Abstract
Anaplasma phagocytophilum is the causative agent of tick-borne fever in sheep, pasture fever in cattle, and granulocytic anaplasmosis in humans. The increasing prevalence and transboundary spread of A. phagocytophilum in livestock, ticks, and wildlife in the UK poses a potential zoonotic risk that has yet to be estimated. Several ecotypes of A. phagocytophilum show variable zoonotic potential. To evaluate the possible risk associated with the transmission of A. phagocytophilum from ruminants to humans, the ecotype was determined by sequencing the groEL gene from 71 positive blood and tissue samples from UK ruminants. Thirty-four groEL sequences were obtained, fourteen of which were identified in multiple samples. Of the 13 nucleotide polymorphisms identified through pairwise comparison, all corresponded to synonymous substitutions. The subsequent phylogenetic estimation of the relationship with other European/world isolates indicated that all the groEL sequences clustered with other ecotype I sequences. The presence of ecotype I closely reflects that observed in ruminants in continental Europe and suggests a lower risk of zoonotic transmission from this reservoir.
1. Introduction
Zoonoses from tick-borne pathogens have increased dramatically in the last few decades [1] and now pose a serious problem worldwide, due to their impact on public health and livestock production, as well as increased morbidity in wildlife [2]. Climate change, globalization, population movements and growth, changes of landscapes and natural habitats, and shifts in host geographic range and population density have led to the emergence of diverse ecotypes of the tick-transmitted bacterium Anaplasma phagocytophilum [3,4].
Anaplasma phagocytophilum is a Gram-negative bacterium (family Anaplasmataceae, order Rickettsiales) which infects lymphoid cells of the immune system (neutrophils and other cells of myeloid and non-myeloid origin) causing mild-to-severe immunosuppression in vertebrate hosts, including humans. Anaplasma phagocytophilum is tick-transmitted and can manifest with different presentations, from a mild febrile-like illness to a severe disease, which can be complicated by secondary infections, and in some species can progress to abortion. Outbreaks of disease associated with A. phagocytophilum infection in ruminants are increasingly being reported in the UK (http://apha.defra.gov.uk//vet-gateway/surveillance/scanning/disease-dashboards.htm accessed on 18 January 2023). Anaplasma phagocytophilum can infect several animal species, including sheep (Ovis aries), cattle (Bos taurus), goat (Capra aegagrus hircus), dog (Canis lupus familiaris), horse (Equus caballus), red deer (Cervus elaphus), roe deer (Capreolus capreolus) and white-tailed deer (Odocoileus virginianus) as well as wild boar (Sus scrofa), wild rodents, hedgehogs, birds, and humans [4]. The role of the tick vectors and different vertebrate hosts as reservoirs of infection remains unclear [5] in our understanding of disease epidemiology and zoonotic risk [6]. For example, ruminant infections are widespread across Europe but not yet reported in the USA, whereas human, equine, and canine infections have been reported worldwide [7].
Transmission of A. phagocytophilum involves many species of Ixodes ticks (I. scapularis, I. pacificus, I. spinipalpis, I. ricinus, I. persulcatus, I. ovatus), with only a small number of additional vectors (e.g. deer keds, Lipoptena cervi) possibly also involved [4]. In Europe, the main vector is I. ricinus [3,8]. Ticks acquire the bacterium from infected vertebrate hosts through a blood meal, and the infection is maintained through trans-stadial transmission, including a trans-ovarian stage [9]. Anaplasma phagocytophilum is known to survive in the salivary glands and midgut cells of the infected ticks, which can transmit the bacterium to other vertebrate hosts during the blood meal.
Anaplasma phagocytophilum is characterized by a high degree of genetic diversity, resulting in variations in pathogenicity and host tropism [6]. Indeed, several studies have demonstrated that different strains are characterized by different host range predilections, and not all strains can infect all hosts [10,11,12]. Due to the existence of diverse genetic variants, vectors, and vertebrate hosts, the ecoepidemiology of the infection caused by A. phagocytophilum is complex, also involving distinct cycles in various geographical locations [13,14].
Several approaches, targeting different genes, have been used to differentiate A. phagocytophilum variants into different clades, namely, ecotypes, clusters, and haplotypes [14]. Initially, Jahfari et al. [14] proposed a division into four ecotypes, based on the sequence of the groEL heat shock operon gene, which are linked to different combinations of mammalian hosts, tick vectors, and geographical regions (Table 1). However, the groEL gene is relatively conserved compared to other markers and does not always show enough discrimination power to segregate some clusters, specifically when short fragments of the gene are analysed. Therefore, more recently, Jaarsma et al. [15] and Grassi et al. [16] further subdivided the four ecotypes identified by Jahfari et al. into clusters and haplotypes (Table 1). Nevertheless, the subdivision into ecotypes is still preferred as it is simpler and has been extensively used by other authors especially from European countries [17,18].

Table 1.
Relationship between A. phagocytophilum ecotypes, haplotypes, clusters, hosts, and vectors observed in distinct geographical locations in samples derived from different vectors and hosts. I. = ixodes; phylogenetic analysis based on the sequence of the groEL heat shock operon gene. From Jaarsma et al. [15], modified.
