Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Expression Analysis in CC Samples Using the TCGA and HPA Datasets
2.2. Correlation Analysis
2.3. Cell Culture
2.4. RNA Extraction
2.5. Determination of Gene Expression in C-33A, SiHa, and Ca Ski Cells by RT-qPCR
2.6. Overall and Relapse-Free Survival Analyses
2.7. Statistical Analysis
3. Results
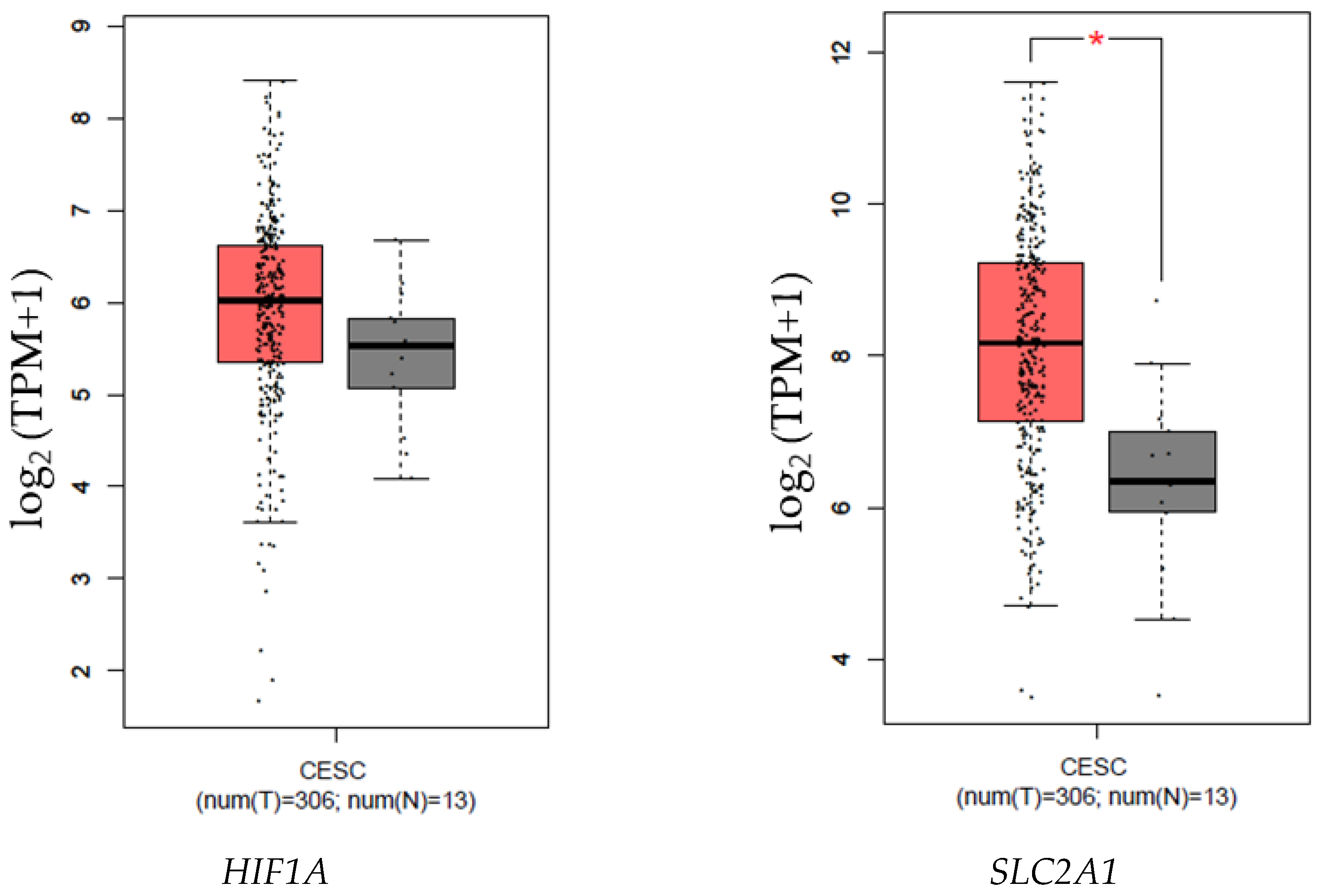
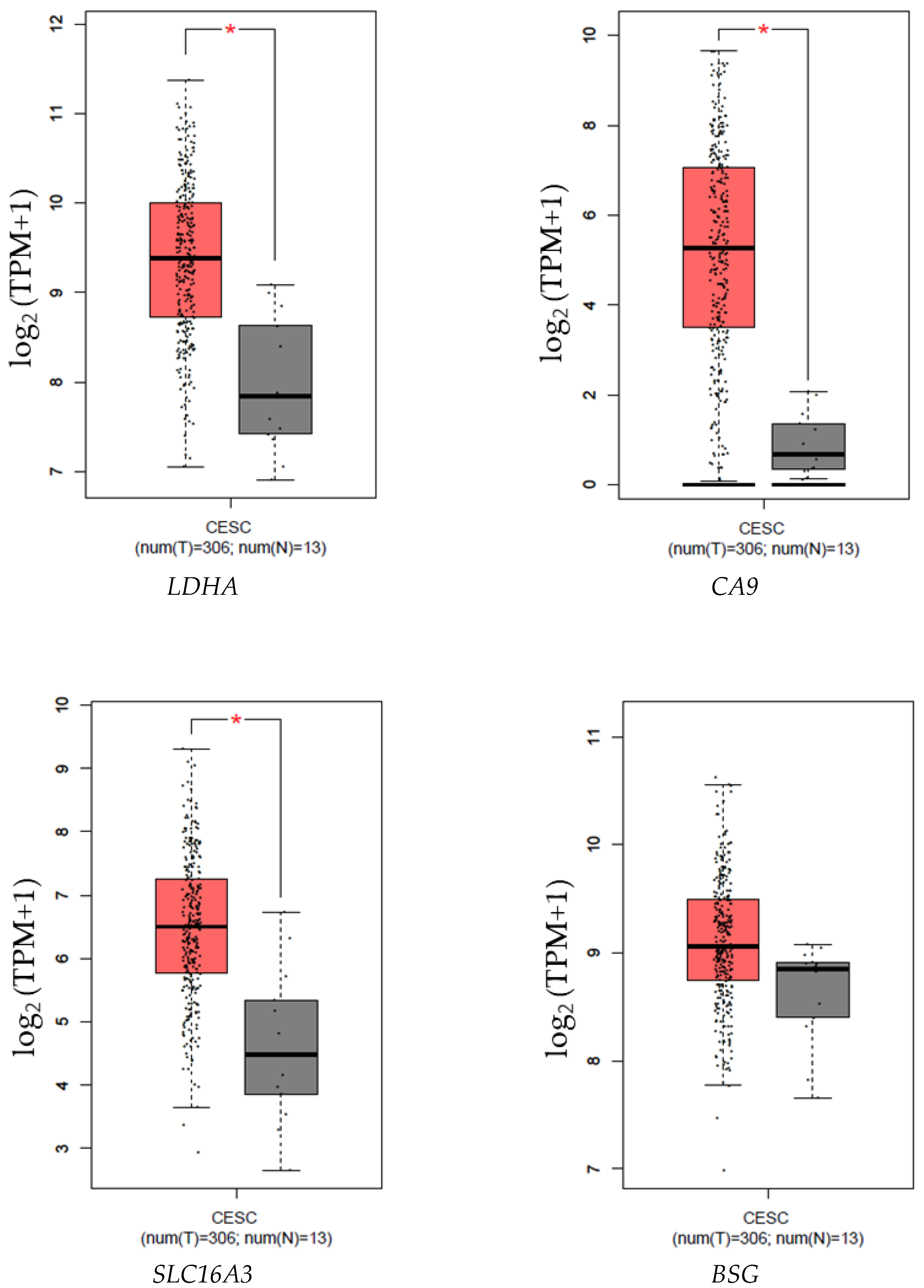
3.1. HIF1A, SLC2A1, LDHA, CA9, SLC16A3, and BSG Expression Is Increased in Samples from Patients with CC
3.2. High Expression of HIF1A Correlates with Increased Expression of SLC2A1, LDHA, CA9, SLC16A3, and BSG in CC
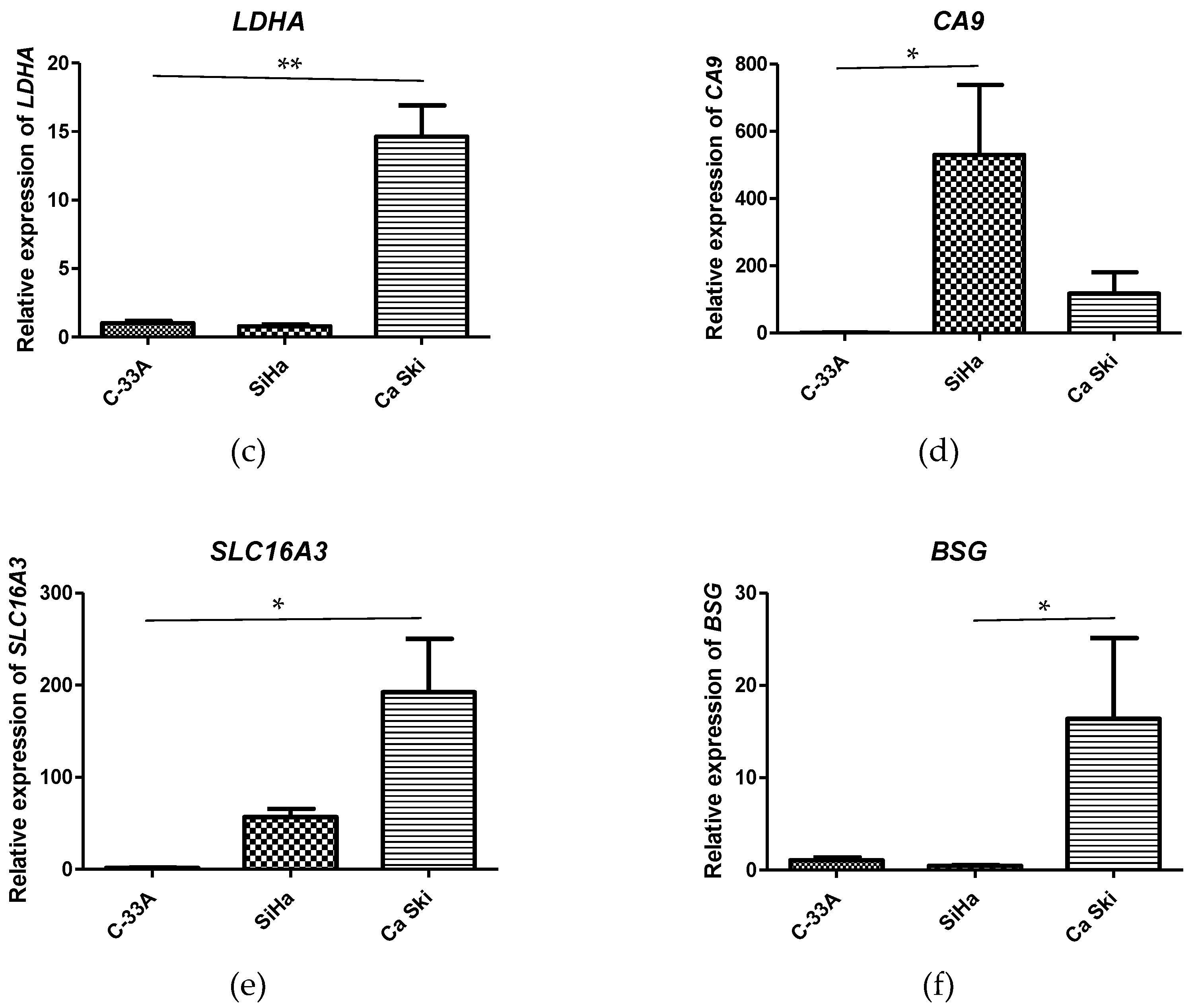
3.3. mRNA Expression of HIF1A, SLC2A1, LDHA, CA9, SLC16A3, and BSG Is Increased in CC Cell Lines
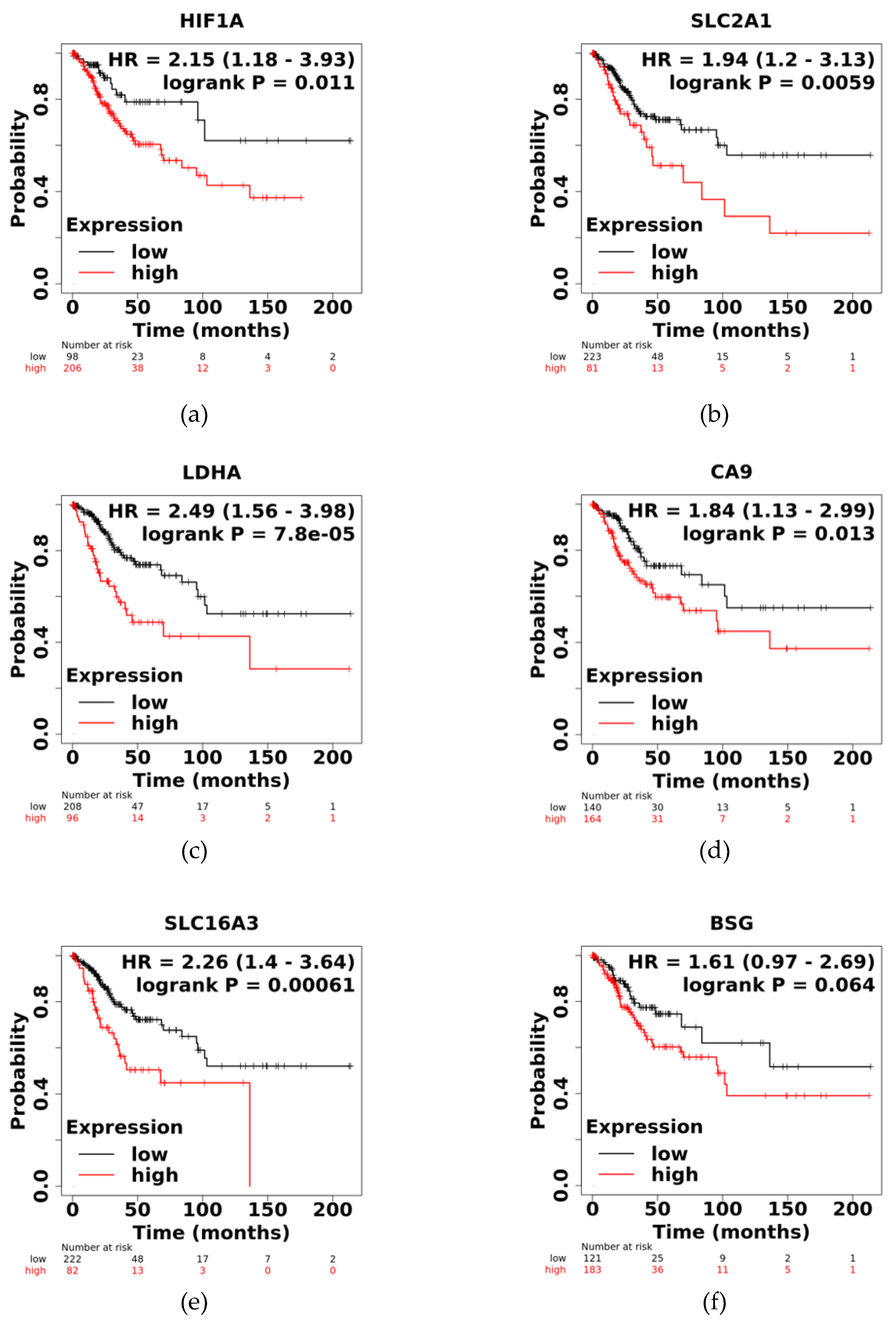
3.4. High Expression of HIF1A, SLC2A1, LDHA, CA9, and SLC16A3 Correlates with Lower Survival in CC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X.; Zhang, Y. Involvement of human papillomaviruses in cervical cancer. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, I.; Carrillo-García, A.; Contreras-Paredes, A.; Ortiz-Sánchez, E.; Cruz-Gregorio, A.; Lizano, M. Regulation of cellular metabolism by high-risk human papillomaviruses. Int. J. Mol. Sci. 2018, 19, 1839. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Long, X.; Guo, X.; Sun, X.; Jin, X.; Li, Z.; Ren, T.; Yuan, P.; Huang, X.; et al. Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene 2017, 36, 4901–4912. [Google Scholar] [CrossRef]
- Park, S.; Smith, C.; Wilbur, R.; Cain, C.; Kallu, S.; Valasapalli, S.; Sahoo, A.; Guda, M.; Tsung, A.; Velpula, K. An overview of MCT1 and MCT4 in GBM: Small molecule transporters with large implications. Am. J. Cancer Res. 2018, 8, 1967–1976. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30416849 (accessed on 10 March 2021).
