A Portable Diagnostic Assay, Genetic Diversity, and Isolation of Seoul Virus from Rattus norvegicus Collected in Gangwon Province, Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Mitochondrial DNA Analysis
2.4. Indirect Immunofluorescence Assay (IFA)
2.5. Reverse Transcription-PCR (RT-PCR)
2.6. qPCR
2.7. Multiplex-PCR-Based NGS
2.8. Rapid Amplification of cDNA Ends (RACE) PCR
2.9. Phylogenetic Analysis
2.10. Cell Lines
2.11. Virus Isolation
2.12. Plaque Assay
2.13. Statistical Analysis
3. Results
3.1. Serological and Molecular Prevalence of SEOV
3.2. Comparison between Handheld and Benchtop qPCR for SEOV
3.3. Whole-Genome Sequencing of SEOV
3.4. Phylogenetic Analysis of SEOV
3.5. Virus Isolation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaheri, A.; Henttonen, H.; Voutilainen, L.; Mustonen, J.; Sironen, T.; Vapalahti, O. Hantavirus infections in Europe and their impact on public health. Rev. Med. Virol. 2013, 23, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Lin, X.D.; Wang, W.; Tian, J.H.; Cong, M.L.; Zhang, H.L.; Wang, M.R.; Zhou, R.H.; Wang, J.B.; Li, M.H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.-K.; Lee, S.-H.; Kim, J.; Lee, J.; Cho, S.; Lee, G.-Y.; No, J.S.; Lee, K.H.; Song, J.-W. A novel genotype of Hantaan orthohantavirus harbored by Apodemus agrarius chejuensis as a potential etiologic agent of hemorrhagic fever with renal syndrome in Republic of Korea. PLoS Negl. Trop. Dis. 2021, 15, e0009400. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.H.; Figueiredo, L.T.M.; Song, J.-W.; Klempa, B. Hantaviruses—Globally emerging pathogens. J. Clin. Virol. 2015, 64, 128–136. [Google Scholar] [CrossRef]
- Kabwe, E.; Davidyuk, Y.; Shamsutdinov, A.; Garanina, E.; Martynova, E.; Kitaeva, K.; Malisheni, M.; Isaeva, G.; Savitskaya, T.; Urbanowicz, R.A.; et al. Orthohantaviruses, Emerging Zoonotic Pathogens. Pathogens 2020, 9, 775. [Google Scholar] [CrossRef]
- Hart, C.A.; Bennett, M. Hantavirus infections: Epidemiology and pathogenesis. Microbes Infect. 1999, 1, 1229–1237. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, C.; Han, J.S.; Kim, S.; Lee, J.S.; Lee, P.W. Hemorrhagic fever with renal syndrome caused by the Seoul virus. Nephron 1995, 71, 419–427. [Google Scholar] [CrossRef]
- Clement, J.; LeDuc, J.W.; Lloyd, G.; Reynes, J.M.; McElhinney, L.; Van Ranst, M.; Lee, H.W. Wild Rats, Laboratory Rats, Pet Rats: Global Seoul Hantavirus Disease Revisited. Viruses 2019, 11, 652. [Google Scholar] [CrossRef]
- Roig, I.L.; Musher, D.M.; Tweardy, D.J. Severe pulmonary involvement in a case attributed to domestically acquired Seoul hantavirus in the United States. Clin. Infect. Dis 2012, 54, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Woods, C.; Palekar, R.; Kim, P.; Blythe, D.; de Senarclens, O.; Feldman, K.; Farnon, E.C.; Rollin, P.E.; Albarino, C.G.; Nichol, S.T.; et al. Domestically acquired seoul virus causing hemorrhagic fever with renal syndrome-Maryland, 2008. Clin. Infect. Dis 2009, 49, e109–e112. [Google Scholar] [CrossRef] [PubMed]
- Shastri, B.; Kofman, A.; Hennenfent, A.; Klena, J.D.; Nicol, S.; Graziano, J.C.; Morales-Betoulle, M.; