Abstract
Cryptosporidium parvum is a significant cause of watery diarrhoea in humans and other animals worldwide. Although hundreds of novel drugs have been evaluated, no effective specific chemotherapeutic intervention for C. parvum has been reported. There has been much recent interest in evaluating plant-derived products in the fight against gastrointestinal parasites, including C. parvum. This study aimed to identify extracts from 13 different plant species that provide evidence for inhibiting the growth of C. parvum in vitro. Efficacy against C. parvum was detected and quantified using quantitative PCR and immunofluorescence assays. All plant extracts tested against C. parvum showed varying inhibition activities in vitro, and none of them produced a cytotoxic effect on HCT-8 cells at concentrations up to 500 µg/mL. Four plant species with the strongest evidence of activity against C. parvum were Curcuma longa, Piper nigrum, Embelia ribes, and Nigella sativa, all with dose-dependent efficacy. To the authors' knowledge, this is the first time that these plant extracts have proven to be experimentally efficacious against C. parvum. These results support further exploration of these plants and their compounds as possible treatments for Cryptosporidium infections.
1. Introduction
Species of the enteric protozoan parasite Cryptosporidium are associated with morbidity and mortality in people, particularly in developing countries. Of the 46 species characterised, Cryptosporidium parvum and Cryptosporidium hominis cause the most human infections [1,2]. In immunocompetent adults, symptoms generally resolve within two weeks; however, the infection can be critical in immunocompromised patients and young children. Recent studies estimate that 30–50% of children under the age of five are infected with Cryptosporidium spp. worldwide [3], and it is the second most common cause of moderate-to-severe diarrhoea in this age group [4]. In addition, Cryptosporidium spp. are among the five pathogens with the highest attributable burden of diarrhoea in the first two years of life [5], with deaths estimated to be 60,000–200,000 annually [6].
Cryptosporidium infection affects many animal hosts worldwide, including livestock such as cattle, sheep, goats, and pigs [7]. Neonatal (<6 weeks old) calves are very vulnerable to cryptosporidiosis [8]. The infected animals suffer from a gastrointestinal disorder that is often accompanied by diarrhoea of variable duration. In livestock, the disease may lead to significant production losses due to retarded growth and mortality of the animals, the cost of drugs, veterinary assistance, and increased staff labour [9]. Zoonotic transmission is evident in many Cryptosporidium spp. [10,11]. Cryptosporidium parvum is the most important zoonotic species [12].
Although hundreds of novel drugs have been evaluated for anti-cryptosporidial activity, the current treatment options are limited, with nitazoxanide the only drug permitted by the US Food and Drug Administration (FDA) [13] for the treatment of cryptosporidiosis. However, this drug has exhibited minimal efficacy in immunocompetent adults and children and is ineffective in immunocompromised patients with acquired immunodeficiency syndrome (AIDS) [14].
Thus, the need for new treatment options against Cryptosporidium spp., which are safe, effective, and cheap, is imperative to deal with the current global burdens of cryptosporidiosis effectively. Natural products with antiparasitic activity could play an important role in combating these protozoan parasites. Traditional medicinal plants and their derivatives have shown favourable results against various parasitic infections [15,16,17].
In vitro screening is a critical step in the early stage of drug development. Compared to animal models, in vitro models are less expensive, less time-consuming, and more convenient for screening drugs for their efficacy against parasites [18,19]. Using in vitro cultures to assess the infectivity and proliferation of the parasite may also help reduce the use of experimental animal models [19,20]. Various cell lines have been successfully applied for in vitro studies on C. parvum, with human ileocaecal adenocarcinoma (HCT-8) cells having been proven to be a suitable cell line for developing the complete life cycle of C. parvum [21,22,23,24].
In searching for new antiparasitic agents, in the current study, we tested thirteen plant extracts for their ability to inhibit the growth of C. parvum, established in vitro on HCT-8 cells. The extracts were chosen because of their commercial availability, diversified pharmacological properties, and traditional use against various diseases, including parasitic gastrointestinal (GI) disorders.
2. Materials and Methods
2.1. Parasites
Cryptosporidium parvum oocysts were purchased from BioPoint Pty. Ltd., Belrose NSW, Australia (Batch No: 859–547) and stored in sterile-filtered phosphate-buffered saline (PBS) containing 100 µg of streptomycin/mL and 100 U penicillin/mL at 5 °C until used. Oocysts were <6 months old when used.
2.2. Plant Material
Thirteen plant extracts were used in this study (Table 1). These were selected based on ethnopharmacological information on their use as antiparasitic treatments in Traditional Indian Medicine (Ayurveda) [25,26]. The dried powder form of plant extracts of Allium sativum (garlic), Cucurbita pepo (pumpkin), Embelia ribes (false black pepper), and Thymus vulgaris (thyme) was commercially purchased from Flamingo Exports, India. The remaining nine dried plant extracts were supplied from Sunpure Extract Ltd., India. All the plant extracts were standardised and validated by the manufacturers using high-performance liquid chromatography (HPLC) and the principal phytochemicals in each extract are shown in Table 1. Dried plant extracts were stored in airtight dark containers at room temperature. The maximum dose range tested for all compounds was 500 µg/mL, as compounds could not be completely dissolved in absolute dimethyl sulfoxide (DMSO) beyond this concentration.

Table 1.
Details of the thirteen plant extracts used in the study.
