NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model
Abstract
1. Introduction
2. Materials and Methods
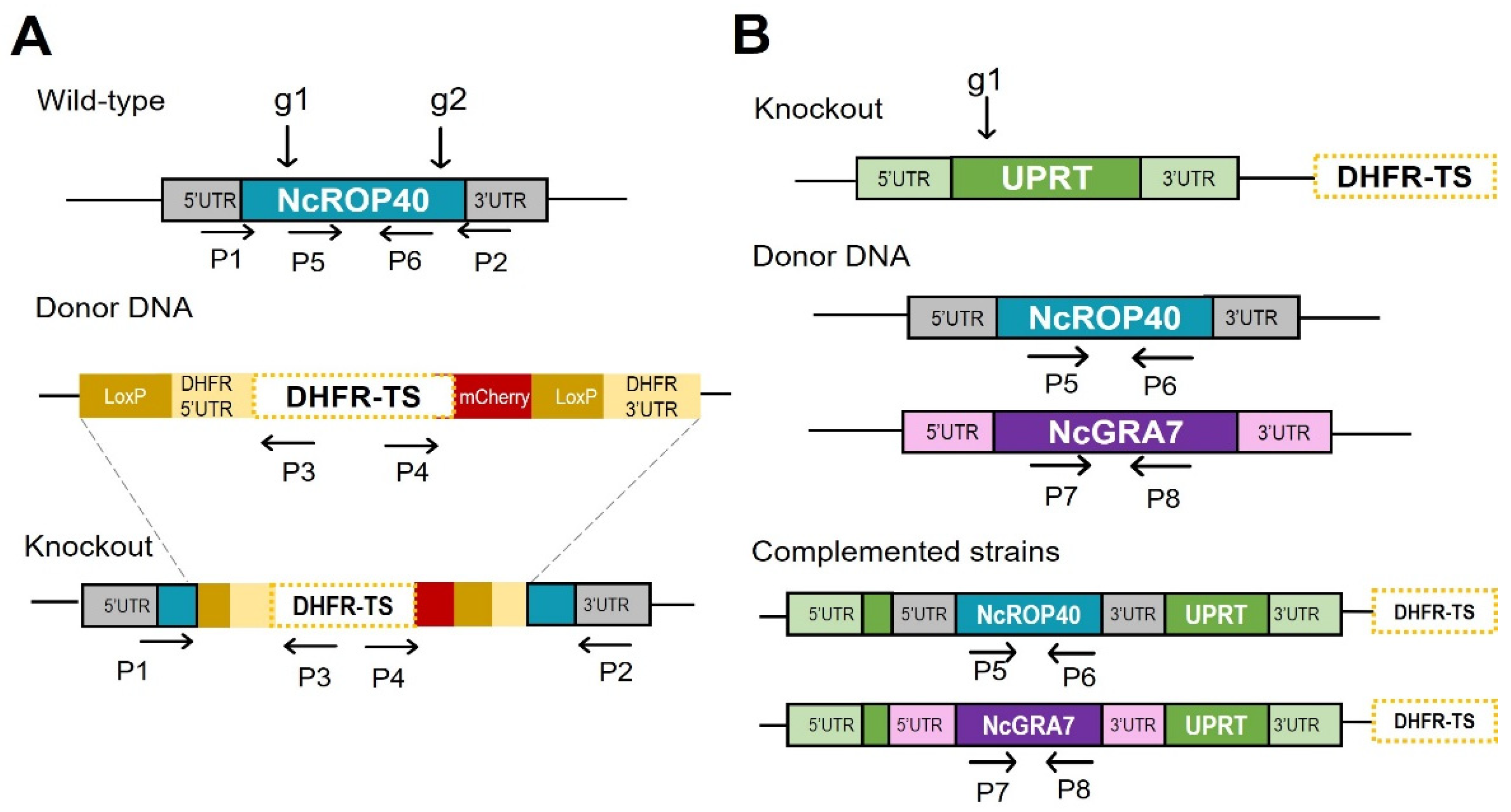
2.1. Generation of Knockout Parasites and Complemented Strains
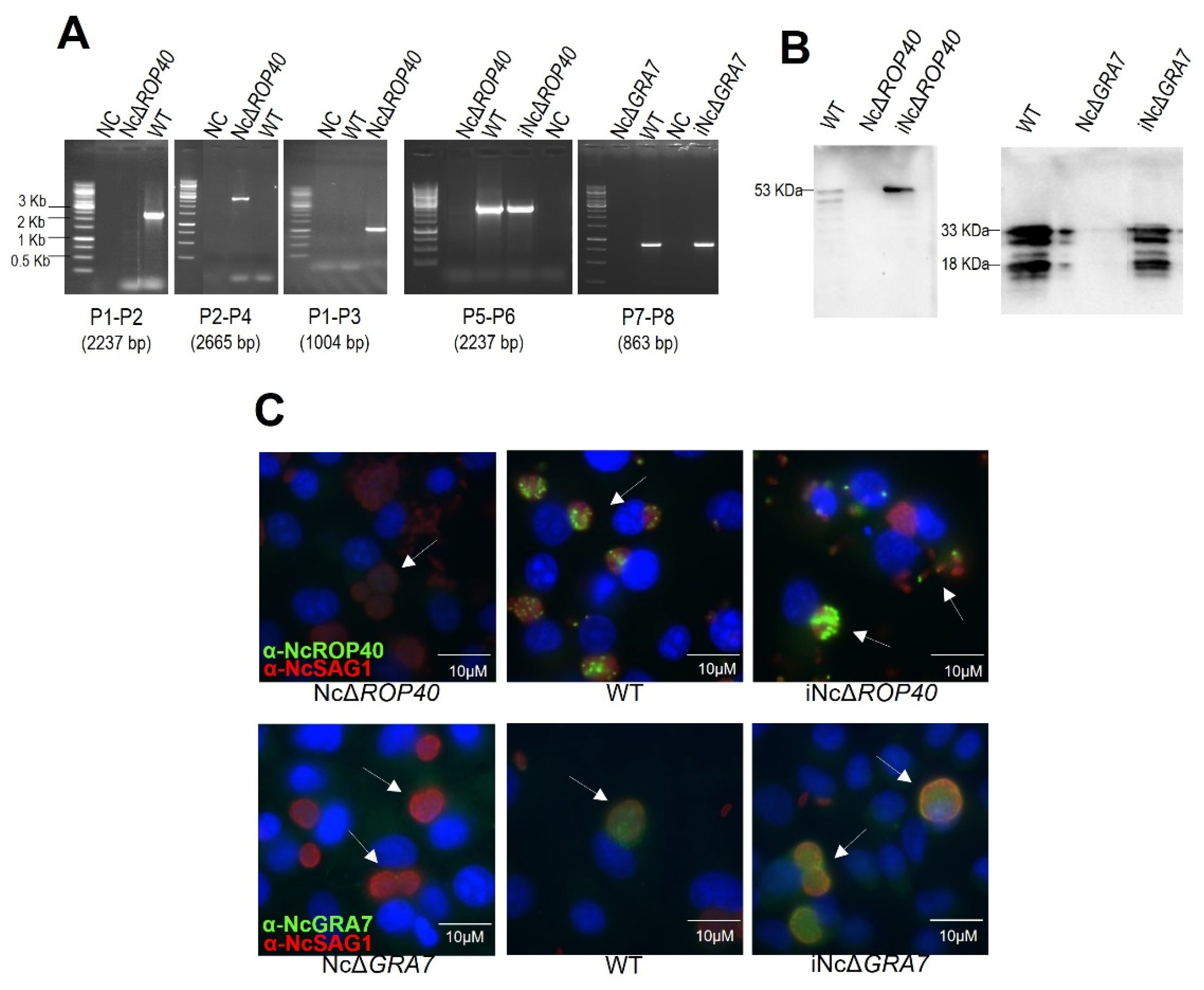
2.1.1. PCR
2.1.2. Western Blot
2.1.3. Immunostaining of NcROP40 and NcGRA7
2.2. Parasite Culture and Inoculum Preparation
2.3. Mice and Ethics Statement
2.4. Assays of Parasite Virulence in Mice
2.4.1. Parasite Detection and Quantification
2.4.2. Real-Time RT–PCR of Cytokine Expression
2.4.3. Humoral Immune Responses
2.5. Statistical Analysis
3. Results
3.1. Successful Construction of the NcROP40 Knockout and NcROP40 and NcGRA7 Complemented Strains
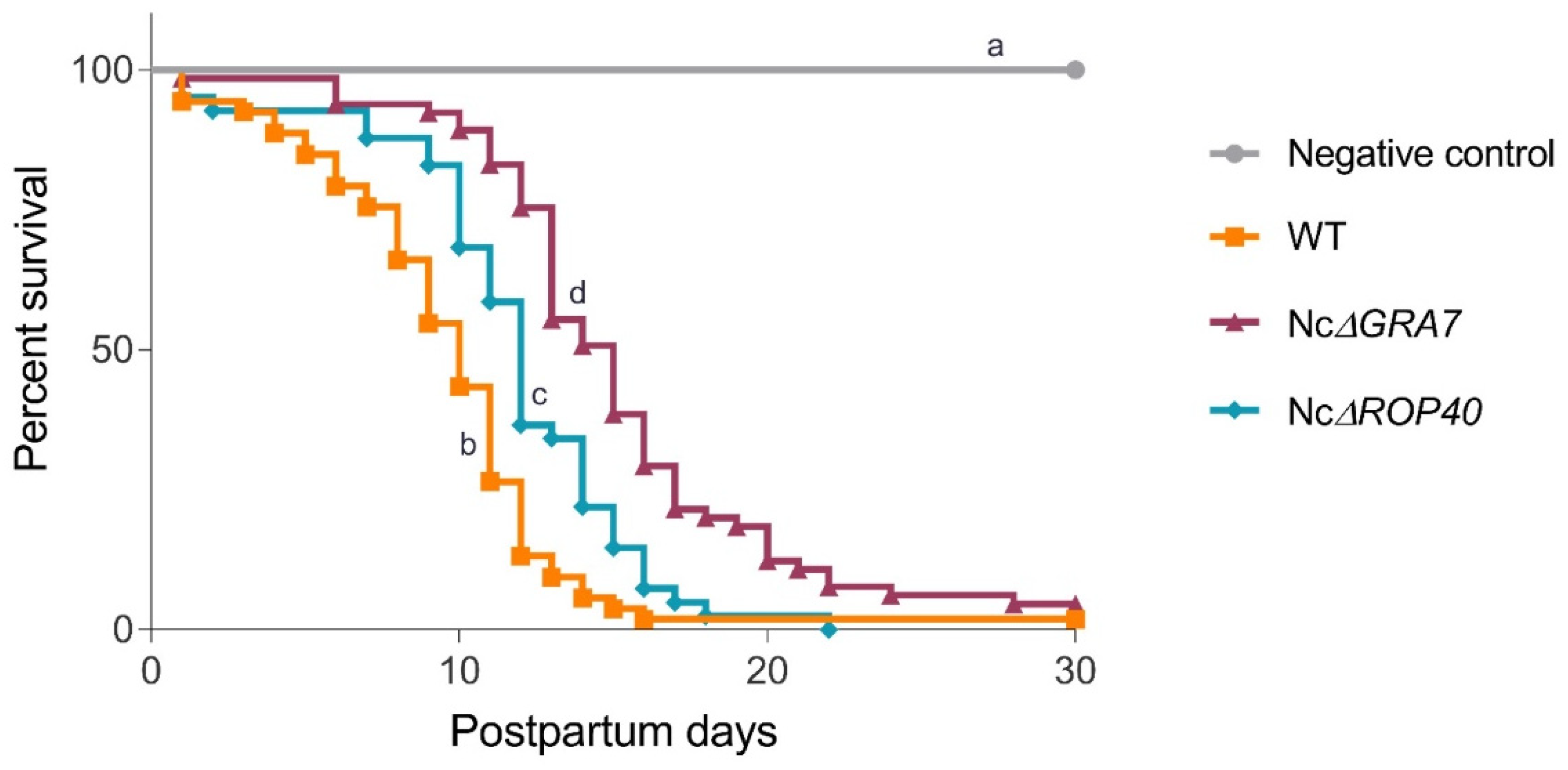
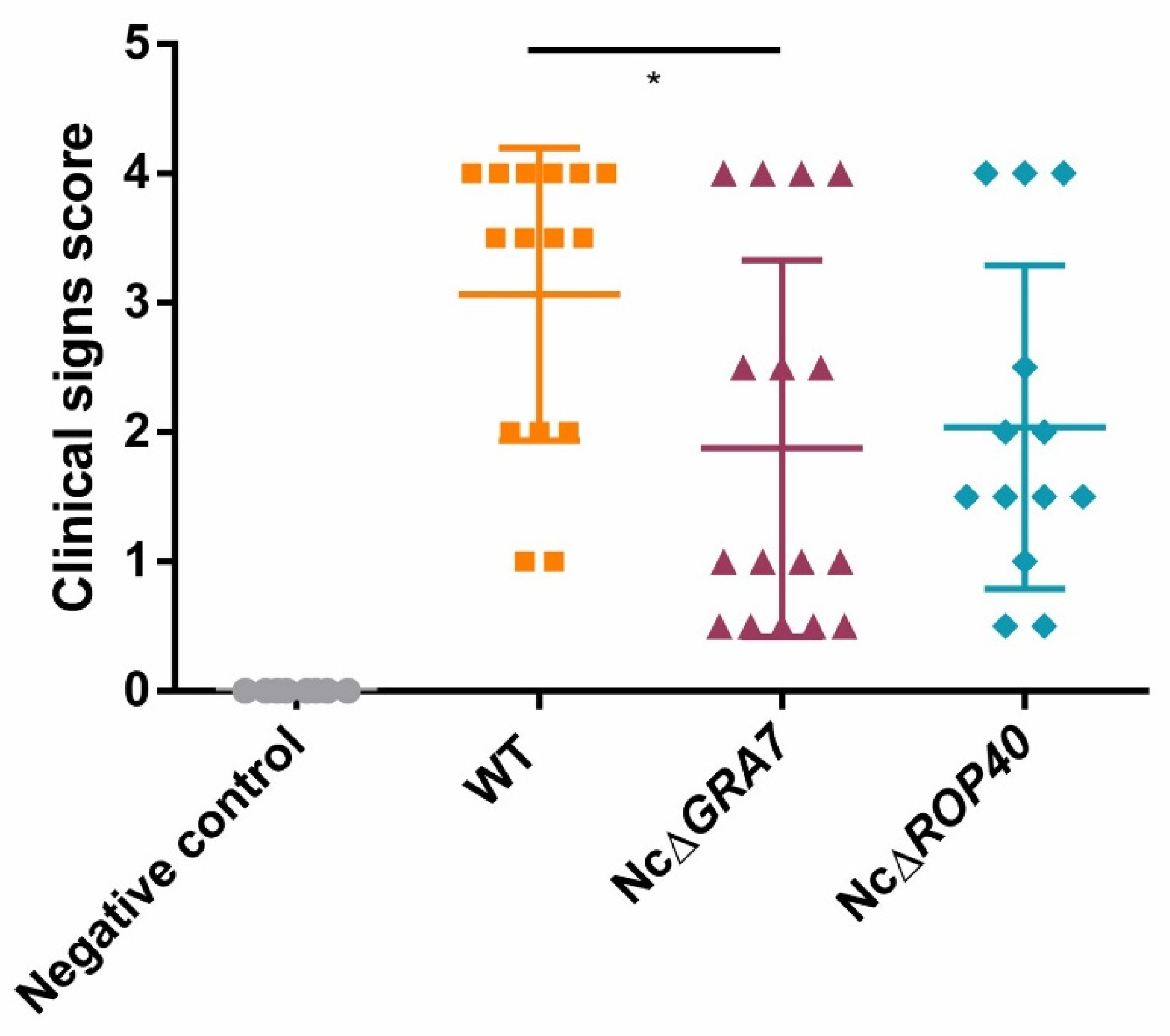
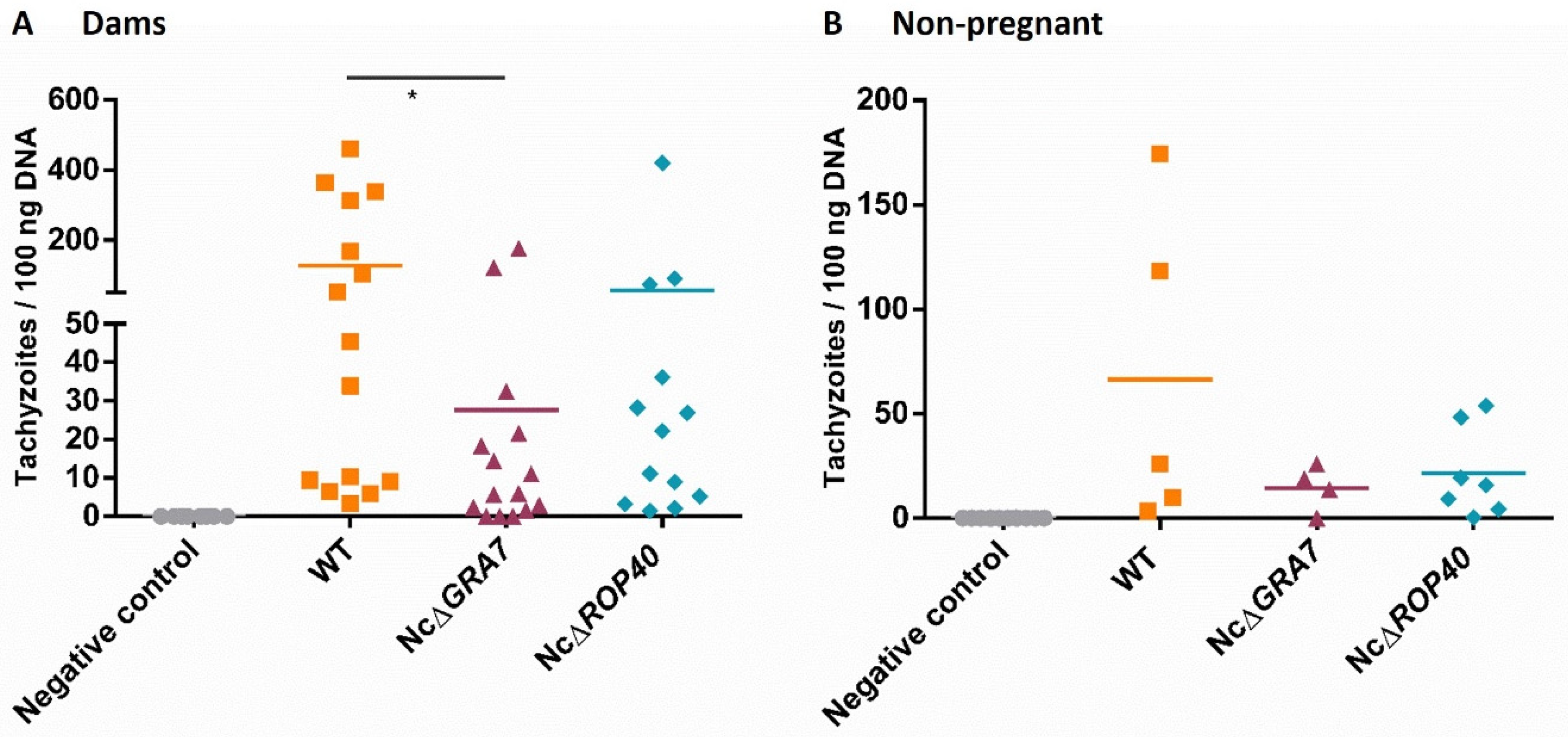
3.2. Evaluation of NcGRA7 and NcROP40 Knockout Parasite Virulence in the BALB/c Model for Congenital and Cerebral Neosporosis
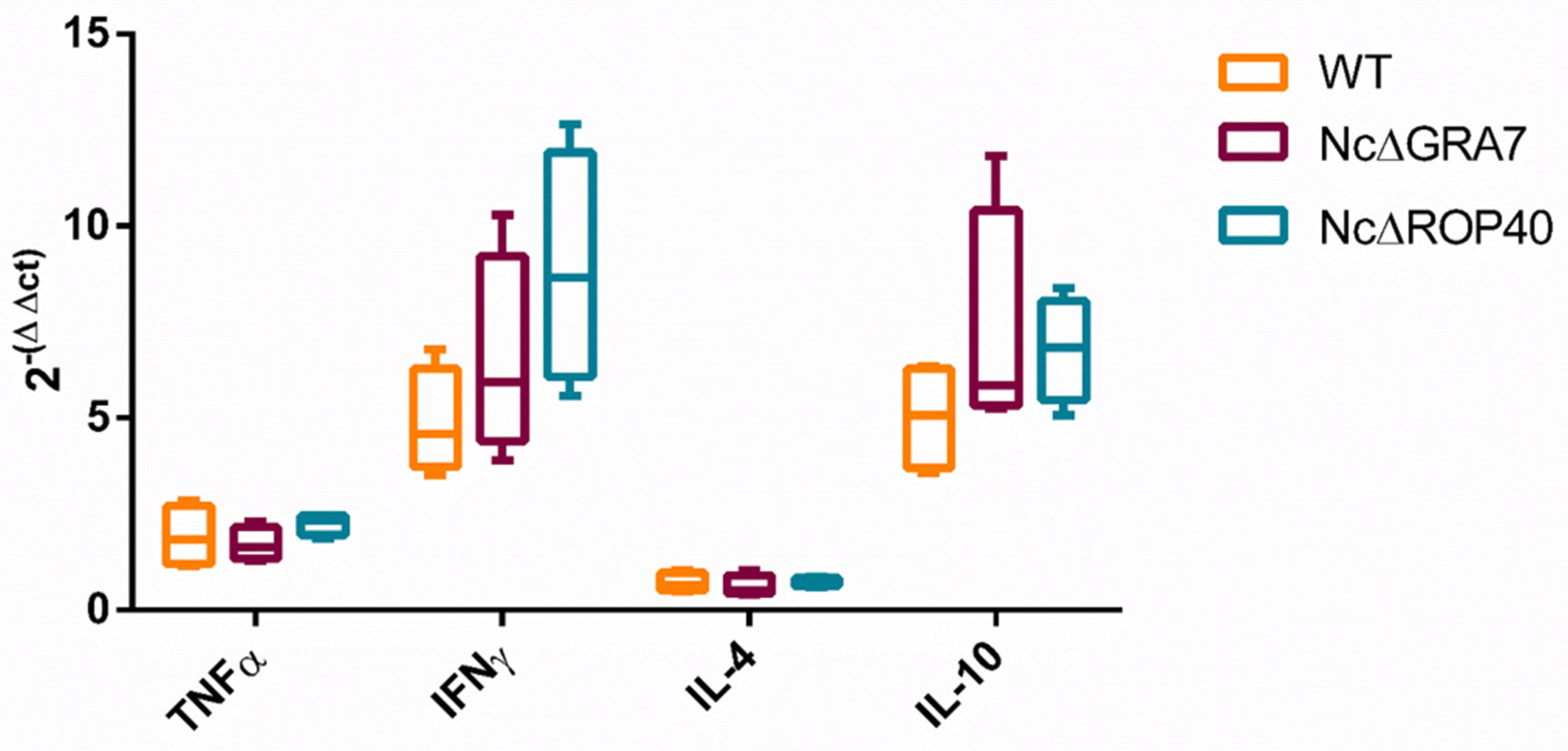
3.3. Similar Cytokine Response Induced by NcGRA7 and NcROP40 Knockout and Wild-Type Parasites during the Acute Phase of Infection
3.4. Humoral Immune Response Induced by NcGRA7 and NcROP40 Knockout and Wild-Type Parasites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Schares, G. Neosporosis in animals-The last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef]
- Reichel, M.P.; Alejandra Ayanegui-Alcérreca, M.; Gondim, L.F.P.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle—The Billion Dollar Question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Montejo, S.; Collantes-Fernández, E.; Regidor-Cerrillo, J.; Álvarez-García, G.; Marugan-Hernández, V.; Pedraza-Díaz, S.; Blanco-Murcia, J.; Prenafeta, A.; Ortega-Mora, L.M. Isolation and characterization of a bovine isolate of Neospora caninum with low virulence. Vet. Parasitol. 2009, 159, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Regidor-Cerrillo, J.; Gámez-Bautista, M.; Sodupe, I.; Aduriz, G.; Álvarez-García, G.; Del Pozo, I.; Ortega-Mora, L. In vitro invasion efficiency and intracellular proliferation rate comprise virulence-related phenotypic traits of Neospora caninum. Vet. Res. 2011, 42, 41. [Google Scholar] [CrossRef] [PubMed]
- Dellarupe, A.; Regidor-Cerrillo, J.; Jiménez-Ruiz, E.; Schares, G.; Unzaga, J.M.; Venturini, M.C.; Ortega-Mora, L.M. Comparison of host cell invasion and proliferation among Neospora caninum isolates obtained from oocysts and from clinical cases of naturally infected dogs. Exp. Parasitol. 2014, 145, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Regidor-Cerrillo, J.; Gómez-Bautista, M.; Del Pozo, I.; Jiménez-Ruiz, E.; Aduriz, G.; Ortega-Mora, L.M. Influence of Neospora caninum intra-specific variability in the outcome of infection in a pregnant BALB/c mouse model. Vet. Res. 2010, 41, 52. [Google Scholar] [CrossRef] [PubMed]
- Dellarupe, A.; Regidor-Cerrillo, J.; Jiménez-Ruiz, E.; Schares, G.; Unzaga, J.M.; Venturini, M.C.; Ortega-Mora, L.M. Clinical outcome and vertical transmission variability among canine Neospora caninum isolates in a pregnant mouse model of infection. Parasitology 2014, 141, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Montejo, S.; Collantes-Fernández, E.; Blanco-Murcia, J.; Rodríguez-Bertos, A.; Risco-Castillo, V.; Ortega-Mora, L.M. Experimental infection with a low virulence isolate of Neospora caninum at 70 days gestation in cattle did not result in foetopathy. Vet. Res. 2009, 40, 49. [Google Scholar] [CrossRef]
- Chryssafidis, A.L.; Cantón, G.; Chianini, F.; Innes, E.A.; Madureira, E.H.; Gennari, S.M. Pathogenicity of Nc-Bahia and Nc-1 strains of Neospora caninum in experimentally infected cows and buffaloes in early pregnancy. Parasitol. Res. 2014, 113, 1521–1528. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Arranz-Solís, D.; Benavides, J.; Gómez-Bautista, M.; Castro-Hermida, J.A.; Mezo, M.; Pérez, V.; Ortega-Mora, L.M.; González-Warleta, M. Neospora caninum infection during early pregnancy in cattle: How the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res. 2014, 45, 10. [Google Scholar] [CrossRef]
- Jiménez-Pelayo, L.; García-Sánchez, M.; Vázquez, P.; Regidor-Cerrillo, J.; Horcajo, P.; Collantes-Fernández, E.; Blanco-Murcia, J.; Gutiérrez-Expósito, D.; Román-Trufero, A.; Osoro, K.; et al. Early Neospora caninum infection dynamics in cattle after inoculation at mid-gestation with high (Nc-Spain7)- or low (Nc-Spain1H)-virulence isolates. Vet. Res. 2019, 50, 72. [Google Scholar] [CrossRef]
- Khan, A.; Fujita, A.W.; Randle, N.; Regidor-Cerrillo, J.; Shaik, J.S.; Shen, K.; Oler, A.J.; Quinones, M.; Latham, S.M.; Akanmori, B.D.; et al. Global selective sweep of a highly inbred genome of the cattle parasite Neospora caninum. Proc. Natl. Acad. Sci. USA 2019, 116, 22764–22773. [Google Scholar] [CrossRef]
- Calarco, L.; Barratt, J.; Ellis, J. Genome wide identification of mutational hotspots in the apicomplexan parasite Neospora caninum and the implications for virulence. Genome Biol. Evol. 2018, 10, 2417–2431. [Google Scholar] [CrossRef]
- Beck, H.P.; Blake, D.; Dardé, M.L.; Felger, I.; Pedraza-Díaz, S.; Regidor-Cerrillo, J.; Gómez-Bautista, M.; Ortega-Mora, L.M.; Putignani, L.; Shiels, B.; et al. Molecular approaches to diversity of populations of apicomplexan parasites. Int. J. Parasitol. 2009, 39, 175–189. [Google Scholar] [CrossRef]
- Adomako-Ankomah, Y.; Wier, G.M.; Borges, A.L.; Wand, H.E.; Boyle, J.P. Differential locus expansion distinguishes Toxoplasmatinae species and closely related strains of Toxoplasma gondii. MBio 2014, 5, e01003-13. [Google Scholar] [CrossRef]
- Lorenzi, H.; Khan, A.; Behnke, M.S.; Namasivayam, S.; Swapna, L.S.; Hadjithomas, M.; Karamycheva, S.; Pinney, D.; Brunk, B.P.; Ajioka, J.W.; et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun. 2016, 7, 10147. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Álvarez-García, G.; Pastor-Fernández, I.; Marugán-Hernández, V.; Gómez-Bautista, M.; Ortega-Mora, L.M. Proteome expression changes among virulent and attenuated Neospora caninum isolates. J. Proteom. 2012, 75, 2306–2318. [Google Scholar] [CrossRef]
- Horcajo, P.; Jiménez-Pelayo, L.; García-Sánchez, M.; Regidor-Cerrillo, J.; Collantes-Fernández, E.; Rozas, D.; Hambruch, N.; Pfarrer, C.; Ortega-Mora, L.M. Transcriptome modulation of bovine trophoblast cells in vitro by Neospora caninum. Int. J. Parasitol. 2017, 47, 791–799. [Google Scholar] [CrossRef]
- Horcajo, P.; Xia, D.; Randle, N.; Collantes-Fernández, E.; Wastling, J.; Ortega-Mora, L.M.; Regidor-Cerrillo, J. Integrative transcriptome and proteome analyses define marked differences between Neospora caninum isolates throughout the tachyzoite lytic cycle. J. Proteom. 2018, 180, 108–119. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Jiménez-Pelayo, L.; Horcajo, P.; Regidor-Cerrillo, J.; Collantes-Fernández, E.; Ortega-Mora, L.M. Gene expression profiling of Neospora caninum in bovine macrophages reveals differences between isolates associated with key parasite functions. Front. Cell. Infect. Microbiol. 2019, 9, 354. [Google Scholar] [CrossRef]
- Rico-San Román, L.; Horcajo, P.; Regidor-Cerrillo, J.; Fernández-Escobar, M.; Collantes-Fernández, E.; Gutiérrez-Blázquez, D.; Hernáez-Sánchez, M.L.; Saeij, J.P.J.; Ortega-Mora, L.M. Comparative tachyzoite proteome analyses among six Neospora caninum isolates with different virulence. Int. J. Parasitol. 2020, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Regidor-Cerrillo, J.; Xia, D.; Jiménez-Pelayo, L.; García-Sánchez, M.; Collantes-Fernández, E.; Randle, N.; Wastling, J.; Ortega-Mora, L.M.; Horcajo, P. Proteomic characterization of host-pathogen interactions during bovine trophoblast cell line infection by Neospora caninum. Pathogens 2020, 9, 749. [Google Scholar] [CrossRef] [PubMed]
- Dubremetz, J.; Garcia-Réguet, N.; Conseil, V.; Fourmaux, M.N. Invited Review Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 1998, 28, 1007–1013. [Google Scholar] [CrossRef]
- Dubremetz, J.F.; Lebrun, M. Virulence factors of Toxoplasma gondii. Microbes Infect. 2012, 14, 1403–1410. [Google Scholar] [CrossRef]
- English, E.D.; Adomako-Ankomah, Y.; Boyle, J.P. Secreted effectors in Toxoplasma gondii and related species: Determinants of host range and pathogenesis? Parasite Immunol. 2015, 37, 127–140. [Google Scholar] [CrossRef]
- Hakimi, M.A.; Bougdour, A. Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr. Opin. Microbiol. 2015, 26, 24–31. [Google Scholar] [CrossRef]
- Fereig, R.M.; Nishikawa, Y. From signaling pathways to distinct immune responses: Key factors for establishing or combating Neospora caninum infection in different susceptible hosts. Pathogens 2020, 9, 384. [Google Scholar] [CrossRef]
- Álvarez-García, G.; Pitarch, A.; Zaballos, A.; Fernández-García, A.; Gil, C.; Gómez-Bautista, M.; Aguado-Martínez, A.; Ortega-Mora, L.M. The NcGRA7 gene encodes the immunodominant 17 KDa antigen of Neospora caninum. Parasitology 2007, 134, 41–50. [Google Scholar] [CrossRef]
- Aguado-Martínez, A.; Álvarez-García, G.; Schares, G.; Risco-Castillo, V.; Fernández-García, A.; Marugán-Hernández, V.; Ortega-Mora, L.M. Characterisation of NcGRA7 and NcSAG4 proteins: Immunolocalisation and their role in the host cell invasion by Neospora caninum tachyzoites. Acta Parasitol. 2010, 55, 304–312. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Wang, J.; Li, F.; Yang, J.; Gong, P.; Li, H.; Zhang, X. Identification and characterization of GRA6/GRA7 of Neospora caninum in MDBK cells. Acta Biochim. Biophys. Sin. 2017, 49, 361–366. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shimoda, N.; Fereig, R.M.; Moritaka, T.; Umeda, K.; Nishimura, M.; Ihara, F.; Kobayashi, K.; Himori, Y.; Suzuki, Y.; et al. Neospora caninum dense granule protein 7 regulates the pathogenesis of neosporosis by modulating host immune response. Appl. Environ. Microbiol. 2018, 84, e01350-18. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, X.; Ma, L.; Yang, J.; Liu, Q.; Liu, J. Function of Neospora caninum dense granule protein 7 in innate immunity in mice. Parasitol. Res. 2021, 120, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Ikeda, R.; Watanabe, K.; Furuoka, H.; Nishikawa, Y. Role of dense granule antigen 7 in vertical transmission of Neospora caninum in C57BL/6 mice infected during early pregnancy. Parasitol. Int. 2022, 89, 102576. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Fernández, I.; Regidor-Cerrillo, J.; Jiménez-Ruiz, E.; Álvarez-Garciá, G.; Marugán-Hernández, V.; Hemphill, A.; Ortega-Mora, L.M. Characterization of the Neospora caninum NcROP40 and NcROP2Fam-1 rhoptry proteins during the tachyzoite lytic cycle. Parasitology 2015, 143, 97–113. [Google Scholar] [CrossRef]
- Ma, L.; Liu, G.; Liu, J.; Li, M.; Zhang, H.; Tang, D.; Liu, Q. Neospora caninum ROP16 play an important role in the pathogenicity by phosphorylating host cell STAT3. Vet. Parasitol. 2017, 243, 135–147. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Li, M.; Fu, Y.; Zhang, X.; Liu, Q. Rhoptry protein 5 (ROP5) is a key virulence factor in Neospora caninum. Front. Microbiol. 2017, 8, 370. [Google Scholar] [CrossRef][Green Version]
- López-Pérez, I.C.; Collantes-Fernández, E.; Aguado-Martínez, A.; Rodríguez-Bertos, A.; Ortega-Mora, L.M. Influence of Neospora caninum infection in BALB/c mice during pregnancy in post-natal development. Vet. Parasitol. 2008, 155, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Solís, D.; Aguado-Martínez, A.; Müller, J.; Regidor-Cerrillo, J.; Ortega-Mora, L.M.; Hemphill, A. Dose-dependent effects of experimental infection with the virulent Neospora caninum Nc-Spain7 isolate in a pregnant mouse model. Vet. Parasitol. 2015, 211, 133–140. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Regidor-Cerrillo, J.; Lourido, S.; Ortega-Mora, L.M.; Saeij, J.P.J. Toxoplasma CRISPR/Cas9 constructs are functional for gene disruption in Neospora caninum. Int. J. Parasitol. 2018, 48, 597–600. [Google Scholar] [CrossRef]
- Sidik, S.M.; Hackett, C.G.; Tran, F.; Westwood, N.J.; Lourido, S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS ONE 2014, 9, e100450. [Google Scholar] [CrossRef]
- Björkman, C.; Hemphill, A. Characterization of Neospora caninum iscom antigens using monoclonal antibodies. Parasite Immunol. 1998, 20, 73–80. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Regidor-Cerrillo, J.; Álvarez-García, G.; Marugán-Hernández, V.; García-Lunar, P.; Hemphill, A.; Ortega-Mora, L.M. The tandemly repeated NTPase (NTPDase) from Neospora caninum is a canonical dense granule protein whose RNA expression, protein secretion and phosphorylation coincides with the tachyzoite egress. Parasites Vectors 2016, 9, 352. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Arranz-Solís, D.; Regidor-Cerrillo, J.; Álvarez-García, G.; Hemphill, A.; García-Culebras, A.; Cuevas-Martín, C.; Ortega-Mora, L.M. A vaccine formulation combining rhoptry proteins NcROP40 and NcROP2 improves pup survival in a pregnant mouse model of neosporosis. Vet. Parasitol. 2015, 207, 203–215. [Google Scholar] [CrossRef]
- Collantes-Fernández, E.; Zaballos, Á.; Álvarez-García, G.; Ortega-Mora, L.M. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J. Clin. Microbiol. 2002, 40, 1194–1198. [Google Scholar] [CrossRef]
- Varona, R.; Cadenas, V.; Gómez, L.; Martínez-A, C.; Márquez, G. CCR6 Regulates CD4+ T-cell–mediated acute graft-versus-host disease responses. Blood 2005, 106, 18–26. [Google Scholar] [CrossRef]
- López-Pérez, I.C.; Collantes-Fernández, E.; Rojo-Montejo, S.; Navarro-Lozano, V.; Risco-Castillo, V.; Pérez-Pérez, V.; Pereira-Bueno, J.; Ortega-Mora, L.M. Effects of Neospora caninum infection at mid-gestation on placenta in a pregnant mouse model. J. Parasitol. 2010, 96, 1017–1020. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hemphill, A.; Debache, K.; Monney, T.; Schorer, M.; Guionaud, C.; Alaeddine, F.; Müller, N.; Müller, J. Proteins mediating the Neospora caninum-host cell interaction as targets for vaccination. Front. Biosci. 2013, E5, E593. [Google Scholar] [CrossRef]
- Bradley, P.J.; Sibley, L.D. Rhoptries: An arsenal of secreted virulence factors. Curr. Opin. Microbiol. 2007, 10, 582–587. [Google Scholar] [CrossRef]
- Boothroyd, J.C.; Dubremetz, J.F. Kiss and Spit: The dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008, 6, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M.; Zhang, Y. Toxoplasma gondii secretory proteins and their role in invasion and pathogenesis. Microbiol. Res. 2019, 227, 126293. [Google Scholar] [CrossRef] [PubMed]
- Cesbron-Delauw, M.-F.; Lecordier, L.; Mercier, C. Role of secretory dense granule organelles in the pathogenesis of toxoplasmosis. In Toxoplasma Gondii; Springer: Berlin/Heidelberg, Germany, 1996; pp. 59–65. [Google Scholar]