Based on the diversity present in the groEL gene, in Europe, multiple ecotypes of A. phagocytophilum are reported to circulate in mammals in three enzootic cycles, one primarily infecting nest-living mammals such as voles and shrews [12,14,15], one host-generalist spreading in livestock, companion animals, and humans [14,19], and a third host-specialist infecting primarily roe deer [20,21]. The host-generalist and host-specialist ecotypes, which are genetically similar, are transmitted by I. ricinus, while A. phagocytophilum infecting burrowing mammals is genetically distant and is mainly transmitted by I. trianguliceps [12,20,21]. Overall, ecotype I (zoonotic) is associated with cattle, horses, mouflons, small ruminants, hedgehogs, red deer, and humans, whereas ecotype II is linked only to ruminants and particularly roe deer; ecotype III is related to rodents, and ecotype IV is associated with birds [14,15]. Other proposed differentiations include clustering based on the sequence of the transcription regulatory protein ankA gene or the major surface protein 2 and 4 genes (msp2 and msp4) [13,14,16].
Few studies have investigated A. phagocytophilum prevalence in the UK. A recently published study on questing ticks revealed a higher prevalence in the recreational area of Northern England (4.7%, corresponding to 38/831 positive nymphs) than in Southern England (1.8%, 44/2385), with the majority (87%, 99/114) of strains belonging to groEL ecotype I and the remaining (13%, 15/114) to groEL ecotype II; only ecotype I was detected in Wales [22]. The Welsh study also revealed a correlation between sheep grazing and a higher prevalence of A. phagocytophilum. Another recently published study, still on questing ticks, revealed a 4.7% prevalence of A. phagocytophilum in Wester Ross, the northwest area of the Scottish Highlands, with A. phagocytophilum being the most prevalent tick-borne pathogen detected. According to the authors, the majority of the strains (86%) were identified as the zoonotic ecotype I, probably maintained by red deer, and the remaining (14%) as the non-zoonotic ecotype II, probably maintained by roe deer [23]. An eco-epidemiological screening of the rodent community in West Wales revealed a low prevalence of A. phagocytophilum, which was detected only at one site in ticks collected from bank voles [24]. To our knowledge, there are no published studies elucidating the ecotypes of A. phagocytophilum present in UK ruminants.
Anaplasma phagocytophilum causes tick-borne fever (TBF) in sheep, pasture fever in cattle, and granulocytic anaplasmosis in humans (HGA), equines (EGA), and canines (CGA) [4,13]. In both sheep and cattle, infections with A. phagocytophilum have a considerable economic impact due to diminished fertility, increased abortions, and lowered milk production and, sometimes, can result in death [4]. Such infections also provide a reservoir for genetic diversification and the spread of the pathogen [8]. Anaplasma phagocytophilum also causes immunosuppression in its host and, consequently, increased incidence of secondary infections by opportunistic pathogens, with outbreaks of diseases such as tick pyemia due to Staphylococcus aureus, pneumonia due to Pasteurella multlocida, Trueperella pyogenes or opportunistic fungi, septicaemic listeriosis, fatal encephalitis due to Louping ill virus, severe orf (contagious ecthyma), and other unusual disease presentations [13,25,26]. Humans are often exposed to ticks and, consequently, to strains of A. phagocytophilum with zoonotic potential. The increasing prevalence of A. phagocytophilum in ticks, livestock, and wildlife is compounded by the lack of vaccines and limited treatment options [4]. Consequently, the characterization of A. phagocytophilum strains in clinical samples and ticks will improve our understanding of the host preference, geographical segregation of ecotypes, and the zoonotic potential of each strain. The aim of this study was to investigate the genetic diversity of the tick-borne pathogen A. phagocytophilum in samples derived from UK ruminants to allow the prediction of its pathogenicity and vertebrate host specificity, as well as to infer its zoonotic potential.
2. Materials and Methods
2.1. Sample Preparation and DNA Extraction
Seventy-one tissues and uncoagulated blood samples were submitted between 2020 and 2022 to the Virus Surveillance Unit at the Moredun Research Institute for investigation using an A. phagocytophilum real-time qPCR targeting a 77-bp fragment of the msp2 gene [27]. Samples originated from the Animal and Plant Health Agency Veterinary Investigation Centres, the SRUC Disease Surveillance Centres, and from private veterinary practitioners. Ethical review and approval were waived for this study due to the nature of the samples and the anonymization of results. All the isolates and fresh samples for A. phagocytophilum isolation originated from clinical diagnostic or pathology submission, where the blood/tissues were collected for non-research purposes as an act of veterinary surgery. The Virus Surveillance Unit submission form specifically requests consent for anonymous surplus sample use, which was granted in all cases. The majority of the samples were represented by ovine spleens (n = 43), followed by ovine EDTA blood (n = 19). A summary of the characteristics of the samples tested is shown in Table 2. Tissue homogenates in Viral Transport Media and buffy coats from uncoagulated blood were processed according to standard protocols. Total DNA extraction was performed using the DNeasy® blood and tissue kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. DNA quality was checked using a NanoDrop one microvolume spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Positive control DNA samples were prepared from the A. phagocytophilum feral goat strain (APFG) provided by the Tick Cell Biobank, University of Liverpool [28]. Samples and positive control were tested using qPCR before use for quality control purposes.