2.3. Cell Culture
Human ileocaecal adenocarcinoma cells, originally obtained from American Type Culture Collection (ATCC; CCL-244™), Manassas, Virginia, were used for the in vitro maintenance of C. parvum infections and cultured in 25 cm2 sterile polystyrene ventilated cell culture flasks (Cellstar®) in growth media consisting of RPMI 1640 (Sigma-Aldrich®) medium (10.3 g/L) supplemented with 10% foetal calf serum (FCS) (Sigma-Aldrich®) and other supplements as previously described by Hijjawi et al. [27]. The flasks were kept in a humidified incubator at 37 °C and 5% (v/v) CO2 and grown for 24 h until monolayers reached 90% confluence.
2.4. Cytotoxicity Assay
The cytotoxicity of all plant extracts was determined before evaluating their anti-cryptosporidial effects.
The cytotoxicity of plant compounds was evaluated on HCT-8 cells using Cell Titer-Blue® cell viability assay (cat # G8081, Promega Corporation, Madison, WI, USA), following the manufacturer’s instructions. This assay offers a homogeneous, fluorometric method to estimate the number of viable cells. The Cell Titer-Blue® (CTB) Reagent contains highly purified resazurin dye, which enables the measurement of cells’ metabolic capacity as an indicator of cell viability. Viable cells can reduce resazurin, which is dark blue in colour and non-fluorescent, into resorufin, which is pink and highly fluorescent. Nonviable cells cannot reduce the indicator dye as they lose metabolic capacity and cannot generate a fluorescent signal [28,29].
To determine cytotoxicity, HCT-8 cells were seeded at a density of 4 × 104 cells/well in 100 µL medium per well and incubated overnight to grow at 37 °C and 5% CO2. Plant extracts were redissolved in 100% DMSO (ChemSupply Australia, Gillman, SA, Australia), and the concentration was adjusted to 500 µg/mL. The plates with 90% confluent cell monolayers were incubated at 37 °C and 5% CO2 in the presence of a three-fold dilution of extracts, ranging from 2–500 µg/mL. After a minimum of 66 h incubation, monolayers were checked for any signs of contamination and 20 µL of CTB was added to each well. The plates were shaken for 10 s and then incubated at 37 °C and 5% CO2 for 4 h. Once the colour change was observed, the plates were shaken for 10 s and read on the DTX 800 Multimode Detector (Beckman Coulter’s Biomek®) at 560 nm excitation/590 nm emission. All plant extracts, at all concentrations, were tested in three independent replicate experiments. In each replicate, cells not exposed to the compounds served as the untreated control, and the wells that contained only the media served as the background control to determine background fluorescence that may be present. Five µL DMSO was added to each untreated and background control well. Each experimental plate had four untreated controls and six background controls.
2.5. Cryptosporidium parvum Growth Inhibition Assay
2.5.1. Pre-Treatment of Oocysts and Infection of Host Cells
The required number of C. parvum oocysts was bleached using 200 µL sodium hypochlorite (Scott Scientific, Western Australia) in 10 mL of water at room temperature for 30 min, centrifuged at 2000 g for 8–10 min, and the supernatant was removed. Bleached oocysts were then placed into 10 mL of freshly prepared, filter sterilised (0.22 μm filter), and warm (37 °C) excystation media composed of acidic water (pH 2.5–3) containing 0.5% trypsin/EDTA (Sigma-Aldrich®). The oocysts were incubated for 30 min at 37 °C with vigorous shaking every 5 min. The excystation suspension was then centrifuged (at 2000× g for 8–10 min) and resuspended in the appropriate volume of maintenance media composed of RPMI 1640 (Sigma-Aldrich®) medium, 1% FCS and other supplements as described previously [27].
Infectivity screens were set up by detaching HCT-8 cell monolayers from culture flasks using 1 mL of trypsin/EDTA (Sigma-Aldrich®) and inoculating into 48-well plates (CELLSTAR®) at a density of 104 cells/well in 500 µL of RPMI growth medium. The plates were then incubated at 37 °C and 5% CO2 overnight to allow them to reach the monolayer stage. Following overnight incubation, the growth medium was removed from the monolayers by aspiration, prepared oocysts were applied to the monolayers (7500 oocysts/well), and the monolayers were incubated overnight at 37 °C and 5% CO2. Infectivity was confirmed using EasyStain™ (BioPoint Pty. Ltd., Belrose, NSW, Australia) (Supplementary Material S1). Two wells on each plate were left uninfected (i.e., oocysts were not added) to serve as negative controls in extract screening tests (see below).
2.5.2. Extract Screening at a Single Concentration
Initially, the efficacy of all 13 plant extracts in inhibiting parasite growth was tested at a standard concentration of 500 ng/mL, with three independent replicate experiments for each extract. Screening plates were prepared as described in Section 2.5.1. Eight plant extracts were tested on one plate. Each experimental plate included two negative control wells (containing uninfected cell monolayers) and six positive control wells (infected but untreated cell monolayers). Five µL DMSO was added to each negative and positive control well. Compound stock solutions with 500 µg/mL initial concentration were diluted 1:10 (10 µL compound + 90 µL DMSO) making them 50 µg/mL. From this, 5 µL was added to the 500 µL RPMI growth media in the well, giving a further dilution of 1:100, making the final concentration 500 ng/mL. The plates were incubated at 37 °C and 5% CO2 for 48 h.
