Indirect Evidence Based on Mating-Type Ratios for the Role of Sexual Reproduction in European and Chinese Populations of Plenodomus biglobosus (Blackleg of Oilseed Rape)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and DNA Extraction
2.2. Design of New Plenodomus biglobosus Mating-Type PCR Diagnostics
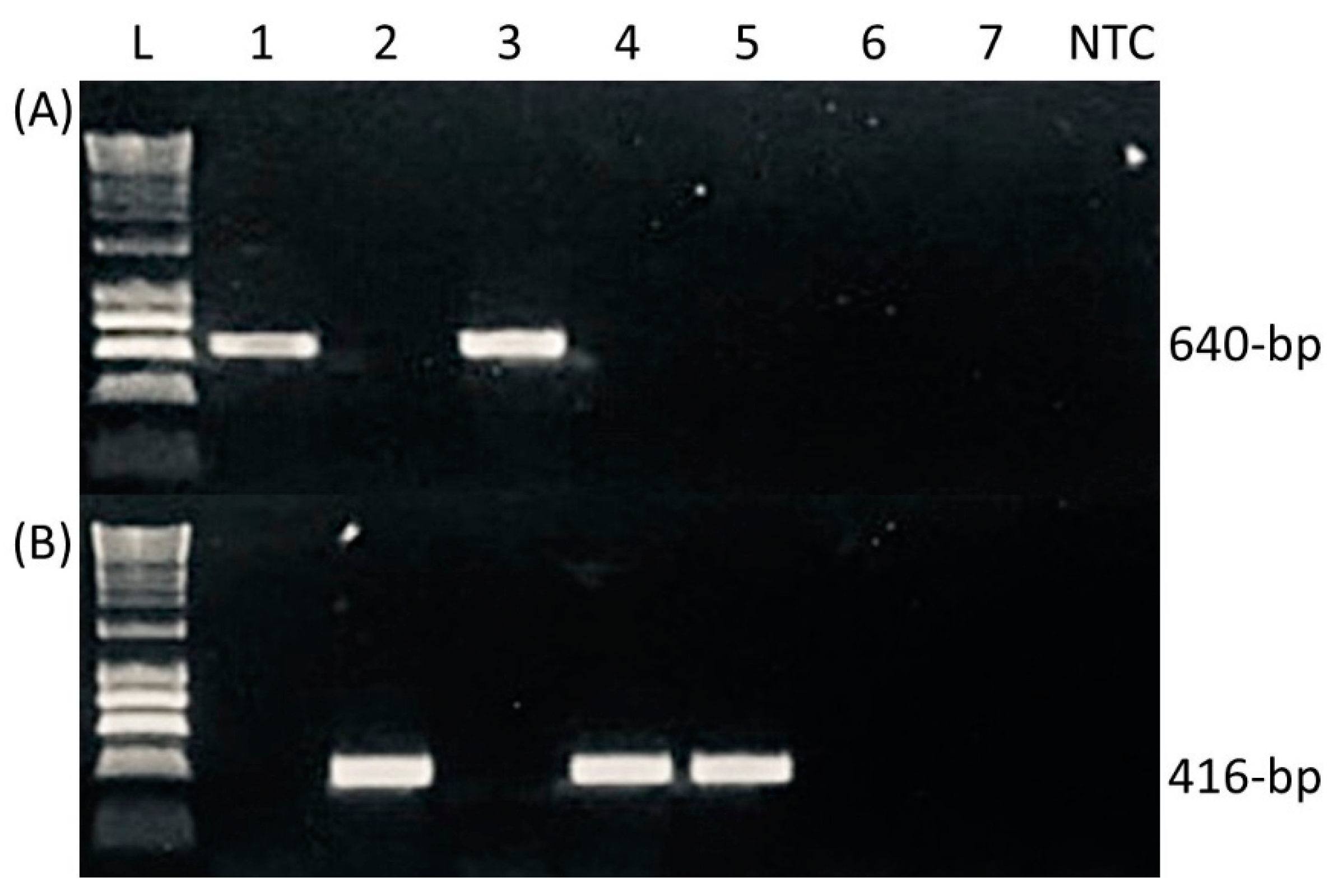
2.3. Validation of New Plenodomus biglobosus PCR Mating-Type Diagnostics
2.4. PCR Testing of European and Chinese Plenodomus biglobosus Isolates for Mating Type
3. Results
3.1. Validation of New Plenodomus biglobosus Mating-Type Diagnostics
3.2. PCR Screening of European and Chinese P. biglobosus Isolates for Mating Type
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Mendes-Pereira, E.; Balesdent, M.-H.; Brun, H.; Rouxel, T. Molecular phylogeny of the Leptosphaeria maculans–L. biglobosa species complex. Mycol. Res. 2003, 107, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- de Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Vincenot, L.; Balesdent, M.H.; Li, H.; Barbetti, M.J.; Sivasithamparam, K.; Gout, L.; Rouxel, T. Occurrence of a new subclade of Leptosphaeria biglobosa in Western Australia. Phytopathology 2008, 98, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, X.; Parks, P.; du Toit, L.J.; Van de Wouw, A.P.; Dilantha Fernando, W.G. A new subclade of Leptosphaeria biglobosa identified from Brassica rapa. Int. J. Mol. Sci. 2019, 20, 1668. [Google Scholar] [CrossRef]
- Liu, Z.; Latunde-Dada, A.O.; Hall, A.M.; Fitt, B.D.L. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur. J. Plant Pathol. 2014, 140, 841–857. [Google Scholar] [CrossRef]