Table 2.
Summary of the characteristics of the samples included in this study. APFG = A. phagocytophilum feral goat (positive control).
2.2. PCR for the Amplification and Sequencing of the groEL Gene
Several primers previously described [29] were investigated to target a 573–666 bp fragment of the A. phagocytophilum groEL gene to select the most sensitive and specific approach for PCR amplification, Sanger sequencing, and phylogenetic analysis. In addition to A. phagocytophilum, some of the primers also anneal with other bacterial targets, such as A. platys, A. bovis, Ehrlichia sp., Lentilitoribacter sp., Neorickettsia findlayensis, Rhodobiaceae bacterium, Erythrobacter sp., and Cohaesibacter sp., and contain degenerate basis. To increase specificity, a second set of non-degenerate primers modified to anneal only to A. phagocytophilum sequences was also tested. The primers selected for this work are shown in Table 3. The following primer combinations were used to test the ruminant samples: 569:1236 and nd643:nd1236. PCR was carried out using the HotStarTaq DNA Polymerase® kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions and containing 3 μL of DNA template. PCR reactions were carried out in a final volume 50 μL to allow electrophoretic analysis, as well as successive fragment isolation. PCR cycling conditions were 10 m at 95 °C, followed by 45 cycles of 1 m at 95 °C, 30 s at 57 °C, and 45 s at 72 °C, with a final elongation of 10 m at 72 °C. PCR products were visualised on a 1.5% TAE agarose gel according to standard procedures. Amplicons were gel-purified using the ChargeSwitch™ PCR clean-up kit (Thermo Fisher) according to manufacturer’s instructions and Sanger sequenced bi-directionally using Eurofins MWG (https://eurofinsgenomics.eu/en/custom-dna-sequencing/additional-services/sample-submission/ accessed on 18 January 2023).

Table 3.
Primers employed in the PCRs for the amplification and sequencing of the groEL gene, including primer specificities. In bold: degenerate bases, named according to the UIPAC coding system [30]. nd = non-degenerate.
2.3. Nucleotide Sequence Analysis
Nucleotide sequences were analysed using SeqMan Pro 17 (DNAStar Lasergene software V17) and BioEdit (V 7.2) software. Following primer removal, a consensus sequence was generated for each of the two primer combinations (569:1236 and nd643:nd1236, respectively); these consensus sequences were aligned to create a final validated consensus sequence representing the product of the four sequencing reactions. The sequences obtained in this study have been deposited in GenBank under accession numbers OQ060727- OQ060798.
The final consensus sequences from each sample were aligned using MegAlign Pro 17 (DNAStar Lasergene software, V17) to identify nucleotide variations. Sequences showing nucleotide variations were translated into amino-acid sequences to identify synonymous and nonsynonymous substitutions using SeqBuilder Pro 17 (DNAStar Lasergene software, V17).
2.4. Phylogenetic Analysis
In total, 1082 groEL sequences, including examples of all four ecotypes, were extracted from the NCBI database and previously published literature [14,15]. A multiple alignment of these sequences was generated using Clustal Omega, (https://www.ebi.ac.uk/Tools/msa/clustalo/ accessed on 18 January 2023). Duplicate sequences were removed, and the remaining 412 groEL sequences, including the newly identified sequences from this study, were re-aligned, then used to generate a maximum likelihood tree in IQ-TREE [31]. The model selection tool in IQ-TREE [32] was employed to choose the optimum substitution model, which was GTR+F+R5 [33]. The ultrafast boot strap method of Minh et al. [34] was used to test tree topology.
A second, simplified phylogenetic tree was prepared for illustration purposes, by removing all the non-European sequences. This resulted in an alignment of 153 sequences, of which 34 were the ones described in this paper. In this case, the substitution model selected was GTR+F+R3. Phylogenetic trees were prepared for publication by importing into Dendroscope 3.8.4 [35].
Finally, to attempt to relate sequence variation of our isolates to ecotype I clusters previously identified as potentially zoonotic, we selected all ecotype I sequences from the initial 1082 extracted and aligned these with those generated in this study. Sequences were aligned in Clustal Omega as described above, and the model selected in IQ-TREE was TN+F+R3. The tree was exported in Newick format and uploaded to Dendroscope for graphical editing.
3. Results
3.1. Primer Selection and groEL PCR Assays on Ruminant Samples
The primer combinations to be used for further analysis were selected based on the quality and specificity of the amplification and quality of the subsequent nucleotide sequence. Both primer combinations tested (degenerate and non-degenerate) showed good amplification of the positive samples and were considered equivalent. All samples in this study were tested with both PCR assays (degenerate and non-degenerate) and produced high quality sequences, with only three samples failing one or the other PCR assay. Forty-four out of forty-six tissue samples and twenty-four out of twenty-five blood samples were positive in both PCRs. For the samples which failed one of the two PCR assays, sequence analysis was, therefore, carried out only on one set of PCR products. There was no difference in PCR performance or sequence quality between matrices or host animal species.
