandt, J.; Mangold, A.J.; Mediannikov, O.; Guglielmone, A.A.; Raoult, D. Relapsing fever Borrelia in Ornithodoros ticks from Bolivia. Ann. Trop. Med. Parasitol. 2011, 105, 407–411. [Google Scholar] [CrossRef]
- Black, W.C.; Piesman JBlack, W.C.; Piesman, J. Phylogeny of hard-and soft-tick taxa (Acari_ Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.V.D.F.; Neves, L.C.; de Paula, L.G.F.; Serpa, M.C.D.A.; de Oliveira, F.P.; Dantas-Torres, F.; Muñoz-Leal, S.; Labruna, M.B.; Krawczak, F.D.S. First molecular detection of Borrelia theileri subclinical infection in a cow from Brazil. Vet. Res. Commun. 2022, 1–5. [Google Scholar] [CrossRef]
- Cordeiro, M.D.; Bahia, M.; Magalhães-Matos, P.C.; Cepeda, M.B.; Guterres, A.; Fonseca, A.H. Morphological, molecular and phylogenetic characterization of Borrelia theileri in Rhipicephalus microplus. Braz. J. Vet. Parasitol. 2018, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; De Salvo, M.N.; Cicuttin, G.; Rossner, V.; Thompson, C.S.; Mangold, A.J.; Nava, S. The presence of Borrelia theileri in Argentina. Veter- Parasitol. Reg. Stud. Rep. 2019, 17, 100314. [Google Scholar] [CrossRef]
- McCoy, B.N.; Maïga, O.; Schwan, T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014, 5, 401–403. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Arroyave, E.; Faccini-Martínez, A.; Martínez-Diaz, H.C.; Betancourt-Ruiz, P.; Olaya-M, L.-A.; Forero-Becerra, E.G.; Hidalgo, M.; Blanton, L.S.; Walker, D.H. Emerging Tickborne Bacteria in Cattle from Colombia. Emerg. Infect. Dis. 2022, 28, 2109–2111. [Google Scholar] [CrossRef]
- Takhampunya, R.; Sakolvaree, J.; Chanarat, N.; Youngdech, N.; Phonjatturas, K.; Promsathaporn, S.; Tippayachai, B.; Tachavarong, W.; Srinoppawan, K.; Poole-Smith, B.K.; et al. The Bacterial Community in Questing Ticks from Khao Yai National Park in Thailand. Front. Vet. Sci. 2021, 8, 1335. [Google Scholar] [CrossRef]
- Fingerle, V.; Pritsch, M.; Wächtler, M.; Margos, G.; Ruske, S.; Jung, J.; Löscher, T.; Wendtner, C.; Wieser, A. “Candidatus Borrelia kalaharica” Detected from a Febrile Traveller Returning to Germany from Vacation in Southern Africa. PLoS Neglected Trop. Dis. 2016, 10, e0004559. [Google Scholar] [CrossRef] [PubMed]
- Stete, K.; Rieg, S.; Margos, G.; Häcker, G.; Wagner, D.; Kern, W.V.; Fingerle, V. Case Report and Genetic Sequence Analysis of Candidatus Borrelia kalaharica, Southern Africa. Emerg. Infect. Dis. 2018, 24, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R.; Moore, S.I.; Wilson, M.L.; Spielman, A.; Mather, T.N.; Iii, S.R.T. Incompetence of Deer as Reservoirs of the Lyme Disease Spirochete. Am. J. Trop. Med. Hyg. 1988, 39, 105–109. [Google Scholar] [CrossRef]
- Gray, J.S.; Kahl, O.; Janetzki, C.; Stein, J. Studies on the Ecology of Lyme Disease in a Deer Forest in County Galway, Ireland. J. Med. Èntomol. 1992, 29, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Heisch, R.B.; Harvey, A.E.C. Is Ornithodoros Savignyi (Audouin) a Vector of Relapsing Fever in Africa? Ann. Trop. Med. Parasitol. 1960, 54, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.M. Report on Relapsing Fever in Burao; 1936; pp. 57–66. Available online: https://www.cabdirect.org/cabdirect/abstract/19372901340 (accessed on 15 November 2022).