2.5.3. Dose–Response Analysis
The four plant extracts showing the greatest anti-cryptosporidial activity (C. parvum inhibition >50%) at 500 ng/mL were further examined to produce a dose–response curve and consequently ascertain an IC50. Three independent replicate experiments were conducted for each extract. Screening plates were prepared as described in Section 2.5.1. A single plant extract was tested on one plate. A three-fold serial dilution (5 µL/well) was created in the screening plate, initiating from 500 ng/mL, giving extract concentrations of 166.7, 55.6, 18.5 and 6.1 ng/mL. Each experimental plate included two negative control wells (containing uninfected cell monolayers) and six positive control wells (infected but untreated cell monolayers). As before, 5 µL DMSO was added to the negative and positive controls. Trifluralin (Sigma-Aldrich®) was used as a standard drug control. Trifluralin is a dinitroaniline, a promising class of anti-cryptosporidial compounds with tubulin-binding properties that were initially recognised for their herbicidal properties [20,30]. Trifluralin has been shown to be effective in inhibiting C. parvum growth in vitro (IC50 = 650.4 mg/L) [30]. A 1 × 105 ng/mL (in acetonitrile) stock solution of trifluralin was diluted 1:10 with DMSO), making it 1 × 104 ng/mL and 5 µL was added to the 500 µL RPMI media in the well. Then, a three-fold serial dilution was created in the screening plate, giving drug concentrations of 3.3 × 103, 1.1 × 103, 3.7 × 102, 1.2 × 102 ng/mL. The plates were then incubated at 37 °C and 5% CO2 for 48 h.
2.5.4. Quantification of Inhibition
Quantitative PCR (qPCR) targeting the 18S rRNA locus was used to assess the in vitro C. parvum growth rate. qPCR is widely applied in Cryptosporidium infectivity-related research to quantify parasite development [31,32,33,34].
After 48 h incubation, the maintenance medium was aspirated from the wells, and the infected monolayers were washed with 500 µL of sterile PBS (Sigma-Aldrich®) twice. Cell monolayers were harvested in 70 µL of 0.5% trypsin/EDTA (Sigma-Aldrich®) (pH 8.0). The total genomic DNA (gDNA) was extracted from the infected cells at 48 h post-infection using DNeasy isolation kits (QIAGEN Inc., Valencia, CA) with minor modifications to the manufacturer’s protocol [35]. Briefly, the lysed cells were added to the labelled microcentrifuge tubes. Then, five freeze–thaw cycles were carried out, freezing in liquid nitrogen for 30 s and thawing at 65 °C for 1 min. The final elution volume was adjusted to 50 µL from the manufacturer’s recommended volume of 200 µL for the DNeasy isolation kits.
Quantitative PCR (qPCR) was performed in triplicate for each DNA sample from each plant extract concentration using primers targeting the 18S rRNA locus (5′ AGTGACAAGAAATAACAATACAGG 3′ and 5′ CCTGCTTTAAGCACTCTAATTTTC 3′) [36], and the 6-carboxyfluorescein (FAM)-labelled TaqMan probe (5’ FAM- AAGTCTGGTGCCAGCAGCCGC-BHQ1 3′) [37]. A standard curve was constructed using five triplicates of recombinant plasmids containing partial fragments of the Cryptosporidium 18S rRNA, serially diluted at a 1:10 ratio and calibrated by digital droplet PCR (ddPCR) as described by Yang et al. [38].
Reaction conditions were a final volume of 15 µL containing 1x Kapa Taq PCR buffer (KAPA Biosystems), 3.75 mM MgCl2, 0.5 µM of each forward (18siF) and reverse (18siR) primers (Fisher Biotec, Australia), 400 µM of each deoxynucleoside triphosphate (dNTP) (Promega, Australia), 0.2 µM Taqman probe, 5 U/µL Kapa Taq DNA polymerase (KAPA Biosystems), and Ultra-Pure PCR grade water (Fisher Biotec, Australia). An aliquot (14 µL) of the template was used in each reaction. Cycling parameters were one pre-melt cycle at 90 °C for 3 min followed by 50 cycles of 94 °C for 20 s and 60 °C for 90 s on a Rotor-Gene Q system (Qiagen, Mortlake, NSW, Australia).
Additionally, an immunofluorescence assay was performed to provide qualitative confirmation of the qPCR results. In this assay, the C. parvum infection intensity was qualitatively evaluated using a fluorescein-conjugated specific polyclonal antibody (Sporo-Glo™, Waterborne Inc., New Orleans, LA, USA) according to the manufacturer’s protocol.
2.5.5. DNA Sequencing
A subset of amplicons was sequenced to confirm the C. parvum strain used, and that no contamination had occurred. Purified PCR products were sequenced independently in both directions using the secondary PCR primers (Fisher Biotec, Australia), and resultant nucleotide sequences were aligned using the BioEdit v. 7.0.1 package and compared with available DNA sequences of Cryptosporidium in the GenBank database using the NCBI BLAST basic local alignment search tool (http://www.ncbi.nlm.nih.gov/BLAST/, accessed on 14 December 2021).
2.6. Data Analysis
Data are presented as the mean percentage (±SEM) of three replicates. For cytotoxicity data, all values obtained from cell monolayers treated with plant compounds were normalised to those obtained from the wells containing only fresh media (background control). The per cent cell viability was calculated relative to the untreated control, which has only the HCT-8 cells, using the following formula:
Relative viability (%) = [Mean Measurement (treatment) − Mean (background control)/Mean (untreated control) − Mean (background)] × 100
For in vitro growth inhibition data, the percentage of endogenous stages of C. parvum in treated groups was compared to untreated control using the following formula:
% inhibition = [(DNA copies in positive control well − DNA copies in treated well)/DNA copies in positive control well] × 100
Fifty per cent inhibitory concentration (IC50) values, with standard errors, were calculated using the non-linear regression function of GraphPad Prism® version 9.4.0 (673) for Windows, GraphPad Software Inc., La Jolla, CA, USA.
3. Results
3.1. Cytotoxicity Assay
No apparent cytotoxicity was detected in HCT-8 cells incubated with plant extracts at concentrations ranging from 2 to 500 µg/mL up to 72 h, with all cell viabilities above 88%. At the highest tested dose (500 µg/mL), the extract of Tribulus terrestris (caltrop) had the maximum cell viability (99.6% ± 0.4), while the Cucurbita pepo (pumpkin) extract had the lowest cell viability (88.1% ± 0.6) (Table S1). The cell viability data were compatible with the microscopic observations, which showed no cytotoxicity effects of plant extracts on the cell monolayers.
3.2. Cryptosporidium Parvum Growth Inhibition Assay
The sequencing results of the randomly selected purified secondary PCR products showed 100% similarity to the C. parvum Iowa strain (CP044422) in the NCBI GenBank database.
The average Ct values obtained for 20,000 and 2 C. parvum oocysts in the standards of the qPCR assays were 21 and 33, respectively. A three-fold increase in Ct values was observed among the standards (Figure 1), confirming the reliability of qPCR measurements. Furthermore, our qPCR measurements of parasitic load in the untreated control reflected the success of infection and the parasite proliferation over the experimental period.
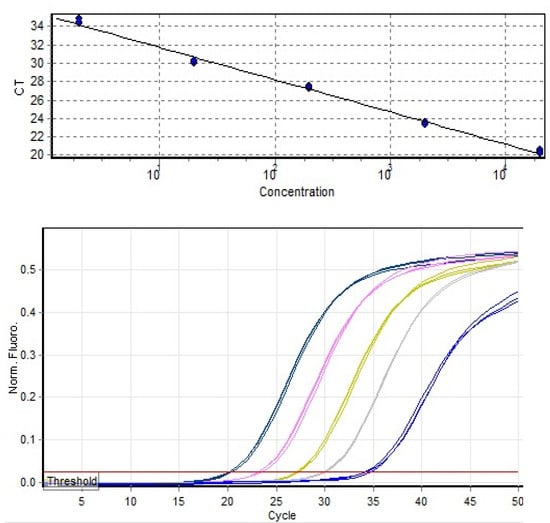
Figure 1.
Standard curve and quantitation data generated from serially diluted Cryptosporidium parvum DNA standards using RotorGene 2000 software Version 4.7, R2 = 0.99.
3.2.1. Growth Inhibition at a Single Concentration
All plant extracts tested against C. parvum demonstrated varying inhibition activities in vitro. Based on the qPCR results, the extracts of C. longa (turmeric), P. nigrum (black pepper), N. sativa (black cumin), and E. ribes (false black pepper) had the maximum C. parvum growth inhibition at 500 ng/mL with 79.6 ± 1.0, 73.6 ± 2.7, 68.1 ± 1.0, and 61.1 ± 1.6, per cent inhibitions, respectively (Table 2). The remaining plant extracts showed growth inhibition values of 50% or less, with Centella asiatica (Gotu kola) having the smallest effect on C. parvum growth in vitro (Table 2).

Table 2.
Effects of the plant extracts on C. parvum oocyst growth inhibition at a single concentration (500 ng/mL) at the end of the 48 h incubation period. Per cent growth inhibition is compared to the negative control (HCT-8 in RPMI and 0.5% DMSO).
3.2.2. Dose–Response Analysis
The extracts of C. longa, P. nigrum, E. ribes, and N. sativa exhibited dose-dependent efficacy on C. parvum growth inhibition in vitro, with IC50 values varying from 3.3 to 141.3 ng/mL. The drug control treatment (trifluralin) had an IC50 of 133.9 ng/mL (Figure 2).
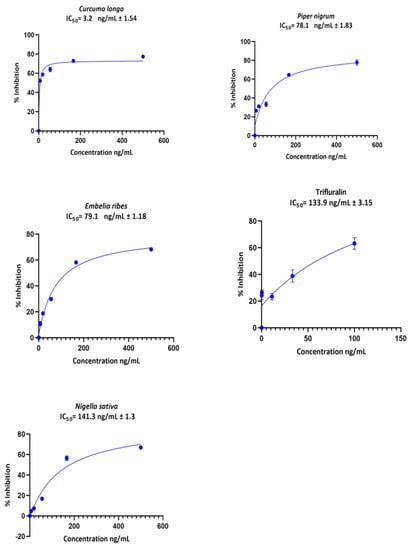
Figure 2.
Cryptosporidium parvum growth inhibition curves derived from non-linear regression in GraphPad Prism® software for the extracts of C. longa, P. nigrum, E. ribes, and N. sativa and the control drug Trifluralin. The bar represents the standard error of means from three replicates.