3.2. Sequence Analysis
All purified PCR products yielded high quality sequences that confirmed the identity of all the PCR products and revealed between 99 to 100% sequence identity to previously deposited A. phagocytophilum groEL reference sequences: AF548386.1, EU246959.1, HM057224.1, HM057231.1, HM057232.1, HM057233.1, KC800986.1, KF312357.1, KF312358.1, KF312359.1, KF312360.1, KF312361.1, KF383241.1, KM215262.1, KM215265.1, MT498616.1, MW732492.1, and OM127910.1. All sequences were trimmed to exclude primers and standardised to a 571 bp amplicon. Alignment of the consensus fragments obtained using the two PCR assays for the same sample produced the final consensus sequences (one per sample). Three out of seventy-two sequences showed a single base difference between the two PCR assays (Supplementary Figure S1). These differences were recorded using the IUPAC coding system for nucleotide nomenclature and retained for the phylogenetic analysis.
We obtained sequence data for all 72 samples tested (71 samples and 1 positive control obtained from the tick-cell biobank). In total, 20 samples yielded sequences represented only once in this dataset, whereas in the remaining 52 samples, we identified 14 sequences which appeared in 2 or more samples. Three sequences (OQ060730, OQ060733, and OQ060739) were found in nine, six, and seven samples, respectively. No duplicate sequences were found in samples originating from the same flock or herd and submitted for testing at the same time.
The alignment of the sequences obtained from all samples tested also identified 13 positions where nucleotide variations (polymorphisms) were present, with 4 found only once, while others were found in up to half of the sequences analysed. When compared to the reference coding sequence (Accn. N. JQ685509) all 13 polymorphic sites represented synonymous substitutions (Supplementary Table S1).
3.3. Phylogenetic Analysis
Phylogenetic estimation of the relationship between the groEL sequences identified in this study and those representing each of the four previously identified ecotypes (Supplementary Table S2) is shown in Figure 1 as a simplified tree. The complete tree is shown in Supplementary Figure S2. The tree estimates that all 34 sequences identified in this study cluster with sequences previously defined as ecotype I in the European samples.
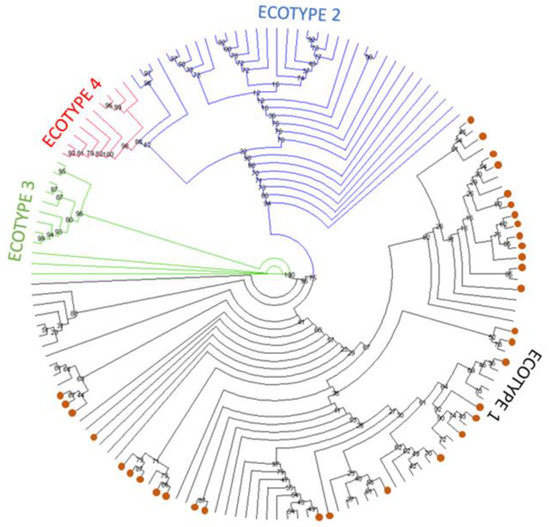
Figure 1.
Simplified maximum likelihood phylogenetic tree presented as a circular cladogram showing the relationship between the 34 groEL sequences generated in this work and representative European sequences from ecotypes I-IV. Phylogenetic analysis was performed using the GTR+F+R5 model in IQ-TREE with 1000 bootstrap replicates. Sequences generated in this work are shown as orange dots, and colours are used to separate sequences in the different ecotypes. Bootstrap values are shown next to the nodes of the trees. The final data set contained 449 positions, and the corresponding tree is shown in Supplementary Figure S2.
The phylogenetic estimation of the relationship between the groEL sequences identified in this study and ecotype I reference sequences, including human-derived sequences, is shown in Figure 2. The tree estimates that all 34 sequences identified in this study are located out with the cluster where the human sequences from the Unites States are present (shadowed). However, there is lack of a clear clustering for the human European and the sequences analysed in the present work. A second cluster is also defined, representing sequences from Croatia and Albania, derived from ovine, caprine, and mouflon (Ovis gmelini) samples.
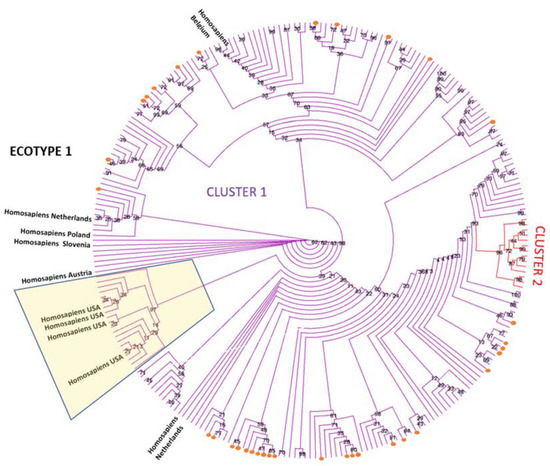
Figure 2.