- Yang, C.; Liu, J.; Ma, L.; Zhang, X.; Zhang, X.; Zhou, B.; Zhu, X.; Liu, Q. NcGRA17 is an important regulator of parasitophorous vacuole morphology and pathogenicity of Neospora caninum. Vet. Parasitol. 2018, 264, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, N.; Dong, J.; Li, J.; Wang, X.; Li, X.; Li, X.; Yang, J.; Gong, P.; Zhang, X. Effects of dense granular protein 6 (GRA6) disruption on Neospora caninum virulence. Front. Vet. Sci. 2020, 7, 562730. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, N.; Zhao, P.; Li, J.; Cao, L.; Wang, X.; Li, X.; Yang, J.; Zhang, X.; Gong, P. Disruption of dense granular protein 2 (GRA2) decreases the virulence of Neospora caninum. Front. Vet. Sci. 2021, 8, 634612. [Google Scholar] [CrossRef]
- Vemulapalli, R.; Sanakkayala, N.; Gulani, J.; Schurig, G.G.; Boyle, S.M.; Lindsay, D.S.; Sriranganathan, N. Reduced cerebral infection of Neospora caninum in BALB/c mice vaccinated with recombinant Brucella abortus RB51 strains expressing N. caninum SRS2 and GRA7 proteins. Vet. Parasitol. 2007, 148, 219–230. [Google Scholar] [CrossRef]
- Aguado-Martínez, A.; Álvarez-García, G.; Fernández-García, A.; Risco-Castillo, V.; Marugán-Hernández, V.; Ortega-Mora, L.M. Failure of a vaccine using immunogenic recombinant proteins rNcSAG4 and rNcGRA7 against neosporosis in mice. Vaccine 2009, 27, 7331–7338. [Google Scholar] [CrossRef]
- Lv, Q.; Xing, S.; Gong, P.; Chang, L.; Bian, Z.; Wang, L.; Zhang, X.; Li, J. A 78 KDa Host cell invasion protein of Neospora caninum as a potential vaccine candidate. Exp. Parasitol. 2015, 148, 56–65. [Google Scholar] [CrossRef]
- Collantes-Fernández, E.; Álvarez-García, G.; Pérez-Pérez, V.; Pereira-Bueno, J.; Ortega-Mora, L.M. Characterization of Pathology and Parasite Load in Outbred and Inbred Mouse Models of Chronic Neospora caninum Infection. J. Parasitol. 2004, 90, 579–583. [Google Scholar] [CrossRef]
- Collantes-Fernández, E.; López-Pérez, I.; Álvarez-García, G.; Ortega-Mora, L.M. Temporal distribution and parasite load kinetics in blood and tissues during Neospora caninum infection in mice. Infect. Immun. 2006, 74, 2491–2494. [Google Scholar] [CrossRef]
- Long, M.T.; Baszler, T.V.; Mathison, B.A. Comparison of Intracerebral Parasite Load, Lesion Development, and Systemic Cytokines in Mouse Strains Infected with Neospora caninum. J. Parasitol. 1998, 84, 316–320. [Google Scholar] [CrossRef]
- Kano, R.; Masukata, Y.; Omata, Y.; Kobayashi, Y.; Maeda, R.; Saito, A. Relationship between type 1/type 2 immune responses and occurrence of vertical transmission in BALB/c mice infected with Neospora caninum. Vet. Parasitol. 2005, 129, 159–164. [Google Scholar] [CrossRef]
- Quinn, H.E.; Miller, C.M.D.; Ellis, J.T. The cell-mediated immune response to Neospora caninum during pregnancy in the mouse is associated with a bias towards production of interleukin-4. Int. J. Parasitol. 2004, 34, 723–732. [Google Scholar] [CrossRef]
- Pereira García-Melo, D.; Regidor-Cerrillo, J.; Collantes-Fernández, E.; Aguado-Martínez, A.; Del Pozo, I.; Minguijn, E.; Gómez-Bautista, M.; Aduriz, G.; Ortega-Mora, L.M. Pathogenic characterization in mice of Neospora caninum isolates obtained from asymptomatic calves. Parasitology 2010, 137, 1057–1068. [Google Scholar] [CrossRef]