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfør, K.; Rofstad, E.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Soga, T. Cancer metabolism: Key players in metabolic reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef]
- Xu, S.; Ying, K. Association between HIF-1α gene polymorphisms and lung cancer: A meta-analysis. Medicine 2020, 99, e20610. [Google Scholar] [CrossRef]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.; Stiehl, D.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, 2005, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1)α: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Payen, V.; Mina, E.; Van Hée, V.; Porporato, P.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2019, 33, 1–19. [Google Scholar] [CrossRef]
- Mulukutla, B.C.; Yongky, A.; Le, T.; Mashek, D.; Hu, W. Regulation of Glucose Metabolism–A Perspective from Cell Bioprocessing. Trends Biotechnol. 2016, 34, 638–651. [Google Scholar] [CrossRef]
- Sowa, T.; Menju, T.; Chen-Yoshikawa, T.; Takahashi, K.; Nishikawa, S.; Nakanishi, T.; Shikuma, K.; Motoyama, H.; Hijiya, K.; Aoyama, A.; et al. Hypoxia-inducible factor 1 promotes chemoresistance of lung cancer by inducing carbonic anhydrase IX expression. Cancer Med. 2017, 6, 288–297. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.; Kunkler, I.; Langdon, S.; Argyle, D. Carbonic anhydrase IX (CAIX), cancer, and radiation responsiveness. Metabolites 2018, 8, 13. [Google Scholar] [CrossRef]
