Triazine-Based Small Molecules: A Potential New Class of Compounds in the Antifungal Toolbox
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Construction of Mutant Strains
2.3. Growth and Minimal Inhibitory Concentration (MIC) Broth Dilution Assays
2.4. Yeast Viability Assays
2.5. Hyphal Assays
2.6. Mitochondrial Assays
3. Results and Discussion
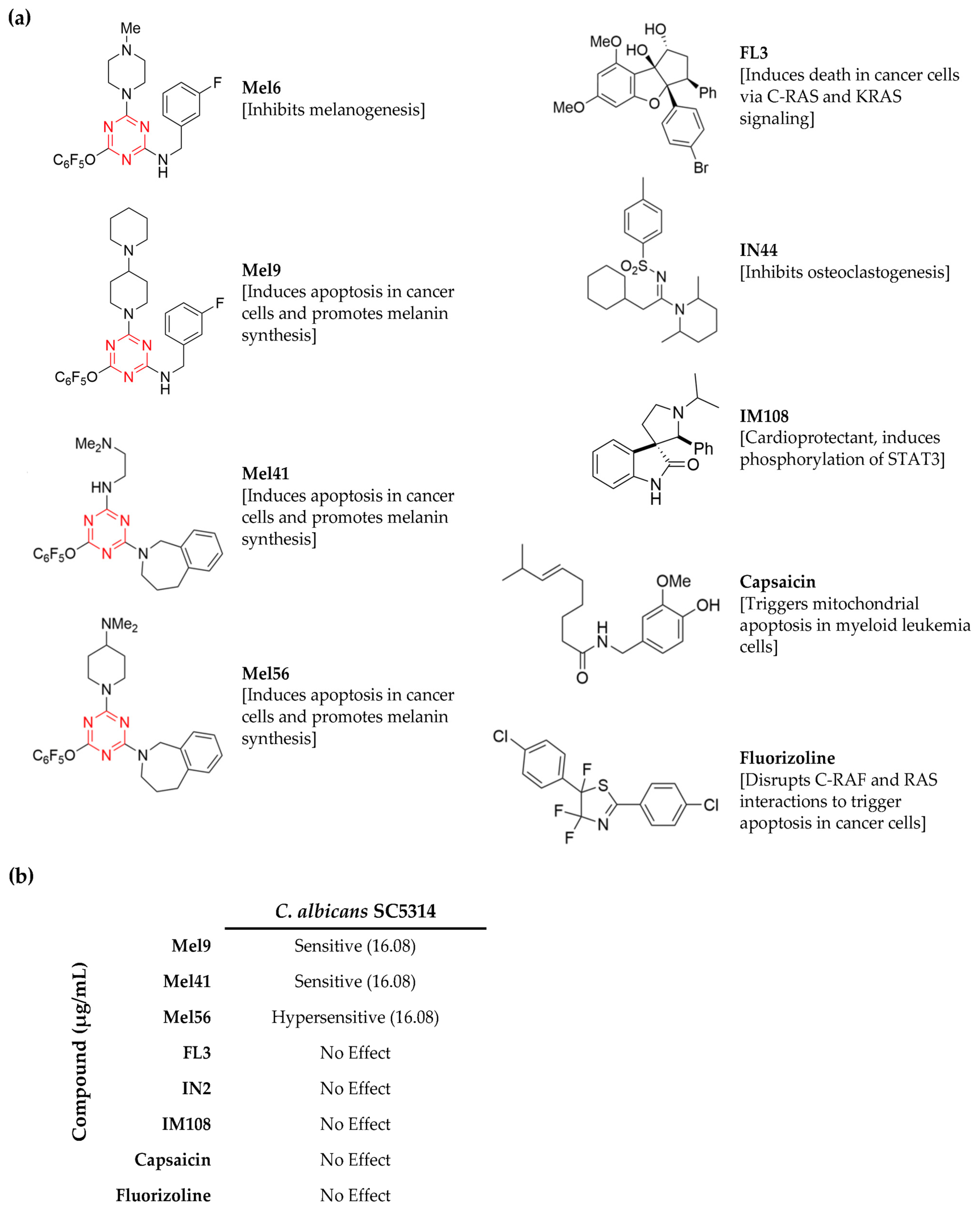
3.1. Identification of C. albicans Chemical Inhibitors
3.2. Determination of Mel56 Minimum Inhibitory Concentration
3.3. General Antifungal Activity of Prohibitin Ligands
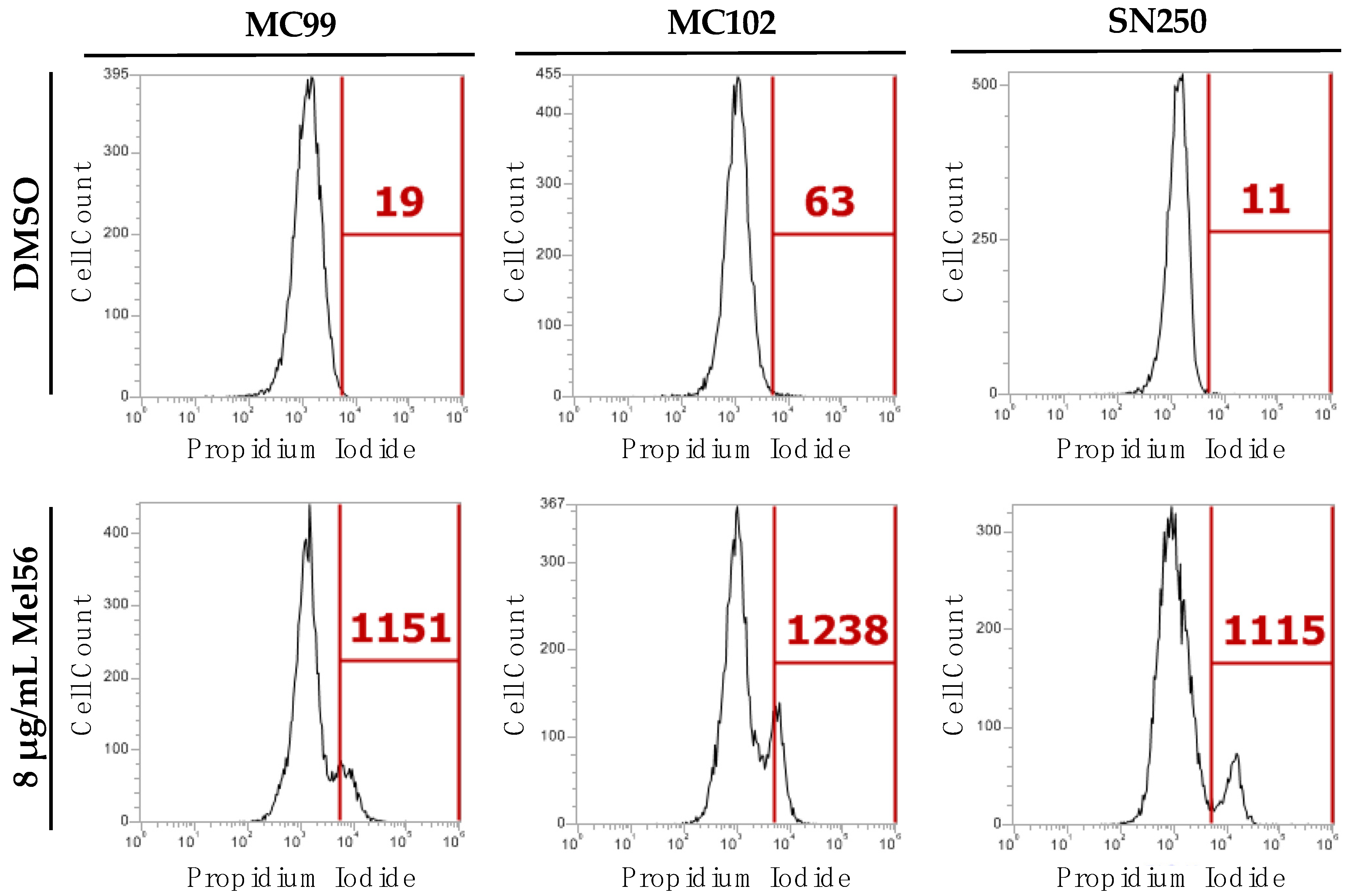
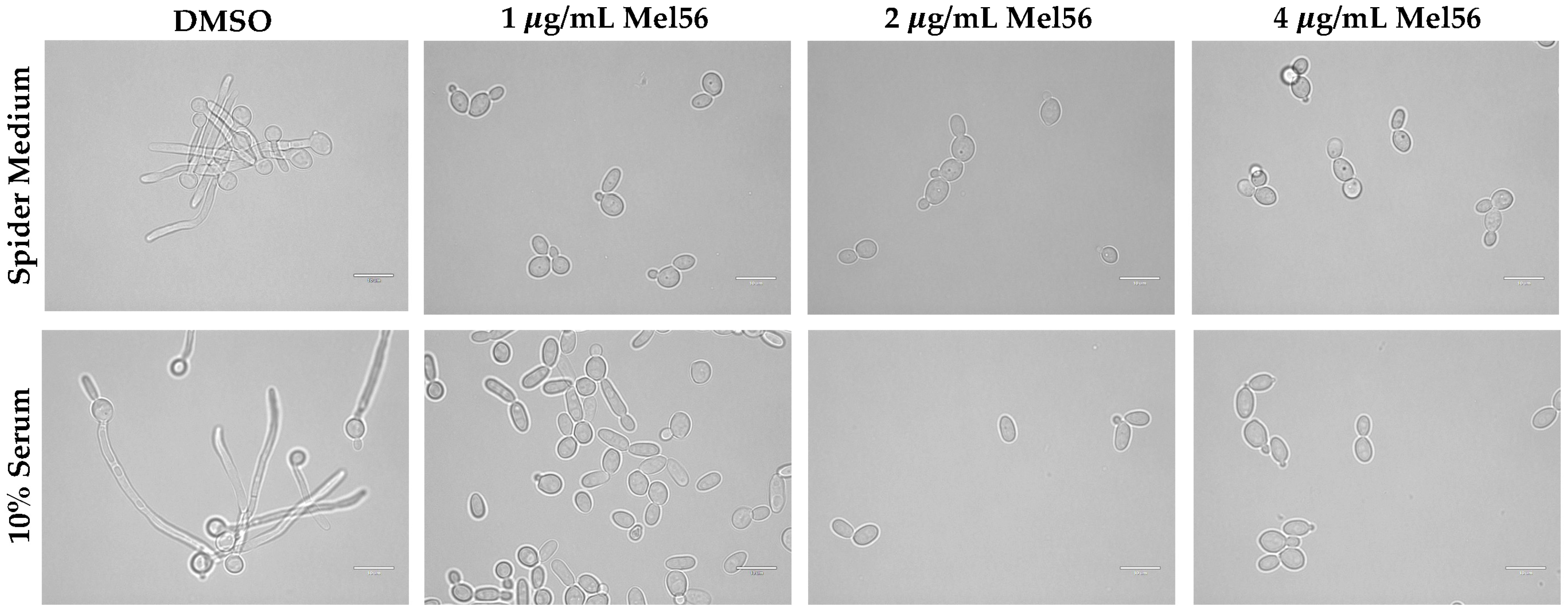
3.4. Cellular Consequences of Mel56 Treatment
3.5. Effect of Mel56 Treatment on C. albicans Prohibitin Mutants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.; Calderone, C.J.C. (Eds.) Candida and Candidiasis, 2nd ed.; ASM Press: Washington, DC, USA, 2012; p. 524. [Google Scholar]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Beattie, S.R.; Krysan, D.J. Antifungal drug screening: Thinking outside the box to identify novel antifungal scaffolds. Curr. Opin. Microbiol. 2020, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watamoto, T.; Egusa, H.; Sawase, T.; Yatani, H. Screening of Pharmacologically Active Small Molecule Compounds Identifies Antifungal Agents Against Candida Biofilms. Front. Microbiol. 2015, 6, 1453. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Hao, Y.; Liu, J.; Bao, J.; Ni, T.; Liu, Y.; Chi, X.; Wang, T.; Yu, S.; Jin, Y.; et al. Discovery of Novel Thiosemicarbazides Containing 1,3,5-Triazines Derivatives as Potential Synergists against Fluconazole-Resistant Candida albicans. Pharmaceutics 2022, 14, 2334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tabti, R.; Elderwish, S.; Djehal, A.; Chouha, N.; Pinot, F.; Yu, P.; Nebigil, C.G.; Desaubry, L. SFPH proteins as therapeutic targets for a myriad of diseases. Bioorg. Med. Chem. Lett. 2020, 30, 127600. [Google Scholar] [CrossRef]