- Hao, L.; Song, P.; Huangfu, H.; Li, Z. Genetic diversity and differentiation of Leptosphaeria biglobosa on oilseed rape in China. Phytoparasitica 2015, 43, 253–263. [Google Scholar] [CrossRef]
- King, K.M.; West, J.S. Detection of the Phoma pathogens Plenodomus biglobosus subclades ‘brassicae’ and ‘canadensis’ on wasabi, and ‘canadensis’ in Europe. Eur. J. Plant Pathol. 2022, 162, 751–756. [Google Scholar] [CrossRef]
- Safi, A.; Mehrabi-Koushki, M.; Farokhinejad, R. Plenodomus dezfulensis sp. nov. causing leaf spot of rapeseed in Iran. Phytotaxa 2021, 523, 141–154. [Google Scholar] [CrossRef]
- Punja, Z.K.; Chandanie, W.A.; Chen, X.; Rodríguez, G. Phoma leaf spot of wasabi (Wasabia japonica) caused by Leptosphaeria biglobosa. Plant Pathol. 2017, 66, 480–489. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Hu, B.C.; Li, Z.Q.; Liu, S.Y.; Lange, R.M.; Kharbanda, P.D.; Butterworth, M.H.; White, R.P. Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 2008, 57, 652–664. [Google Scholar] [CrossRef]
- West, J.S.; Balesdent, M.-H.; Rouxel, T.; Narcy, J.P.; Huang, Y.J.; Roux, J.; Steed, J.M.; Fitt, B.D.L.; Schmit, J. Colonization of winter oilseed rape tissues by A/Tox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathol. 2002, 51, 311–321. [Google Scholar] [CrossRef]
- Li, Q.; Rong, S.; Hu, B.; Jiang, Y.; Hou, S.; Fei, W.; Chen, F.; Wu, X.; Fan, Z.; Lei, W. Distribution of blackleg disease on oilseed rape in China and its pathogen identification. Chin. J. Oil Crop Sci. 2013, 35, 415–423. Available online: http://www.jouroilcrops.cn/EN/Y2013/V35/I4/415 (accessed on 7 December 2022).