- Marugán-Hernández, V.; Ortega-Mora, L.M.; Aguado-Martínez, A.; Álvarez-García, G. Genetic manipulation of Neospora caninum to express the bradyzoite-specific protein NcSAG4 in tachyzoites. Parasitology 2011, 138, 472–480. [Google Scholar] [CrossRef]
- Bartley, P.M.; Wright, S.; Sales, J.; Chianini, F.; Buxton, D.; Innes, E.A. Long-term passage of tachyzoites in tissue culture can attenuate virulence of Neospora caninum in vivo. Parasitology 2006, 133, 421–432. [Google Scholar] [CrossRef]
- Hermanns, T.; Müller, U.B.; Könen-Waisman, S.; Howard, J.C.; Steinfeldt, T. The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell. Microbiol. 2016, 18, 244–259. [Google Scholar] [CrossRef]
- Alaganan, A.; Fentress, S.J.; Tang, K.; Wang, Q.; Sibley, L.D. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc. Natl. Acad. Sci. USA 2014, 111, 1126–1131. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.T.; Elsheikha, H.M.; Chen, K.; Zhu, W.N.; Yue, D.M.; Zhu, X.Q.; Huang, S.Y. Functional characterization of rhoptry kinome in the virulent Toxoplasma gondii RH strain. Front. Microbiol. 2017, 8, 84. [Google Scholar] [CrossRef]
- Fox, B.A.; Rommereim, L.M.; Guevara, R.B.; Falla, A.; Hortua Triana, M.A.; Sun, Y.; Bzik, D.J. The Toxoplasma gondii rhoptry kinome is essential for chronic infection. MBio 2016, 7, e00193-16. [Google Scholar] [CrossRef]
- Liew, F.Y. TH1 and TH2 cells: A historical perspective. Nat. Rev. Immunol. 2002, 2, 55–60. [Google Scholar] [CrossRef]
- Long, M.T.; Baszler, T.V. Neutralization of maternal IL-4 modulates congenital protozoal transmission: Comparison of innate versus acquired immune responses. J. Immunol. 2000, 164, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Vonlaufen, N.; Naguleswaran, A. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology 2006, 133, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Quinn, H.E.; Miller, C.M.D.; Ryce, C.; Windsor, P.A.; Ellis, J.T. Characterization of an outbred pregnant mouse model of Neospora caninum infection. J. Parasitol. 2002, 88, 691–696. [Google Scholar] [CrossRef]
- Baszler, T.V.; Long, M.T.; Mcelwain, T.F.; Mathison, B.A. Interferon-g and Interleukin-12 Mediate Protection to Acute Neospora caninum Infection in BALB/c Mice. Int. J. Parasitol. 1999, 29, 1635–1646. [Google Scholar] [CrossRef]

| Purpose | Primer Name | Sequence | Used to |
|---|---|---|---|
| NcROP40 disruption | ROP40 gRNA1 | 5′ GATATTTGCACTCGAATGCT 3′ | Disrupt ROP40 gene in 5′ and 3′ terminal ends |
| ROP40 gRNA2 | 5′ GTCGCGGTGCGTTCAGTGGTG 3′ | ||
| ROP40 Fw (P1) | 5′ TAAGAACGCATGGCTGACTG 3′ | Amplify and sequence the gRNA targeted region of the ROP40 gene together with donor template | |
| ROP40 Rv (P2) | 5′ CGGTTCGGACAAAACGTATAC 3′ | ||
| DHFR Fw (P4) | 5′ GGCGTGAAGATCTGGGACAA 3′ | Amplify and sequence the gRNA targeted region of the ROP40 gene together with DHFR–TS donor template | |
| DHFR Rv (P3) | 5′ GCCTGGTATCTTTATAGTCC 3′ | ||