- Wang, D.; Tabti, R.; Elderwish, S.; Abou-Hamdan, H.; Djehal, A.; Yu, P.; Yurugi, H.; Rajalingam, K.; Nebigil, C.G.; Desaubry, L. Prohibitin ligands: A growing armamentarium to tackle cancers, osteoporosis, inflammatory, cardiac and neurological diseases. Cell. Mol. Life Sci. 2020, 77, 3525–3546. [Google Scholar] [CrossRef]
- Lapatsina, L.; Brand, J.; Poole, K.; Daumke, O.; Lewin, G.R. Stomatin-domain proteins. Eur. J. Cell Biol. 2012, 91, 240–245. [Google Scholar] [CrossRef]
- Mitsopoulos, P.; Chang, Y.H.; Wai, T.; Konig, T.; Dunn, S.D.; Langer, T.; Madrenas, J. Stomatin-like protein 2 is required for in vivo mitochondrial respiratory chain supercomplex formation and optimal cell function. Mol. Cell. Biol. 2015, 35, 1838–1847. [Google Scholar] [CrossRef]
- Matz, J.M.; Goosmann, C.; Matuschewski, K.; Kooij, T.W.A. An Unusual Prohibitin Regulates Malaria Parasite Mitochondrial Membrane Potential. Cell Rep. 2018, 23, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gong, L.; Chen, L.; Xu, M.; Abou-Hamdan, H.; Tang, M.; Desaubry, L.; Song, Z. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 2020, 16, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.A.; Rodriguez, R.; Salcedo, E.C.; Rauceo, J.M. The Candida albicans stress response gene Stomatin-Like Protein 3 is implicated in ROS-induced apoptotic-like death of yeast phase cells. PLoS ONE 2018, 13, e0192250. [Google Scholar] [CrossRef]
- Mitsopoulos, P.; Lapohos, O.; Weraarpachai, W.; Antonicka, H.; Chang, Y.H.; Madrenas, J. Stomatin-like protein 2 deficiency results in impaired mitochondrial translation. PLoS ONE 2017, 12, e0179967. [Google Scholar] [CrossRef]
- Djehal, A.; Krayem, M.; Najem, A.; Hammoud, H.; Cresteil, T.; Nebigil, C.G.; Wang, D.; Yu, P.; Bentouhami, E.; Ghanem, G.E.; et al. Targeting prohibitin with small molecules to promote melanogenesis and apoptosis in melanoma cells. Eur. J. Med. Chem. 2018, 155, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Chouha, N.; Abou-Hamdan, H.; Yurugi, H.; Yoshii, R.; Ii, H.; Najem, A.; Ghanem, G.E.; Nakata, S.; Rajalingam, K.; Peng, Y.; et al. Development of fluorizoline analogues as prohibitin ligands that modulate C-RAF signaling, p21 expression and melanogenesis. Eur. J. Med. Chem. 2022, 242, 114635. [Google Scholar] [CrossRef] [PubMed]