- Ullah, M.; Davies, A.; Halestrap, A. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, X.; Ma, J.; Zheng, Y.; Wang, Q.; Wang, Y.; Shang, H. Human papillomavirus 16 E6 contributes HIF-1α induced warburg effect by attenuating the VHL-HIF-1α interaction. Int. J. Mol. Sci. 2014, 15, 7974–7986. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Garcia, E.; Morais-Santos, F.; Scapulatempo-Neto, C.; Mafra, A.; Steenbergen, R.; Boccardo, E.; Villa, L.; Baltazar, F.; Longatto-Filho, A. Lactate transporters and vascular factors in HPV-induced squamous cell carcinoma of the uterine cervix. BMC Cancer 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sui, L. Metabolic reprogramming in cervical cancer and metabolomics perspectives. Nutr. Metab. 2021, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-based survival analysis tool tailored for medical research (KMplot): Development and implementation. J. Med. Internet Res. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Liao, D.; Johnson, R. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Chan, D.; Giaccia, A. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007, 26, 333–339. [Google Scholar] [CrossRef]
- Sullivan, R.; Graham, C. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007, 26, 319–331. [Google Scholar] [CrossRef]
- Reyna-Hernández, M.A.; Alarcón-Romero, L.D.C.; Ortiz-Ortiz, J.; Illades-Aguiar, B.; Jiménez-López, M.A.; Ocampo-Bárcenas, A.; Morrugares-Ixtepan, M.O.; Torres-Rojas, F.I. GLUT1, LDHA, and MCT4 Expression Is Deregulated in Cervical Cancer and Precursor Lesions. J. Histochem. Cytochem. 2022, 70, 437–446. [Google Scholar] [CrossRef]
- Yang, Y.; Su, D.; Zhao, L.; Zhang, D.; Xu, J.; Wan, J.; Fan, S.; Chen, M. Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget 2014, 5, 11886–11896. [Google Scholar] [CrossRef] [PubMed]