Maximum likelihood phylogenetic tree presented as a circular cladogram showing the relationship between the 34 groEL sequences generated in this work and representative ecotype I sequences. Phylogenetic analysis was performed using the TN+F+R3 model in IQ-TREE with 1000 bootstrap replicates. Sequences generated in this work are shown as orange dots, and colours are used to differentiate sequences in the different clusters. Bootstrap values are shown next to the nodes of the trees. The final data set contained 260 positions. Human sequences derived from samples from the United States are located in the shadowed box.
4. Discussion
This study aimed to identify the A. phagocytophilum ecotypes present in 72 samples from UK ruminants by sequence-based typing of the groEL gene. Diversity in the groEL gene is one of the few markers used to differentiate between A. phagocytophilum ecotypes, and its use in this study allows comparison with other studies from different geographical regions. The sequence and phylogenetic analysis revealed the presence of a single A. phagocytophilum ecotype (ecotype I) in the ruminant samples confirming previous observations on the host and geographical restriction of these variants within Europe [22]. To the author’s knowledge, this is the first study describing A. phagocytophilum ecotypes in ruminant species in the UK. For data protection reasons, we are not able to disclose the precise location of individual samples across the UK.
The initial primer selection, based on published information, allowed identification of the optimal primer combinations for the PCR assay. When two primer sets yielded identical results, we chose the combination offering the longest sequence to increase the probability of identifying groEL allelic polymorphism among isolates. The use of two primer sets allowed the preparation of a final consensus sequence for each sample based on four sequencing reactions, increasing accuracy, and eliminating potential PCR and sequencing errors. There was no difference in sequence quality in relation to host species or sample type, indicating that this method may be applied to samples from living or post-mortem cases.
The groEL gene was amplified by one of the two PCR reactions in 69 of the 72 samples tested. Only three samples failed amplification in one of the two PCR reactions. The lack of amplification in these three samples indicates subtle differences in the performance of the two PCR reactions, which may be associated with unknown polymorphism in the primer binding sites and indicates that both approaches should be adopted if a negative result is obtained at the first attempt. Most nucleotide sequences obtained from the same sample were identical; however, three samples showed a single nucleotide difference between the two PCR reactions. This might indicate PCR errors and shows the need for confirmatory reactions when carrying out amplicon sequencing for ecotype identification. As two of the samples showed polymorphism at the same position, this is unlikely to be a PCR or sequencing artefact and could be due to a recent mutation within the host or the presence of two haplotypes associated with co-infection by two bacterial strains. Co-infection with multiple strains has previously been reported in roe deer and sheep [36].
Among the samples tested, we identified 13 single nucleotide polymorphisms or SNPs in different positions of the groEL gene, with some sequences presenting more than one. Our results show significant allelic diversity in the groEL gene, however, all nucleotide polymorphisms were synonymous, with no changes in the predicted amino acid sequence. GroEL is a highly conserved gene coding for an essential chaperon protein found in many bacteria and which, together with its co-chaperonin groES, plays an essential role in protein folding [37,38] and Anaplasma-tick interactions [39]. The protein is translocated into host cell nuclei, eventually altering the phenotype of the infected neutrophils, and the lack of non-synonymous substitutions suggests that the purifying selection is acting to maintain the essential function of this protein.
The prevalence and genotype of A. phagocytophilum strains in vector and host populations have been investigated in several countries to assess their zoonotic potential [7]. Anaplasma phagocytophilum infection has been identified throughout Europe in domestic ruminants, with variable rates according to the species. A seroprevalence of 80% was described in Norwegian sheep [40]. No Anaplasma phagocytophilum infection has been reported in the USA to date, suggesting that North American strains may not be very pathogenic for ruminants. In contrast, equine granulocytic anaplasmosis and granulocytic anaplasmosis in dogs are widely reported in the USA, South America, Europe, Asia, and Africa [7].
The groEL gene is recognised as an appropriate marker for phylogenetic analysis to distinguish between A. phagocytophilum ecotypes, host preference, and their pathogenicity, especially in relation to their zoonotic potential [15]. It is accepted that ecotype I represents strains present in several hosts, including zoonotic strains identified in humans and variants present in domestic animals [14,15,41], whereas ecotypes II, III, and IV do not include zoonotic strains and are mainly associated with ruminants, rodents, and non-human primates. Using groEL gene sequences, ecotype I was linked to red deer and ecotype II exclusively to roe deer in Norway [42], whereas in Poland, Germany, and the Czech Republic, the presence of all ecotypes except III [39,41,43] was detected in several host species, including roe deer, wild and domestic large ungulates, and brown hares [44,45]. All ecotypes were detected in Spain [2], Belgium, and the Netherlands in several domestic and wild species [14]. In Italy, a high prevalence of both ecotypes I and II was identified among wild ruminants and wild boars [16]. In France, using a different genetic marker belonging to the ankA gene cluster I, two different genetic groups were identified, mainly infecting humans and companion and farm animals [46]. Surveys have been conducted in countries in central and southern China, demonstrating that ecotype I is present in almost all Eurasia, whereas the remaining ecotypes (II–IV) have a more restricted distribution [47]. However, ecotype attribution often depends on the gene used to carry out the classification. For example, some strains detected in sheep, goats, cows, hedgehogs, wild carnivores, and other species might cluster into different genetic groups using different markers [7].
Despite the widespread presence of A. phagocytophilum in Europe, with varied rates of seropositivity recorded in different human populations (on average ~ 8.3%, reaching up to 31%) [48], human disease seems to be limited and less prevalent than in the United States, where it has been on the increase for some time [49]. This difference seems to be linked to presence of specific sub-clusters or haplotypes within ecotype I, showing different zoonotic potential ([7]). For example, Rar et al. [7], using groEL gene sequence analysis, described the presence of three different sub-clusters within ecotype I isolates. These included clusters containing sequences of (i) European isolates from horses, dogs, wild boars, wild and domestic ruminants, a raccoon, bears, and I. ricinus, (ii) American isolates from humans, horses, dogs, a cat, a rabbit, woodrats, and I. pacificus and (iii) a small Brazilian cluster with sequences derived only from dogs. Further clustering delineated a division between a European zoonotic group and North American group. Furthermore, according to Matei et al. [48] and based on the sequence of the 16S rRNA gene, North American strains seem to show less diversity compared to European strains and mainly belong to two variants, of which the Ap-ha represents the zoonotic group. Collectively, strains with clear zoonotic potential correspond to a minority of A. phagocytophilum sequences, and evidence is emerging indicating that a simple, one marker analysis is not sufficient to assign an isolate to a specific genogroup [7]. The attribution of zoonotic potential to a specific isolate is still work in progress and requires more epidemiological, clinical, and ecological information than phylogenetic analysis alone.
The results of the work presented here, albeit limited to a relatively small number of samples collected in three consecutive years, indicate the presence of ecotype I in UK ruminants and confirms that the ecotype distribution in the UK reflects closely the European situation. A recent UK tick survey [22] identified both ecotypes I and II, with a higher prevalence of ecotype I in ticks surveyed in different areas of England and Wales with the absence of ecotypes III and IV. The same study suggested a positive association between the presence of sheep in a specific area and a higher prevalence of A. phagocytophilum, rather than a specific environment. It is likely that the isolates typed in this work will represent strains of low zoonotic potential, similar to those already identified in the rest of Europe, as suggested by the phylogenetic analysis, where our sequences clustered independently of the North American zoonotic group. Lack of zoonotic potential seems also to be confirmed by the fact that very few instances of human granulocytic anaplasmosis have been reported in Europe [48]. However, it is unclear if this observation is due to lack of exposure in specific geographical areas, underreporting of the symptoms to medical practitioners, or to a true lack of pathogenicity even when exposure is high, as reported by a Swedish study [50]. Finally, the zoonotic importance of strains not associated with human disease and belonging to different ecotypes is unclear and requires further investigation to confirm their absence of pathogenicity.
In summary, we have demonstrated the presence of an ecotype of A. phagocytophilum in the UK ruminant population closely clustering with strains identified in Europe with zoonotic potential. Its zoonotic potential should be considered until additional data are obtained. Meanwhile, better public information and medical awareness is required to limit exposure to tick bites as well as an epidemiological evaluation of the potential for A. phagocytophilum infection to cause immunosuppression in the human population. Definitive information on these strains’ correlation with pathogenicity and vector and host interaction, as well as serological and humoral immune responses, are still knowledge gaps that need to be filled.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12020216/s1, Table S1: Nucleotide polymorphisms detected in the sequence of the groEL gene 571 bp fragment and predicted amino-acid changes. Table S2: Sequences used for phylogenetic analysis with duplicates removed (excel file). Figure S1: Alignment of the three consensus sequences showing polymorphism. Figure S2: Complete phylogenetic tree.