- Kuramori, C.; Azuma, M.; Kume, K.; Kaneko, Y.; Inoue, A.; Yamaguchi, Y.; Kabe, Y.; Hosoya, T.; Kizaki, M.; Suematsu, M.; et al. Capsaicin binds to prohibitin 2 and displaces it from the mitochondria to the nucleus. Biochem. Biophys. Res. Commun. 2009, 379, 519–525. [Google Scholar] [CrossRef]
- Tabti, R.; Lamoureux, F.; Charrier, C.; Ory, B.; Heymann, D.; Bentouhami, E.; Desaubry, L. Development of prohibitin ligands against osteoporosis. Eur. J. Med. Chem. 2021, 210, 112961. [Google Scholar] [CrossRef]
- Elderwish, S.; Audebrand, A.; Nebigil, C.G.; Desaubry, L. Discovery of 3,3′-pyrrolidinyl-spirooxindoles as cardioprotectant prohibitin ligands. Eur. J. Med. Chem. 2020, 186, 111859. [Google Scholar] [CrossRef]
- Noble, S.M.; French, S.; Kohn, L.A.; Chen, V.; Johnson, A.D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590–598. [Google Scholar] [CrossRef]
- Davis, D.; Edwards, J.E., Jr.; Mitchell, A.P.; Ibrahim, A.S. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 2000, 68, 5953–5959. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Davis, D.; Mitchell, A.P. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 1999, 181, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Quail, M.M.F.; Hernday, A.D. An Efficient, Rapid, and Recyclable System for CRISPR-Mediated Genome Editing in Candida albicans. mSphere 2017, 2, e00149-17. [Google Scholar] [CrossRef] [PubMed]
- Seher, T.D.; Nguyen, N.; Ramos, D.; Bapat, P.; Nobile, C.J.; Sindi, S.S.; Hernday, A.D. AddTag, a two-step approach with supporting software package that facilitates CRISPR/Cas-mediated precision genome editing. G3 2021, 11, jkab216. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.S.; Yao, Q.H.; Peng, R.H.; Li, X.; Fan, H.Q.; Cheng, Z.M.; Li, Y. A simple, rapid, high-fidelity and cost-effective PCR-based two-step DNA synthesis method for long gene sequences. Nucleic Acids Res. 2004, 32, e98. [Google Scholar] [CrossRef] [PubMed]