- Maftouh, M.; Avan, A.; Sciarrillo, R.; Granchi, C.; Leon, L.G.; Rani, R.; Funel, N.; Smid, K.; Honeywell, R.; Boggi, U.; et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br. J. Cancer 2014, 110, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Hu, X.; Lian, Y.; Ding, C.; Ming, L. Inhibition of LDHA suppresses cell proliferation and increases mitochondrial apoptosis via the JNK signaling pathway in cervical cancer cells. Oncol. Rep. 2022, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, M.; Roszak, A.; Pawlik, P.; Kedzia, H.; Lianeri, M.; Jagodziński, P.P. Increased expression of HIF-1A and its implication in the hypoxia pathway in primary advanced uterine cervical carcinoma. Oncol. Rep. 2011, 26, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Woś, J.; Bryś, M.; Lewy-Trenda, I.; Stasikowska, O.; Papie, P.; Papierz, W.; Starska, K. Analiza ekspresji HIF-1α i COX-2 w utkaniu guza oraz korelacja ze stopniem inwazyjności zmian nowotworowych u chorych z rakiem krtani-Badania wstpne. Otolaryngol. Pol. 2011, 65, 102–108. [Google Scholar] [CrossRef]
- Kim, B.; Chang, J. Differential effect of GLUT1 overexpression on survival and tumor immune microenvironment of human papilloma virus type 16-positive and -negative cervical cancer. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Feng, W.; Cui, G.; Tang, C.; Zhang, X.; Dai, C.; Xu, Y.; Gong, H.; Xue, T.; Guo, H.; Bao, Y. Role of glucose metabolism related gene GLUT1 in the occurrence and prognosis of colorectal cancer. Oncotarget 2017, 8, 56850–56857. [Google Scholar] [CrossRef]
- Yu, C.; Hou, L.; Cui, H.; Zhang, L.; Tan, X.; Leng, X.; Li, Y. LDHA upregulation independently predicts poor survival in lung adenocarcinoma, but not in lung squamous cell carcinoma. Futur. Oncol. 2018, 14, 2483–2492. [Google Scholar] [CrossRef]
- Tafreshi, N.; Lloyd, M.; Proemsey, J.; Bui, M.; Kim, J.; Gillies, R.; Morse, D. Evaluation of CAIX and CAXII Expression in Breast Cancer at Varied O2 Levels: CAIX is the Superior Surrogate Imaging Biomarker of Tumor Hypoxia. Mol. Imaging Biol. 2016, 18, 219–231. [Google Scholar] [CrossRef]