Author Contributions
Conceptualisation, L.T. and M.S.R.; methodology, L.B., M.M., K.B., K.A. and M.S.R.; validation, L.B.; resources, Virus Surveillance Unit at Moredun Research Institute for clinical samples, Liverpool University for APFG; data curation, L.B. and M.M.; writing original draft preparation, all authors; funding acquisition, L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a transnational access grant from the European Union’s Horizon 2020 research and innovation programme under grant agreement N731014 (VetBioNet).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the nature of the samples and the anonymisation of results.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
L. Bianchessi is in receipt of a doctoral training fellowship from the University of Milan. M. Rocchi, M. Maley, K. Ballingall, and K. Allen are funded by the Scottish Government Rural & Environment Science & Analytical Services. L. Turin is financed by University of Milan.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Remesar, S.; Díaz, P.; Prieto, A.; García-Dios, D.; Fernández, G.; López, C.M.; Panadero, R.; Díez-Baños, P.; Morrondo, P. Prevalence and molecular characterization of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) from Spain. Ticks Tick Borne Dis. 2020, 11, 101351. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Mechanism of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum-a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Keesing, F.; Hersh, M.H.; Tibbetts, M.; McHenry, D.J.; Duerr, S.; Brunner, J.; Killilea, M.; LoGiudice, K.; Schmidt, K.A.; Ostfeld, R.S. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 2012, 18, 2013–2016. [Google Scholar] [CrossRef]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Hauck, D.; Jordan, D.; Springer, A.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasites Vectors 2020, 13, 176. [Google Scholar] [CrossRef]
- Morissette, E.; Massung, R.F.; Foley, J.E.; Alleman, A.R.; Foley, P.; Barbet, A.F. Diversity of Anaplasma phagocytophilum strains, USA. Emerg. Infect. Dis. 2009, 15, 928–931. [Google Scholar] [CrossRef]
- Foley, J.; Nieto, N.C.; Madigan, J.; Sykes, J. Possible differential host tropism in Anaplasma phagocytophilum strains in the Western United State. Ann. N. Y. Acad. Sci. 2008, 1149, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Bown, K.J.; Lambin, X.; Ogden, N.H.; Begon, M.; Telford, G.; Woldehiwet, Z.; Birtles, R.J. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg. Infect. Dis. 2009, 15, 1948–1954. [Google Scholar] [CrossRef]
- Dugat, T.; Lagrée, A.; Maillard, R.; Boulouis, H.; Haddad, N. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell. Infect. Microbiol. 2015, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; Maanen, C.V.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, R.I.; Sprong, H.; Takumi, K.; Kazimirova, M.; Silaghi, C.; Mysterud, A.; Rudolf, I.; Beck, R.; Földvári, G.; Tomassone, L.; et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasites Vectors 2019, 12, 328. [Google Scholar] [CrossRef]
- Grassi, L.; Franzo, G.; Martini, M.; Mondin, A.; Cassini, R.; Drigo, M.; Pasotto, D.; Vidorin, E.; Menandro, M.L. Ecotyping of Anaplasma phagocytophilum from wild ungulates and ticks shows circulation of zoonotic strains in northeastern Italy. Animals 2021, 11, 310. [Google Scholar] [CrossRef]
- Katargina, O.; Geller, J.; Alekseev, A.; Dubinina, H.; Efremova, G.; Mishaeva, N.; Vasilenko, V.; Kuznetsova, T.; Järvekülg, L.; Vene, S.; et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin. Microbiol. Infect. 2012, 18, 40–46. [Google Scholar] [CrossRef]
- von Loewenich, F.D.; Baumgarten, B.U.; Schröppel, K.; Geissdörfer, W.; Röllinghoff, M.; Bogdan, C. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. J. Clin. Microbiol. 2003, 41, 5033–5040. [Google Scholar] [CrossRef]
- Langenwalder, D.B.; Schmidt, S.; Silaghi, C.; Skuballa, J.; Pantchev, N.; Matei, I.A.; Mihalca, A.D.; Gilli, U.; Zajkowska, J.; Ganter, M.; et al. The absence of the drhm gene is not a marker for human-pathogenicity in European Anaplasma phagocytophilum strains. Parasites Vectors 2020, 13, 238. [Google Scholar] [CrossRef]
- Aardema, M.L.; von Loewenich, F.D. Varying influences of selection and demography in host-adapted populations of the tick-transmitted bacterium, Anaplasma phagocytophilum. BMC Evol. Biol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Aardema, M.L.; Bates, N.V.; Archer, Q.E.; von Loewenich, F.D. Demographic expansions and the emergence of host specialization in genetically distinct ecotypes of the tick-transmitted bacterium Anaplasma phagocytophilum. Appl. Environ. Microbiol. 2022, 88, e0061722. [Google Scholar] [CrossRef] [PubMed]
- Gandy, S.; Hansford, K.; McGinley, L.; Cull, B.; Smith, R.; Semper, A.; Brooks, T.; Fonville, M.; Sprong, H.; Phipps, P.; et al. Prevalence of Anaplasma phagocytophilum in questing Ixodes ricinus nymphs across twenty recreational areas in England and Wales. Ticks Tick Borne Dis. 2022, 13, 101965. [Google Scholar] [CrossRef] [PubMed]
- Olsthoorn, F.; Sprong, H.; Fonville, M.; Rocchi, M.; Medlock, J.; Gilbert, L.; Ghazoul, J. Occurrence of tick-borne pathogens in questing Ixodes ricinus ticks from Wester Ross, Northwest Scotland. Parasites Vectors 2021, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Occhibove, F.; McKeown, N.J.; Risley, C.; Ironside, J.E. Eco-epidemiological screening of multi-host wild rodent communities in the UK reveals pathogen strains of zoonotic interest. Int. J. Parasitol. Parasites Wildl. 2022, 17, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, M.; Dagleish, M.; McInnes, C. Tick bites and tick-transmitted diseases. Vet. Rec. 2018, 182, 609. [Google Scholar] [CrossRef]
- Daniel, R.; Hopkins, B.A.M.; Rocchi, M.S.; Wessels, M.; Floyd, T. High mortality in a sheep flock caused by coinfection of coinfection of louping ill virus and Anaplasma phagocytophilum. Vet. Rec. Case Rep. 2020, 8, e000980. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef]
- Woldehiwet, Z.; Horrocks, B.K. Antigenicity of ovine strains of Anaplasma phagocytophilum grown in tick cells and ovine granulocytes. J. Comp. Pathol. 2005, 132, 322–328. [Google Scholar] [CrossRef]
- Alberti, A.; Addis, M.F.; Sparagano, O.; Zobba, R.; Chessa, B.; Cubeddu, T.; Parpaglia, M.L.; Ardu, M.; Pittau, M. Anaplasma phagocytophilum, Sardinia, Italy. Emerg. Infect. Dis. 2005, 11, 1322–1324. [Google Scholar] [CrossRef]
- IUPAC-IUB Comm. on Biochem. Nomenclature (CBN). Abbreviations and symbols for nucleic acids, polynucleotides, and their constituents. Biochemistry 1970, 9, 4022–4027. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.F.K.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, S.; Miura, R.M. Some probabilistic and statistical problems in the analysis of DNA sequences. Lectures on mathematics in the life sciences. Am. Math. Soc. 1986, 17, 57–86. [Google Scholar]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Scornavacca, C. Dendroscope 3- An interactive viewer for rooted phylogenetic trees and networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef]
- Jouglin, M.; Chagneau, S.; Faille, F.; Verheyden, H.; Bastian, S.; Malandrin, L. Detecting and characterizing mixed infections with genetic variants of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) by developing an ankA cluster-specific nest-ed PCR. Parasites Vectors 2017, 10, 377. [Google Scholar] [CrossRef]
- Crosby, F.L.; Eskeland, S.; Bø-Granquist, E.G.; Munderloh, U.G.; Price, L.D.; Al-Khedery, B.; Stuen, S.; Barbet, A.F. Comparative Whole Genome Analysis of an Anaplasma phagocytophilum Strain Isolated from Norwegian Sheep. Pathogens 2022, 11, 601. [Google Scholar] [CrossRef]
- Zeilstra-Ryalls, J.; Fayet, O.; Georgopoulos, C. The universally conserved GroE (Hsp60) chaperonins. Annu. Rev. Microbiol. 1991, 45, 301–325. [Google Scholar] [CrossRef]
- Villar, M.; Ayllón, N.; Kocan, K.M.; Bonzón-Kulichenko, E.; Alberdi, P.; Blouin, E.F.; Weisheit, S.; Mateos-Hernández, L.; Cabezas-Cruz, A.; Bell-Sakyi, L.; et al. Identification and Characterization of Anaplasma phagocytophilum proteins involved in infection of the tick vector, Ixodes scapularis. PLoS ONE 2015, 10, e0137237. [Google Scholar] [CrossRef]
- Stuen, S.; Bergström, K. Serological investigation of granulocytic Ehrlichia infection in sheep in Norway. Acta Vet. Scand. 2001, 42, 331–338. [Google Scholar] [CrossRef]
- Hamšíková, Z.; Silaghi, C.; Takumi, K.; Rudolf, I.; Gunár, K.; Sprong, H.; Kazimírová, M. Presence of roe deer affects the occurrence of Anaplasma phagocytophilum ecotypes in questing Ixodes ricinus in different habitat types of Central Europe. Int. J. Environ. Res. Public Health 2019, 16, 4725. [Google Scholar] [CrossRef] [PubMed]
- Stigum, V.M.; Jaarsma, R.I.; Sprong, H.; Rolandsen, C.M.; Mysterud, A. Infection prevalence and ecotypes of Anaplasma phagocytophilum in moose Alces alces, red deer Cervus elaphus, roe deer Capreolus capreolus, and Ixodes ricinus ticks from Norway. Parasites Vectors 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Dwużnik-Szarek, D.; Kowalec, M.; Alsarraf, M.; Bajer, A. Contribution of tick-borne diseases to mortality in juvenile free-living cervids. Ann. Agric. Environ. Med. 2022, 29, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Myczka, A.W.; Kaczor, S.; Filip-Hutsch, K.; Czopowicz, M.; Plis-Kuprianowicz, E.; Laskowski, Z. Prevalence and genotyping of Anaplasma phagocytophilum strains from wild animals, European Bison (Bison bonasus) and Eurasian Moose (Alces alces) in Poland. Animals 2022, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Lesiczka, P.M.; Modry, D.; Sprong, H.; Fonville, M.; Pikula, J.; Piacek, V.; Heger, T.; Hrazdilova, K. Detection of Anaplasma phagocytophilum in European brown hares (Lepus europaeus) using three different methods. Zoonoses Public Health 2021, 68, 917–925. [Google Scholar] [CrossRef]
- Dugat, T.; Leblond, A.; Keck, N.; Lagrée, A.C.; Desjardins, I.; Joulié, A.; Pradier, S.; Durand, B.; Boulouis, H.J.; Haddad, N. One particular Anaplasma phagocytophilum ecotype infects cattle in the Camargue, France. Parasites Vectors 2017, 10, 371. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Han, R.; Guan, G.; Li, Y.; Liu, G.; Luo, J.; Yin, H. Anaplasma phagocytophilum in sheep and goats in central and southeastern China. Parasites Vectors 2016, 9, 593. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Dumler, J.S.; Dotevall, L.; Gustafson, R.; Granström, M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J. Infect. Dis. 1997, 175, 720–722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).