The fluorescence-microscope observation revealed the presence of intracellular stages in the infected and treated monolayers and the untreated control wells. Compared to the untreated controls, a higher inhibition of C. parvum growth was observed with C. longa, P. nigrum, E. ribes, and N. sativa (Supplementary Material S2).
4. Discussion
Toxicity is a primary concern when testing the clinical efficacy of any new drug candidate [39]. In the present study, all thirteen tested plant extracts had low toxicity profiles, with more than 85% cell viability at the highest dose (500 μg/mL). All these extracts also showed some ability to inhibit the in vitro growth of C. parvum, with four (C. longa, P. nigrum, E. ribes and N. sativa) having dose-dependent activity similar to or better than the standard drug control, trifluralin.
We emphasise that these results provide only the first step in the search for potential new drug candidates for cryptosporidiosis. The extracts have not yet been fully characterised for their chemical composition. This is an essential next step in determining the mode of action of the extracts and identifying the specific compounds within them which are responsible for the antiparasitic activity.
In the present study, qPCR targeting the 18S rRNA locus [36] was used to assess the multiplication of C. parvum in cell culture. The calculation of a standard curve in qPCR is crucial to validate the quality of each experiment and to obtain accurate results. In the current study, a standard curve was constructed using five triplicates of recombinant plasmids containing partial fragments of the Cryptosporidium 18S rRNA, serially diluted at a 1:10 ratio and calibrated by digital droplet PCR (ddPCR) [38]. The use of digital droplet PCR enabled an accurate count of the copy numbers of standards and, thereby, the copy number of unknown concentrations (in the samples treated with plant extracts and in the controls).
The aqueous extract of C. longa was the most effective plant product in inhibiting C. parvum growth in cell culture, with an IC50 of 3.2 ng/mL. The effect of C. longa crude extracts has not previously been studied on protozoan parasites, although inhibitory activity has been reported against model nematodes and different helminth parasites. Ethanol extract of C. longa rhizome had dose-dependent anthelmintic activity against Haemonchus contortus, with 78% worm mortality at 2 × 108 ng/mL after 24 h exposure [40] and performed better than the standard anthelmintic drug, piperazine citrate at 1 × 107 ng/mL against Indian earthworm, Pheretima posthuma [41]. Methanol extract of C. longa rhizome also showed moderate anthelmintic activity against Caenorhabditis elegans with IC50 of 1.2 × 105 ng/mL [42]. Curcumin, the most abundant phytochemical in the C. longa rhizome and a natural polyphenolic compound from Curcuma species, was effective against C. parvum in vitro with an IC50 value of 4.8 × 106 ng/mL [43].
The potent anticryptosporidial effect of C. longa in cell culture may be explained by various mechanisms of action. Species of Cryptosporidium can generate reactive oxygen species and inflammation as a part of defence mechanisms against host cells [44]. Curcumin has antioxidant and anti-inflammatory properties [45], which may be responsible for the inhibitory activity of C. longa on C. parvum. Curcumin was also reported to reduce mammalian cellular phospholipase [46,47], and inhibition of phospholipases and arachidonic acid production has previously been shown to reduce infectivity in Cryptosporidium spp. [48]. Curcumin is a known histone deacetylation inhibitor [49]. A member of the apicomplexan histone deacetylase family has been identified in C. parvum [50]. Histone deacetylase regulates transcription and is considered one of the novel therapeutic targets for antiprotozoal agents [51]. Therefore, it can be argued that C. longa extracts (which contain 95% curcuminoids) may inhibit histone deacetylation and thereby alter the proliferation of C. parvum.
Crude extracts of P. nigrum have not previously been evaluated for their efficacy on GI parasites. However, Chouhan et al. [52] demonstrated significant in vitro inhibitory actions of P. nigrum hexane seed extract and ethanolic fractions against the intracellular protozoan parasite Leishmania donovani. The activity of P. nigrum extracts against L. donovani promastigotes is presumed to be mediated through apoptosis, as evidenced by DNA fragmentation and loss of mitochondrial membrane potential [52]. This may be due to a range of bioactive compounds in Piper species, such as flavanones, neolignans, dihydrochalcones, chalcones, and alkaloids, which have been claimed to possess antiprotozoal activities [53]. Piperine, an alkaloid, is well known for its potent antioxidant and anti-inflammatory activities [54]. Therefore, piperine has the ability to scavenge free radicals, mainly reactive oxygen species (ROS) [55]. A significant protective role of ROS has been identified in experimental cryptosporidiosis [44]. It seems to be possible that a similar mechanism is involved in the inhibition of growth of the C. parvum by piperine in the P. nigrum extract.
To our knowledge, extracts of E. ribes have not been previously studied for their antiprotozoal activity, although they have previously been reported to show anthelmintic activities. Aqueous and alcoholic extracts of E. ribes seed extracts caused a moderate reduction in faecal egg count in sheep experimentally infected (at 6 mL/sheep) with H. contortus [56]. An aqueous extract of E. ribes fruit, in 3% and 5% concentrations, caused significantly greater in vitro mortality of P. posthuma compared to the same concentrations of the anthelmintic drug piperazine citrate [57]. The seed oil of E. ribes also caused mortality of P. posthuma in vitro at 1 × 107 ng/mL, and the effect was not significantly different from piperazine citrate at the same concentration [58]. Embelin, the main active ingredient of E. ribes, showed significantly greater anthelmintic activity against P. posthuma at 1 × 107 ng/mL than albendazole at 1.5 × 107 ng/mL [59].
As for E. ribes, extracts of N. sativa (black cumin) have not previously been tested against protozoan parasites but have been studied for their anthelmintic activities. Al-Shaibani et al. [60] found that aqueous and ethanol extracts of N. sativa had ED50 values on in vitro egg hatching of a mixture of GI sheep nematodes, including H. contortus, of 2.2 × 107 ng/mL and 2 × 107 ng/mL, respectively, and both extracts reduced in vivo egg counts by 200 mg/kg p.o compared to untreated control sheep. In another study, the essential oil of N. sativa at 9 × 108 ng/mL significantly reduced the survival of C. elegans larval and adult stages compared to negative (1% ethanol) and positive (levamisole at 8 × 106 ng/mL) controls, with larvae being more sensitive than adult worms [61]. The seeds of N. sativa contain fixed and volatile oils, rich sources of quinones, unsaturated fatty acids, amino acids and proteins, and traces of alkaloids and terpenoids. Thymoquinone, the active ingredient in volatile oil, has induced apoptosis in cancerous cells through DNA damage [62] and is believed to cause DNA damage to parasite cells through the increased number of free radicals [63]. Another mechanism that inhibits the parasite’s DNA synthesis may be by obstructing the interaction of the histone deacetylase enzyme with chromosomes [64]. Seeds of N. sativa also exhibit an immunomodulatory effect by stimulating CD4-positive T-cells and macrophages [65], which may reduce inflammatory damage.
5. Conclusions
Our results demonstrate that the crude extracts of C. longa, P. nigrum, E. ribes and N. sativa inhibited C. parvum intracellular growth with promising IC50 values. These plants offer a great hope to obtain anti-cryptosporidial compounds that are more promising and more effective than current treatments. Based on the sensitivity of parasites to these plant extracts in cell culture, it can be argued that the target sites could be developmental stages. The findings of the cytotoxicity assay showed that the host cells are unlikely to be targeted. However, it is possible that the extracts could target the infected host cells in preference to uninfected host cells. However, these encouraging findings are limited to the in vitro model of C. parvum infection. Further studies with appropriate in vivo models of cryptosporidiosis are required to confirm whether these plants could be developed into new anti-cryptosporidial drugs. Moreover, the exact mechanisms of C. parvum inhibitory actions of these plants remain to be explained. Future studies to characterise the mode of action of these compounds could lead to the identification of novel Cryptosporidium drug targets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12010061/s1, Supplementary Material S1: Confirmation of Cryptosporidium parvum infectivity; Supplementary Material S2: Immunofluorescence assay; Table S1: Mean % Cell viability of HCT-8 cells exposed to plant extracts.
Author Contributions
Conceptualization, A.A. (Anthony Armson) and S.R.; methodology, S.R., A.A. (Anthony Armson) and A.Z.; software, S.R. and A.Z.; validation, S.R. and A.Z.; formal analysis, S.R.; investigation, S.R.; resources, A.A. (Amanda Ash); data curation, S.R.; writing—original draft preparation, S.R.; writing—review and editing, A.J.L., A.Z., A.A. (Amanda Ash) and A.A. (Anthony Armson); supervision, A.A. (Amanda Ash), A.Z., A.A. (Anthony Armson) and A.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Murdoch University Research Training Program (RTP) Scholarship, 2019. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Sunpure Extract Ltd., India, for their generous support in providing plant extracts. The authors also wish to thank Jill Austen and Huda Al-Shalan for their assistance with fluorescence microscopy imaging.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ryan, U.; Paparini, A.; Oskam, C. New technologies for detection of enteric parasites. Trends. Parasitol. 2017, 33, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.T. Infectious diseases: Decrypting Cryptosporidium. Nat. Rev. Drug. Discov. 2017, 16, 527. [Google Scholar] [CrossRef]
- Tamomh, A.G.; Agena, A.M.; Elamin, E.; Suliman, M.A.; Elmadani, M.; Omara, A.B.; Musa, S.A. Prevalence of cryptosporidiosis among children with diarrhoea under five years admitted to Kosti teaching hospital, Kosti City, Sudan. BMC Infect. Dis. 2021, 21, 349. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef] [PubMed]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.; Faruque, A.S.; et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS. Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef]
- Robertson, L.J.; Björkman, C.; Axén, C.; Fayer, R. Cryptosporidiosis in farmed animals. In Cryptosporidium: Parasite and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 149–235. [Google Scholar]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef]
- Sanford, S.; Josephson, G. Bovine cryptosporidiosis: Clinical and pathological findings in forty-two scouring neonatal calves. Can. Vet. J. 1982, 23, 343. [Google Scholar] [PubMed]