- Eckert, A.; Horter, S.; Bethmann, D.; Kotrba, J.; Kaune, T.; Rot, S.; Bache, M.; Bilkenroth, U.; Reich, W.; Greither, T.; et al. Investigation of the prognostic role of carbonic anhydrase 9 (CAIX) of the cellular mRNA/protein level or soluble CAIX protein in patients with oral squamous cell carcinoma. Int. J. Mol. Sci. 2019, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B.; Yan, W.; Xia, Y.; Wang, Z.; Zheng, G.; Da Wang, W.; Zhang, Y. Integrative Analysis Identified MCT4 as an Independent Prognostic Factor for Bladder Cancer. Front. Oncol. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhang, J.; Lou, J.; Wang, S.; Jiang, Y.; Wu, F.; Wang, S. Comprehensive Analysis of Monocarboxylate Transporter 4 (MCT4) expression in breast cancer prognosis and immune infiltration via integrated bioinformatics analysis. Bioengineered 2021, 12, 3850–3863. [Google Scholar] [CrossRef] [PubMed]
- Łacina, P.; Butrym, A.; Turlej, E.; Stachowicz-Suhs, M.; Wietrzyk, J.; Mazur, G.; Bogunia-Kubik, K. BSG (CD147) Serum Level and Genetic Variants Are Associated with Overall Survival in Acute Myeloid Leukaemia. J. Clin. Med. 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pang, X.; Zhang, Z.; Liu, Q.; Zhang, H.; Xiang, Q.; Cui, Y. Co-expression and prognosis analyses of GLUT1–4 and RB1 in breast cancer. BMC Cancer 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guddeti, R.; Bali, P.; Karyala, P.; Pakala, S. MTA1 coregulator regulates LDHA expression and function in breast cancer. Biochem. Biophys. Res. Commun. 2019, 520, 54–59. [Google Scholar] [CrossRef]
- Bonatelli, M.; Fornari, I.; Bernécule, P.; Pinheiro, L.; Costa, R.; Longatto-Filho, A.; Junior, J.; Silva, E.; Cárcano, F.; Pinheiro, C. Expression of Glycolysis-Related Proteins in Cancer of Unknown Primary Origin. Front. Oncol. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Mansour, R.; Enderami, S.; Ardeshirylajimi, A.; Fooladsaz, K.; Fathi, M.; Ganji, S. Evaluation of hypoxia inducible factor-1 alpha gene expression in colorectal cancer stages of Iranian patients. J. Cancer Res. Ther. 2016, 12, 1313–1317. [Google Scholar] [CrossRef]
- Semenza, G. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Zhang, R.; Su, J.; Xue, S.; Yang, H.; Ju, L.; Ji, Y.; Wu, K.; Zhang, Y. Original Article inhibits Warburg effect through targeting LDHA in cervical cancer. Am. J. Cancer Res. 2016, 6, 312–320. [Google Scholar] [PubMed]
- Shagieva, G.; Domnina, L.; Makarevich, O.; Chernyak, B.; Skulachev, V.; Dugina, V. Depletion of mitochondrial reactive oxygen species downregulates epithelial-to-mesenchymal transition in cervical cancer cells. Oncotarget 2017, 8, 4901–4913. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.; Hussa, R.; Story, M.; Ruckert, A.; Shalaby, M.; Mattingly, R. Tumor Antigen and Human Chorionic Gonadotropin in CaSki Cells: A New Epidermoid Cervical Cancer Cell Line Abstract. Obstet. Gynecol. Surv. 1978, 33, 56–57. [Google Scholar] [CrossRef]
- Meissner, J. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J. Gen. Virol. 1999, 80, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Phelps, W.; Lindgren, V.; Braun, M.; Gonda, M.; Howley, P. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 1987, 61, 962–971. [Google Scholar] [CrossRef]