| NcROP40 and NcGRA7 complementation | UPRT gRNA | 5′ GCAGGAGGAAAGCATTCTGC 3′ | Disrupt UPRT gene in 5′ terminal end |
| KpnI_ROP40 Fw | 5′ AGTCGAggtaccGAGTGCATGAGGGAGTTCAAG 3′ | Amplify the ROP40 gene with its promoters for cloning it into the pUC19-plasmid; the restriction sequence is shown in lower case | |
| KpnI_ROP40 Rv | 5′ TCGACTggtaccGCAGTCAGAACCACGTTTTCC 3′ | ||
| iROP40 Fw (P5) | 5′ TAAGAACGCATGGCTGACTG 3′ | Amplify and sequence the ROP40 complemented gene donor template | |
| iROP40 Rv2 (P6) | 5′ CGGTTCGGACAAAACGTATA 3′ | ||
| KpnI_GRA7 Fw | 5′ AGTCGAggtaccAAACACAGGTTCGTTCCTGCC 3′ | Amplify the GRA7 gene with its promoters for cloning it into the pUC19-plasmid; the restriction sequence is shown in lower case | |
| KpnI_GRA7 Rv | 5′ TCGACTggtaccTCGAAGCAGAGAGAAGCTTC 3′ | ||
| iGRA7 5UTR Fw (P7) | 5′ TCGCTGTTCCTGTAGGCTTT 3′ | Amplify and sequence the GRA7 complemented gene donor template | |
| iGRA7 3UTR Rv (P8) | 5′ CTGTCATCTGGGACACGAAA 3′ |
| Group | Fertility Rate a | Litter Size b | Mortality of Dams c | Parasite Presence in Dams’ Brains d | Early Pup Mortality e | Neonatal Mortality f | Median Survival Time of Pups g |
|---|---|---|---|---|---|---|---|
| Negative control | 8/20 | 5.38 ± 2.13 | 0/8 | 0/8 | 2/42 | 0/40 | Undefined |
| (40%) | (0%) | (0%) | (4.8%) | (0%) | |||
| WT | 15/20 | 4.64 ± 2.10 | 7/15 | 15/15 | 14/65 | 50/51 | 10 days |
| (75%) | (46.6%) | (100%) | (21.5%) | (98%) | |||
| NcΔGRA7 | 16/20 | 4.69 ± 1.74 | 4/16 | 13/16 | 11/75 | 61/64 | 15 days |
| (80%) | (25%) | (81.25%) | (14.7%) | (95.3%) | |||
| NcΔROP40 | 13/20 | 3.54 ± 1.61 | 3/13 | 13/13 | 8/46 | 38/38 | 12 days |
| (65%) | (23.1%) | (100%) | (17.4%) | (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-San Román, L.; Amieva, R.; Regidor-Cerrillo, J.; García-Sánchez, M.; Collantes-Fernández, E.; Pastor-Fernández, I.; Saeij, J.P.J.; Ortega-Mora, L.M.; Horcajo, P. NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model. Pathogens 2022, 11, 998. https://doi.org/10.3390/pathogens11090998
Rico-San Román L, Amieva R, Regidor-Cerrillo J, García-Sánchez M, Collantes-Fernández E, Pastor-Fernández I, Saeij JPJ, Ortega-Mora LM, Horcajo P. NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model. Pathogens. 2022; 11(9):998. https://doi.org/10.3390/pathogens11090998
Chicago/Turabian StyleRico-San Román, Laura, Rafael Amieva, Javier Regidor-Cerrillo, Marta García-Sánchez, Esther Collantes-Fernández, Iván Pastor-Fernández, Jeroen P. J. Saeij, Luis Miguel Ortega-Mora, and Pilar Horcajo. 2022. "NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model" Pathogens 11, no. 9: 998. https://doi.org/10.3390/pathogens11090998
APA StyleRico-San Román, L., Amieva, R., Regidor-Cerrillo, J., García-Sánchez, M., Collantes-Fernández, E., Pastor-Fernández, I., Saeij, J. P. J., Ortega-Mora, L. M., & Horcajo, P. (2022). NcGRA7 and NcROP40 Play a Role in the Virulence of Neospora caninum in a Pregnant Mouse Model. Pathogens, 11(9), 998. https://doi.org/10.3390/pathogens11090998