- Barrera, J.P.; Carmena, D.; Rodríguez, E.; Checa, R.; López, A.M.; Fidalgo, L.E.; Gálvez, R.; Marino, V.; Fuentes, I.; Miró, G. The red fox (Vulpes vulpes) as a potential natural reservoir of human cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020, 67, 2172–2182. [Google Scholar] [CrossRef]
- Silverlås, C.; Mattsson, J.G.; Insulander, M.; Lebbad, M. Zoonotic transmission of Cryptosporidium meleagridis on an organic Swedish farm. Int. J. Parasitol. 2012, 42, 963–967. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Xiao, L.; Feng, Y. Molecular epidemiology of human cryptosporidiosis in low-and middle-income countries. Clin. Microbiol. Rev. 2021, 34, e00087-19. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.V.; Corcoran, G.D. New drugs and treatment for cryptosporidiosis. Curr. Opin. Infect. Dis. 2004, 17, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sparks, H.; Nair, G.; Castellanos-Gonzalez, A.; White, A.C., Jr. Treatment of Cryptosporidium: What we know, gaps, and the way forward. Curr. Trop. Med. Rep. 2015, 2, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Aleem, M.T.; Shaukat, A.; Khan, A.; Mohsin, M.; Rehman, T.u.; Abbas, R.Z.; Saleemi, M.K.; Khatoon, A.; Babar, W.; et al. Medicinal plants as an alternative to control poultry parasitic diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef]
- Hussain, K.; Abbas, R.; Abbas, A.; Samiullah, K.; Ahmed, T.; Siddique, F.; Mohsin, M.; Rehman, A.; Rahman, A.U.; Waqas, M. Anticoccidial potential of Ageratum conyzoides and its effect on Blood parameters of experimentally infected Broiler Chickens. J. Hell. Vet. Med. Soc. 2021, 72, 3085–3090. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal activity against Entamoeba histolytica of flavonoids isolated from Lippia graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro experiments to in vivo and clinical studies; pros and cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Armson, A.; Sargent, K.; MacDonald, L.M.; Finn, M.P.; Thompson, R.C.A.; Reynoldson, J.A. A comparison of the effects of two dinitroanilines against Cryptosporidium parvum in vitro and in vivo in neonatal mice and rats. FEMS Immunol. Med. Microbiol. 1999, 26, 109–113. [Google Scholar] [CrossRef]
- Arrowood, M.J. In Vitro cultivation of Cryptosporidium Species. Clin. Microbiol. Rev. 2002, 15, 390. [Google Scholar] [CrossRef]
- Hijjawi, N.S. Successful in vitro cultivation of Cryptosporidium andersoni: Evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. Int. J. Parasitol. 2002, 32, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Borowski, H.; Thompson, R.; Armstrong, T.; Clode, P. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 2010, 137, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Karanis, P.; Aldeyarbi, H. Evolution of Cryptosporidium in vitro culture. Int. J. Parasitol. 2011, 41, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Warrier, P.K. Indian Medicinal Plants: A. Compendium of 500 Species; Orient Blackswan: Hyderabad, India, 1993; Volume 5. [Google Scholar]
- Yelne, M.; Sharma, P.; Dennis, T. Database on medicinal plants used in Ayurveda. Cent. Counc. Res. Ayurveda. Siddha. New Delhi 2000, 2, 69–73. [Google Scholar]
- Hijjawi, N.; Meloni, B.; Morgan, U.; Thompson, R. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 2001, 31, 1048–1055. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef]
- Arrowood, M.J.; Mead, J.R.; Xie, L.; You, X. In vitro anticryptosporidial activity of dinitroaniline herbicides. FEMS Microbiol. Lett. 1996, 136, 245–249. [Google Scholar] [CrossRef][Green Version]
- MacDonald, L.M.; Sargent, K.; Armson, A.; Thompson, R.C.A.; Reynoldson, J.A. The development of a real-time quantitative-PCR method for characterisation of a Cryptosporidium parvum in vitro culturing system and assessment of drug efficacy. Mol. Biochem. Parasitol. 2002, 121, 279–282. [Google Scholar] [CrossRef]
- Di Giovanni, G.D.; LeChevallier, M.W. Quantitative-PCR assessment of Cryptosporidium parvum cell culture infection. Appl. Environ. Microbiol. 2005, 71, 1495–1500. [Google Scholar] [CrossRef][Green Version]
- Zahedi, A.; Greay, T.L.; Paparini, A.; Linge, K.L.; Joll, C.A.; Ryan, U.M. Identification of eukaryotic microorganisms with 18S rRNA next-generation sequencing in wastewater treatment plants, with a more targeted NGS approach required for Cryptosporidium detection. Water Res. 2019, 158, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Certad, G.; Zahedi, A.; Gantois, N.; Sawant, M.; Creusy, C.; Duval, E.; Benamrouz, S.; Ryan, U.; Viscogliosi, E. Molecular characterization of novel Cryptosporidium fish genotypes in edible marine fish. Microorganisms 2020, 8, 2014. [Google Scholar] [CrossRef]
- Cai, X.; Woods, K.M.; Upton, S.J.; Zhu, G. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob. Agents. Chemother. 2005, 49, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Morgan, U.M.; Constantine, C.C.; Forbes, D.A.; Thompson, R.C. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J. Parasitol. 1997, 83, 825–830. [Google Scholar] [CrossRef] [PubMed]
- King, B.J.; Keegan, A.R.; Monis, P.T.; Saint, C.P. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 2005, 71, 3848–3857. [Google Scholar] [CrossRef]
- Yang, R.; Paparini, A.; Monis, P.; Ryan, U. Comparison of next-generation droplet digital PCR (ddPCR) with quantitative PCR (qPCR) for enumeration of Cryptosporidium oocysts in faecal samples. Int. J. Parasitol. 2014, 44, 1105–1113. [Google Scholar] [CrossRef]
- Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80. [Google Scholar] [CrossRef]
- Nasai, N.B.; Abba, Y.; Abdullah, F.F.J.; Marimuthu, M.; Tijjani, A.; Sadiq, M.A.; Mohammed, K.; Chung, E.L.T.; Omar, M.A.B. In vitro larvicidal effects of ethanolic extract of Curcuma longa Linn. on Haemonchus larval stage. Vet. World 2016, 9, 417. [Google Scholar] [CrossRef][Green Version]
- Raul, S.K.; Padhy, G.K.; Charly, J.P.; Kumar, K.V. An in-vitro evaluation of the anthelmintic activity of rhizome extracts of Zingiber officinalis, Zingiber zerumbet and Curcuma longa, a comparative study. J. Pharm. Res. 2012, 5, 3813–3814. [Google Scholar]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P.J. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J. Ethnopharmacol. 2009, 123, 475–482. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Dyachenko, V.; Khalafalla, R.E.; Desouky, A.Y.; Daugschies, A. Effects of curcumin on Cryptosporidium parvum in vitro. Parasitol. Res. 2009, 105, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; He, Q. Reactive nitrogen and oxygen species ameliorate experimental cryptosporidiosis in the neonatal BALB/c mouse model. Infect. Immun. 1999, 67, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Ramsewak, R.S.; DeWitt, D.L.; Nair, M.G. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine 2000, 7, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150. [Google Scholar] [CrossRef]
- Milacic, V.; Banerjee, S.; Landis-Piwowar, K.R.; Sarkar, F.H.; Majumdar, A.P.; Dou, Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells In vitro and In vivo. Cancer Res. 2008, 68, 7283–7292. [Google Scholar] [CrossRef]
- Pollok, R.C.; McDonald, V.; Kelly, P.; Farthing, M.J. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol. Res. 2003, 90, 181–186. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 427–433. [Google Scholar] [CrossRef]
- Rider, S.D., Jr.; Zhu, G. An apicomplexan ankyrin-repeat histone deacetylase with relatives in photosynthetic eukaryotes. Int. J. Parasitol. 2009, 39, 747–754. [Google Scholar] [CrossRef]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef]
- Chouhan, G.; Islamuddin, M.; Want, M.Y.; Ozbak, H.A.; Hemeg, H.A.; Sahal, D.; Afrin, F. Leishmanicidal activity of Piper nigrum bioactive fractions is interceded via apoptosis In Vitro and substantiated by Th1 immunostimulatory potential In Vivo. Front. Microbiol. 2015, 6, 1368. [Google Scholar] [CrossRef]
- Ali, B.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-induced hepatotoxicity and neurotoxicity in mice. Oxidative Med. Cell. Longev. 2019, 2019, 1309175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sasmal, D.; Sharma, N. Mechanism of deltamethrin induced thymic and splenic toxicity in mice and its protection by piperine and curcumin: In Vivo study. Drug. Chem. Toxicol. 2018, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, C.P.; Singh, D.; Khan, F.A.; Bhagwan, P.S.K. Anthelmintic potential of Embelia ribes seeds against Haemonchus contortus of sheep. Indian. J. Anim. Sci. 2009, 79, 167–170. [Google Scholar]
- Kekuda Prashith, T.; Kumar, P.; Nishanth, B.; Sandeep, M. In vitro anthelmintic activity of aqueous extract of Embelia ribes. Biotechnol. Indian J. 2009, 3, 87–89. [Google Scholar]
- Jalalpure, S.; Alagawadi, K.; Mahajanashetti, C.; Shah, B.; Singh, V.; Patil, J. In vitro anthelmintic property of various seed oils against Pheritima posthuma. Indian J. Pharm. Sci. 2007, 69, 158. [Google Scholar] [CrossRef]
- Ghugarkar, P.G.; Nupur, A.; Inamdar, N.; Tarkase, K. In vitro evaluation of anthelmintic activity of Embelin. World J. Pharm. Res. 2015, 4, 1433–1437. [Google Scholar]
- Al-Shaibani, I.; Phulan, M.; Arijo, A.; Qureshi, T.; Kumbher, A. Anthelmintic activity of Nigella sativa L., seeds on gastrointestinal nematodes of sheep. Pak. J. Nematol. 2008, 26, 207–218. [Google Scholar]
- Sen, E.; Ogut, T.; Olgun, A.; Kisa, O. Anthelmintic activity of Nigella sativa against Caenorhabditis elegans. Adv. Pharmacol. Pharm. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- El-Baba, C.; Mahadevan, V.; Fahlbusch, F.B.; Rau, T.T.; Gali-Muhtasib, H.; Schneider-Stock, R. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signaling in colorectal cancer. Mol. Cancer 2014, 13, 201. [Google Scholar] [CrossRef]
- Ullah, R.; Rehman, A.; Zafeer, M.F.; Rehman, L.; Khan, Y.A.; Khan, M.A.H.; Khan, S.N.; Khan, A.U.; Abidi, S.M.A. Anthelmintic potential of thymoquinone and curcumin on Fasciola gigantica. PLoS ONE 2017, 12, e0171267. [Google Scholar] [CrossRef] [PubMed]
- Raval, B.P.; Shah, T.G.; Suthar, M.P.; Ganure, A.L. Screening of Nigella sativa seeds for antifungal activity. Ann. Biol. Res. 2010, 1, 164–171. [Google Scholar]
- Haq, A.; Lobo, P.I.; Al-Tufail, M.; Rama, N.R.; Al-Sedairy, S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int. J. Immunopharmacol. 1999, 21, 283–295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).