- Zacapala-Gómez, A.E.; Del Moral-Hernández, O.; Villegas-Sepúlveda, N.; Hidalgo-Miranda, A.; Romero-Córdoba, S.L.; Beltrán-Anaya, F.O.; Leyva-Vázquez, M.A.; Alarcón-Romero, L.D.C.; Illades-Aguiar, B. Changes in global gene expression profiles induced by HPV 16 E6 oncoprotein variants in cervical carcinoma C33-A cells. Virology 2016, 488, 187–195. [Google Scholar] [CrossRef]
- Cui, X.; Han, Z.; He, S.; Da Wu, X.; Chen, T.; Shao, C.; Chen, D.; Su, N.; Chen, Y.; Wang, T.; et al. HIF1/2α mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 2017, 8, 24840–24852. [Google Scholar] [CrossRef]
- Hsin, M.; Hsieh, Y.; Hsiao, Y.; Chen, P.; Wang, P.; Yang, S. Carbonic anhydrase ix promotes human cervical cancer cell motility by regulating pfkfb4 expression. Cancers 2021, 13, 1174. [Google Scholar] [CrossRef]
- Ju, X.; Liang, S.; Zhu, J.; Ke, G.; Wen, H.; Wu, X. Extracellular matrix metalloproteinase inducer (CD147/ BSG/EMMPRIN)-induced radioresistance in cervical cancer by regulating the percentage of the cells in the G2/m phase of the cell cycle and the repair of DNA Double-strand Breaks (DSBs). Am. J. Transl. Res. 2016, 8, 2498–2511. [Google Scholar]
- Shamis, S.; McMillan, D.; Edwards, J. The relationship between hypoxia-inducible factor 1α (HIF-1α) and patient survival in breast cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 159, 103231. [Google Scholar] [CrossRef]
- Chen, Z.; Ai, L.; Mboge, M.; Tu, C.; McKenna, R.; Brown, K.; Heldermon, C.; Frost, S. Differential expression and function of CAIX and CAXII in breast cancer: A comparison between tumorgraft models and cells. PLoS ONE 2018, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tsang, J.; Lee, M.; Ni, Y.; Chan, S.; Cheung, S.; Hu, J.; Hu, H.; Tse, G. CD147 expression is associated with poor overall survival in chemotherapy treated triple-negative breast cancer. J. Clin. Pathol. 2018, 71, 1007–1014. [Google Scholar] [CrossRef] [PubMed]