- Bakkes, D.K.; De Klerk, D.; Latif, A.A.; Mans, B.J. Integrative taxonomy of Afrotropical Ornithodoros (Ornithodoros) (Acari: Ixodida: Argasidae). Ticks Tick-Borne Dis. 2018, 9, 1006–1037. [Google Scholar] [CrossRef] [PubMed]
- Kisinza, W.N.; McCall, P.; Mitani, H.; Talbert, A.; Fukunaga, M. A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet 2003, 362, 1283–1284. [Google Scholar] [CrossRef]
- Cutler, S.J. Relapsing fever—A forgotten disease revealed. J. Appl. Microbiol. 2010, 108, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Talbert, A.; Fukunaga, M. New World Relapsing Fever Borrelia Found in Ornithodoros porcinus Ticks in Central Tanzania. Microbiol. Immunol. 2004, 48, 501–505. [Google Scholar] [CrossRef]
- Munson, E. Moving Targets of Bacterial Taxonomy Revision: What Are They and Why Should We Care? Clin. Microbiol. Newsl. 2020, 42, 111–120. [Google Scholar] [CrossRef]
- Pegram, R.G.; Hoogstraal, H.; Wassef, H.Y. Ticks (Acari: Ixodoidea) of Ethiopia. I. Distribution, ecology and host relationships of species infesting livestock. Bull. Èntomol. Res. 1981, 71, 339–359. [Google Scholar] [CrossRef]
- Mans, B.J.; Featherston, J.; Kvas, M.; Pillay, K.-A.; de Klerk, D.G.; Pienaar, R.; de Castro, M.H.; Schwan, T.G.; Lopez, J.; Teel, P.; et al. Argasid and ixodid systematics: Implications for soft tick evolution and systematics, with a new argasid species list. Ticks Tick-Borne Dis. 2019, 10, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Ogo, N.I.; de Mera, I.G.F.; Galindo, R.C.; Okubanjo, O.O.; Inuwa, H.M.; Agbede, R.I.; Torina, A.; Alongi, A.; Vicente, J.; Gortázar, C.; et al. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet. Parasitol. 2012, 187, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, G.; Eshed, T.; Nachum-Biala, Y.; King, R.; Baneth, G. Transmission of the Human Relapsing Fever Spirochete Borrelia persica by the Argasid Tick Ornithodoros tholozani Involves Blood Meals from Wildlife Animal Reservoirs and Mainly Transstadial Transfer. Appl. Environ. Microbiol. 2021, 87, e03117-20. [Google Scholar] [CrossRef] [PubMed]
- Marshall, I.W.F.; Telford, I.S.R.; Rys, P.N.; Rutledge, B.J.; Mathiesen, D.; Malawista, S.E.; Spielman, A.; Persing, D.H. Detection of Borrelia Burgdorferi Dna In Museum Specimens of Peromyscus. J. Infect. Dis. 1994, 170, 1027–1032. [Google Scholar] [CrossRef]
- Amanzougaghene, N.; Akiana, J.; Mongo Ndombe, G.; Davoust, B.; Nsana, N.S.; Parra, H.J.; Fenollar, F.; Raoult, D.; Mediannikov, O. Head Lice of Pygmies Reveal the Presence of Relapsing Fever Borreliae in the Republic of Congo. PLoS Negl. Trop. Dis. 2016, 10, e0005142. [Google Scholar] [CrossRef]
- Mathisson, D.C.; Kross, S.M.; Palmer, M.I.; Diuk-Wasser, M.A. Effect of Vegetation on the Abundance of Tick Vectors in the Northeastern United States: A Review of the Literature. J. Med. Èntomol. 2021, 58, 2030–2037. [Google Scholar] [CrossRef]
- Kleinerman, G.; Baneth, G. Ornithodoros (Pavlovskyella) tholozani. In Ticks of Europe and North Africa; Springer: Cham, Switzerland, 2018; pp. 67–74. [Google Scholar]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia. Biology 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Assous, M.; Wilamowski, A. Relapsing fever borreliosis in Eurasia—Forgotten, but certainly not gone! Clin. Microbiol. Infect. 2009, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Reháçek, J. Ecological relationships between ticks and rickettsiae. Eur. J. Epidemiology 1989, 5, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H. Argasid and Nuttalliellid Ticks as Parasites and Vectors. Adv. Parasitol. 1985, 24, 135–238. [Google Scholar] [CrossRef] [PubMed]