- Huang, Y.J.; Karandeni-Dewage, C.S.; Fitt, B.D.L. Importance of Leptosphaeria biglobosa as a cause of phoma stem canker on winter oilseed rape in the UK. Asp. Appl. Biol. 2014, 127, 117–122. [Google Scholar]
- Zhang, X.; White, R.P.; Demir, E.; Jedryczka, M.; Lange, R.M.; Islam, M.; Li, Z.Q.; Huang, Y.J.; Hall, A.M.; Zhou, G.; et al. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 2014, 63, 598–612. [Google Scholar] [CrossRef]
- Dilmaghani, A.; Gladieux, P.; Gout, L.; Giraud, T.; Brunner, P.C.; Stachowiak, A.; Balesdent, M.-H.; Rouxel, T. Migration patterns and changes in population biology associated with the worldwide spread of the oilseed rape pathogen Leptosphaeria maculans. Mol. Ecol. 2012, 21, 2519–2533. [Google Scholar] [CrossRef]
- Zhang, X.; Dilantha Fernando, W.G. Insights into fighting against blackleg disease of Brassica napus in Canada. Crop Pasture Sci. 2018, 69, 40–47. [Google Scholar] [CrossRef]
- Huang, Y.J.; Fitt, B.D.L.; Jedryczka, M.; Dakowska, S.; West, J.S.; Gladders, P.; Steed, J.M.; Li, Z.Q. Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). Eur. J. Plant Pathol. 2005, 111, 263–277. [Google Scholar] [CrossRef]
- Stonard, J.F.; Marchant, B.P.; Latunde-Dada, A.O.; Liu, Z.; Evans, N.; Gladders, P.; Eckert, M.R.; Fitt, B.D.L. Geostatistical analysis of the distribution of Leptosphaeria species causing phoma stem canker on winter oilseed rape (Brassica napus) in England. Plant Pathol. 2010, 59, 200–210. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Kaczmarek, J.; Jedryczka, M. The effect of winter weather conditions on the ability of pseudothecia of Leptosphaeria maculans and L. biglobosa to release ascospores. Eur. J. Plant Pathol. 2012, 134, 329–343. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Jedryczka, M.; Cools, H.J.; Fitt, B.D.; Lucas, J.A.; Latunde-Dada, A.O. Quantitative PCR analysis of abundance of airborne propagules of Leptosphaeria species in air samples from different regions of Poland. Aerobiologia 2012, 28, 199–212. [Google Scholar] [CrossRef][Green Version]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Cozijnsen, A.J.; Howlett, B.J. Characterisation of the mating-type locus of the plant pathogenic ascomycete Leptosphaeria maculans. Curr. Genet. 2003, 43, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Barrins, J.M.; Ades, P.K.; Salisbury, P.A.; Howlett, B.J. Genetic diversity of Australian isolates of Leptosphaeria maculans, the fungus that causes blackleg of canola (Brassica napus). Australas. Plant Pathol. 2004, 33, 529–536. [Google Scholar] [CrossRef]
- Gout, L.; Eckert, M.; Rouxel, T.; Balesdent, M.H. Genetic variability and distribution of mating type alleles in field populations of Leptosphaeria maculans from France. Appl. Environ. Microbiol. 2006, 72, 185–191. [Google Scholar] [CrossRef]
- Pickard, J.E. Investigating the Distribution and Diversity of Leptosphaeria maculans in Northern Idaho. Master’s Thesis, University of Idaho, Moscow, ID, USA, December 2018. [Google Scholar]
- Dilmaghani, A.; Gout, L.; Moreno-Rico, O.; Dias, J.S.; Coudard, L.; Castillo-Torres, N.; Balesdent, M.-H.; Rouxel, T. Clonal populations of Leptosphaeria maculans contaminating cabbage in Mexico. Plant Pathol. 2013, 62, 520–532. [Google Scholar] [CrossRef]
- Lob, S. Leptosphaeria diseases of oilseed rape and swede: Identification and epidemiology, Doctoral thesis, Lincoln University, Canterbury, New Zealand. 2014. Available online: https://hdl.handle.net/10182/6592 (accessed on 7 December 2022).
- Voigt, K.; Cozijnsen, A.J.; Kroymann, J.; Pöggeler, S.; Howlett, B.J. Phylogenetic relationships between members of the crucifer pathogenic Leptosphaeria maculans species complex as shown by mating type (MAT1-2), actin, and β-tubulin sequences. Mol. Phylogenet. Evol. 2005, 37, 541–557. [Google Scholar] [CrossRef]
- Oilseed Rape Enhanced Genetic Improvement Network (OREGIN). Available online: https://www.herts.ac.uk/oregin (accessed on 7 December 2022).