| HIF-1α Target Genes | CESC (Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma) | |
|---|---|---|
| Tumor | ||
| R | p | |
| SLC2A1 | 0.27 | *** |
| LDHA | 0.41 | *** |
| CA9 | 0.14 | ** |
| SLC16A3 | 0.31 | *** |
| BSG | −0.26 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priego-Hernández, V.D.; Arizmendi-Izazaga, A.; Soto-Flores, D.G.; Santiago-Ramón, N.; Feria-Valadez, M.D.; Navarro-Tito, N.; Jiménez-Wences, H.; Martínez-Carrillo, D.N.; Salmerón-Bárcenas, E.G.; Leyva-Vázquez, M.A.; et al. Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines. Pathogens 2023, 12, 33. https://doi.org/10.3390/pathogens12010033
Priego-Hernández VD, Arizmendi-Izazaga A, Soto-Flores DG, Santiago-Ramón N, Feria-Valadez MD, Navarro-Tito N, Jiménez-Wences H, Martínez-Carrillo DN, Salmerón-Bárcenas EG, Leyva-Vázquez MA, et al. Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines. Pathogens. 2023; 12(1):33. https://doi.org/10.3390/pathogens12010033
Chicago/Turabian StylePriego-Hernández, Víctor D., Adán Arizmendi-Izazaga, Diana G. Soto-Flores, Norma Santiago-Ramón, Milagros D. Feria-Valadez, Napoleón Navarro-Tito, Hilda Jiménez-Wences, Dinorah N. Martínez-Carrillo, Eric G. Salmerón-Bárcenas, Marco A. Leyva-Vázquez, and et al. 2023. "Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines" Pathogens 12, no. 1: 33. https://doi.org/10.3390/pathogens12010033
APA StylePriego-Hernández, V. D., Arizmendi-Izazaga, A., Soto-Flores, D. G., Santiago-Ramón, N., Feria-Valadez, M. D., Navarro-Tito, N., Jiménez-Wences, H., Martínez-Carrillo, D. N., Salmerón-Bárcenas, E. G., Leyva-Vázquez, M. A., Illades-Aguiar, B., Alarcón-Romero, L. d. C., & Ortiz-Ortiz, J. (2023). Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines. Pathogens, 12(1), 33. https://doi.org/10.3390/pathogens12010033