- Yurugi, H.; Marini, F.; Weber, C.; David, K.; Zhao, Q.; Binder, H.; Desaubry, L.; Rajalingam, K. Targeting prohibitins with chemical ligands inhibits KRAS-mediated lung tumours. Oncogene 2017, 36, 5914. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Arendrup, M.C.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P.; Dromer, F.; et al. Definitive Document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts: Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Boussemart, L.; Malka-Mahieu, H.; Girault, I.; Allard, D.; Hemmingsson, O.; Tomasic, G.; Thomas, M.; Basmadjian, C.; Ribeiro, N.; Thuaud, F.; et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 2014, 513, 105–109. [Google Scholar] [CrossRef]
- Polier, G.; Neumann, J.; Thuaud, F.; Ribeiro, N.; Gelhaus, C.; Schmidt, H.; Giaisi, M.; Kohler, R.; Muller, W.W.; Proksch, P.; et al. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem. Biol. 2012, 19, 1093–1104. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Vautier, S.; Potrykus, J.; Walker, L.A.; Shepardson, K.M.; Hopke, A.; Mora-Montes, H.M.; Kerrigan, A.; Netea, M.G.; Murray, G.I.; et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013, 9, e1003315. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, K.; Raja Kumar, S.; Puri, S.K.; Chauhan, P.M. Synthesis of 9-anilinoacridine triazines as new class of hybrid antimalarial agents. Bioorg. Med. Chem. Lett. 2009, 19, 6996–6999. [Google Scholar] [CrossRef]
- El-Faham, A.; Farooq, M.; Almarhoon, Z.; Alhameed, R.A.; Wadaan, M.A.M.; de la Torre, B.G.; Albericio, F. Di- and tri-substituted s-triazine derivatives: Synthesis, characterization, anticancer activity in human breast-cancer cell lines, and developmental toxicity in zebrafish embryos. Bioorg. Chem. 2020, 94, 103397. [Google Scholar] [CrossRef]