- Dutreux, F.; Da Silva, C.; d’Agata, L.; Couloux, A.; Gay, E.J.; Istace, B.; Lapalu, N.; Lemainque, A.; Linglin, J.; Noel, B.; et al. De novo assembly and annotation of three Leptosphaeria genomes using Oxford Nanopore MinION sequencing. Sci. Data. 2018, 5, 180235. [Google Scholar] [CrossRef]
- Zamanmirabadi, A.; Hemmati, R.; Dolatabadian, A.; Batley, J. Genetic structure and phylogenetic relationships of Leptosphaeria maculans and L. biglobosa in Northern regions of Iran. Arch. Phytopathol. Pflanzenschutz. 2022, 55, 1062–1081. [Google Scholar] [CrossRef]
- West, J.S.; Evans, N.; Liu, S.; Hu, B.; Peng, L. Leptosphaeria maculans causing stem canker of oilseed rape in China. New Dis. Rep. 2000, 1, 3. [Google Scholar] [CrossRef][Green Version]
- Petrie, G.A. Long-term survival and sporulation of Leptosphaeria maculans (blackleg) on naturally-infected rapeseed/canola stubble in Saskatchewan. Can. Plant Dis. Surv. 1995, 75, 23–34. [Google Scholar]
| Isolates Origin | Total Isolates Tested a | MAT1-1:MAT1-2 Frequency | X2 Value | p Value |
|---|---|---|---|---|
| China: | ||||
| North (Inner Mongolia, Shaanxi) | 22 (OSR: 22) | 20:2 | 14.72 | <0.01 |
| East (Anhui, Jiangsu) | 46 (OSR: 18; BR: 28) | 23:23 | 0 | 1.00 |
| Southwest (Chongqing, Guizhou, Hubei, Hunan, Sichuan) | 89 (OSR: 56; BR: 33) | 32:57 | 7.02 | <0.01 |
| Grand total China: | 157 (OSR: 96; BR: 61) | 75:82 | 0.31 | 0.576 |
| Europe: | ||||
| Austria | 5 (OSR: 5) | 3:2 | - | - |
| France | 13 (OSR: 13) | 5:8 | 0.69 | 0.405 |
| Poland | 15 (OSR: 15) | 5:10 | 1.67 | 0.197 |
| United Kingdom | 26 (OSR: 26) | 15:11 | 0.62 | 0.433 |
| Grand total Europe: | 59 (OSR: 59) | 28:31 | 0.15 | 0.691 |
| China + Europe datasets combined: | ||||
| Grand total (China + Europe) | 216 (OSR: 155; BR: 61) | 103:113 | 0.46 | 0.496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, K.M.; Canning, G.; Zhou, K.; Liu, Z.; Wu, M.; West, J.S. Indirect Evidence Based on Mating-Type Ratios for the Role of Sexual Reproduction in European and Chinese Populations of Plenodomus biglobosus (Blackleg of Oilseed Rape). Pathogens 2023, 12, 3. https://doi.org/10.3390/pathogens12010003
King KM, Canning G, Zhou K, Liu Z, Wu M, West JS. Indirect Evidence Based on Mating-Type Ratios for the Role of Sexual Reproduction in European and Chinese Populations of Plenodomus biglobosus (Blackleg of Oilseed Rape). Pathogens. 2023; 12(1):3. https://doi.org/10.3390/pathogens12010003
Chicago/Turabian StyleKing, Kevin M., Gail Canning, Kang Zhou, Zekuan Liu, Mingde Wu, and Jonathan S. West. 2023. "Indirect Evidence Based on Mating-Type Ratios for the Role of Sexual Reproduction in European and Chinese Populations of Plenodomus biglobosus (Blackleg of Oilseed Rape)" Pathogens 12, no. 1: 3. https://doi.org/10.3390/pathogens12010003
APA StyleKing, K. M., Canning, G., Zhou, K., Liu, Z., Wu, M., & West, J. S. (2023). Indirect Evidence Based on Mating-Type Ratios for the Role of Sexual Reproduction in European and Chinese Populations of Plenodomus biglobosus (Blackleg of Oilseed Rape). Pathogens, 12(1), 3. https://doi.org/10.3390/pathogens12010003