- Sage, K.M.; Johnson, T.L.; Teglas, M.B.; Nieto, N.C.; Schwan, T.G. Ecological niche modeling and distribution of Ornithodoros hermsi associated with tick-borne relapsing fever in western North America. PLoS Neglected. Trop. Dis. 2017, 11, e0006047. [Google Scholar] [CrossRef]





| Genome Target | Nucleotide Sequences | Band Size | Reference |
|---|---|---|---|
| 16S–23S intergenic spacer region (IGS) (Ethiopia soft ticks) | First round: F: GTATGTTTAGTGAGGGGGGTG R: GGATCATAGCTCAGGTGGTTAG Nested: F: AGGGGGGTGAAGTCGTAACAAG R: GTCTGATAAACCTGAGGTCGGA | 750 bp | [17] |
| Flagellin B (flaB) (Ethiopia soft ticks) | F: TAATACGTCAGCCATAAATGC R: GCTCTTTGATCAGTTATCATTC | 770 bp | [33] |
| Flagellin B (flaB) (Nigerian livestock) | First round: F: GATCARGCWCAAYATAACCAWATGCA R: AGATTCAAGTCTGTTTTGGAAAGC Nested: F: GCTGAAGAGCTTGGAATGCAACC R: TGATCAGTTATCATTCTAATAGCA | 350 bp | [34] |
| 16S rRNA (Nigerian livestock) | First round: F: GCGAACGGGTGAGTAACG R: CCTCCCTTACGGGTTAGAA Nested: F: GAGGCGAAGGCGAACTTCTG R: CTAGCGATTCCAACTTCATGAAG | 650 bp | [35] |
| RT-PCR 16S rRNA (All samples) | F: AGCCTTTAAAGCTTCGCTTGTAG R: GCCTCCCGTAGGAGTCTGG P [FAM] CCGGCCTGAGAGGGTGAACGG | 148 bp | [36] |
| Tick 16S rDNA (All tick samples) | F: CTGCTCAATGATTTTTTAAATTGC R: CCGGTCTGAACTCAGATCATGTA | 450 bp | [37] |
| Species | RT-PCR | Flagellin B | 16S–23S IGS | 16S rRNA |
|---|---|---|---|---|
| O. savignyi (Ethiopia) | 3.5% (11/312) | 4/11 | 4/11 | NA |
| O. savignyi (Nigeria) | 0/77 | NA | NA | NA |
| Cattle | 5% (18/350) | 0/18 | 0/18 | 0/18 |
| Goat | 1.5% (3/200) | 3/3 | 3/3 | 3/3 |
| Stage/State | 16S RT-PCR | Flagellin | IGS | |||
|---|---|---|---|---|---|---|
| Borrelia infection vs. total ticks screened | Borrelia positive (engorged ticks) | Borrelia positive (non-engorged ticks) | Amplified Borrelia sequences | Amplified Borrelia sequences | p-value | |
| Adult ticks | 10% (6/60) | 36% (4/11) | 18% (2/11) | 27% (3/11) | 27% (3/11) | p * > 0.25 |
| Nymphs | 2% (5/252) | 27% (3/11) | 18% (2/11) | 9% (1/11) | 9% (1/11) | p ** > 0.51 |
| Total screened | 11/312 | 7/11 | 4/11 | 36% (4/11) | 36% (4/11) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankole, A.A.; Kumsa, B.; Mamo, G.; Ogo, N.I.; Elelu, N.; Morgan, W.; Cutler, S.J. Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria. Pathogens 2023, 12, 81. https://doi.org/10.3390/pathogens12010081
Bankole AA, Kumsa B, Mamo G, Ogo NI, Elelu N, Morgan W, Cutler SJ. Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria. Pathogens. 2023; 12(1):81. https://doi.org/10.3390/pathogens12010081
Chicago/Turabian StyleBankole, Adefolake A., Bersissa Kumsa, Gezahegne Mamo, Ndudim I. Ogo, Nusirat Elelu, Winston Morgan, and Sally J. Cutler. 2023. "Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria" Pathogens 12, no. 1: 81. https://doi.org/10.3390/pathogens12010081
APA StyleBankole, A. A., Kumsa, B., Mamo, G., Ogo, N. I., Elelu, N., Morgan, W., & Cutler, S. J. (2023). Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria. Pathogens, 12(1), 81. https://doi.org/10.3390/pathogens12010081