- Xiong, Y.Z.; Chen, F.E.; Balzarini, J.; De Clercq, E.; Pannecouque, C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: Structural modulations of diaryltriazines with potent anti-HIV activity. Eur. J. Med. Chem. 2008, 43, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Singh, U.P.; Bhat, H.R.; Gahtori, P. Antifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivatives. J. Mycol. Med. 2012, 22, 134–141. [Google Scholar] [CrossRef]
- Haiba, N.S.; Khalil, H.H.; Moniem, M.A.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinical isolates. Bioorg. Chem. 2019, 89, 103013. [Google Scholar] [CrossRef]
- Abd Alhameed, R.; Almarhoon, Z.; NSholkamy, E.; Ali Khan, S.; Ul-Haq, Z.; Sharma, A.; G de la Torre, B.; Albericio, F.; El-Faham, A. Novel 4,6-Disubstituted s-Triazin-2-yl Amino Acid Derivatives as Promising Antifungal Agents. J. Fungi 2020, 6, 237. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Hernday, A.D.; Homann, O.R.; Deneault, J.S.; Nantel, A.; Andes, D.R.; Johnson, A.D.; Mitchell, A.P. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009, 7, e1000133. [Google Scholar] [CrossRef]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef]
- Osman, C.; Wilmes, C.; Tatsuta, T.; Langer, T. Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol. Biol. Cell 2007, 18, 627–635. [Google Scholar] [CrossRef]
- Younes, N.; Alsahan, B.S.; Al-Mesaifri, A.J.; Da’as, S.I.; Pintus, G.; Majdalawieh, A.F.; Nasrallah, G.K. JC-10 probe as a novel method for analyzing the mitochondrial membrane potential and cell stress in whole zebrafish embryos. Toxicol. Res. 2022, 11, 77–87. [Google Scholar] [CrossRef]
- Heredia, M.Y.; Rauceo, J.M. The SPFH Protein Superfamily in Fungi: Impact on Mitochondrial Function and Implications in Virulence. Microorganisms 2021, 9, 2287. [Google Scholar] [CrossRef]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, D592–D596. [Google Scholar] [CrossRef]
- Maciel, E.I.; Jiang, C.; Barghouth, P.G.; Nobile, C.J.; Oviedo, N.J. The planarian Schmidtea mediterranea is a new model to study host-pathogen interactions during fungal infections. Dev. Comp. Immunol. 2019, 93, 18–27. [Google Scholar] [CrossRef]
| Compound MIC [µg/mL] | |
|---|---|
| Yeast Strain | Mel56 |
| C. albicans SC5314 | 16 |
| C. albicans SN250 | 8 |
| C. albicans DAY185 | 8 |
| C. albicans DAY 286 | 8 |
| C. albicans MC99 | 8 |
| C. albicans MC102 | 8 |
| C. albicans 3147 | 8 |
| C. glabrata | 4 |
| C. tropicalis | 8 |
| C. parapsilosis | 8 |
| C. dubliniensis | 8 |
| S. cerevisiae BY4741 | 4 |
| S. cerevisiae BY4742 | 4 |
| S. cerevisiae W303-1A | 4 |
| S. cerevisiae W303-1B | 4 |
| Compound MIC [µg/mL] | |
|---|---|
| Yeast Strain | Mel56 |
| C. albicans phb1Δ/Δ | 8 |
| C. albicans phb2Δ/Δ | 8 |
| C. albicans phb1Δ/Δ-phb2Δ/Δ | 8 |
| C. albicans phb1Δ/Δ-phb2Δ/Δ-phb12Δ/Δ | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conrad, K.A.; Kim, H.; Qasim, M.; Djehal, A.; Hernday, A.D.; Désaubry, L.; Rauceo, J.M. Triazine-Based Small Molecules: A Potential New Class of Compounds in the Antifungal Toolbox. Pathogens 2023, 12, 126. https://doi.org/10.3390/pathogens12010126
Conrad KA, Kim H, Qasim M, Djehal A, Hernday AD, Désaubry L, Rauceo JM. Triazine-Based Small Molecules: A Potential New Class of Compounds in the Antifungal Toolbox. Pathogens. 2023; 12(1):126. https://doi.org/10.3390/pathogens12010126
Chicago/Turabian StyleConrad, Karen A., Hyunjeong Kim, Mohammad Qasim, Amel Djehal, Aaron D. Hernday, Laurent Désaubry, and Jason M. Rauceo. 2023. "Triazine-Based Small Molecules: A Potential New Class of Compounds in the Antifungal Toolbox" Pathogens 12, no. 1: 126. https://doi.org/10.3390/pathogens12010126
APA StyleConrad, K. A., Kim, H., Qasim, M., Djehal, A., Hernday, A. D., Désaubry, L., & Rauceo, J. M. (2023). Triazine-Based Small Molecules: A Potential New Class of Compounds in the Antifungal Toolbox. Pathogens, 12(1), 126. https://doi.org/10.3390/pathogens12010126








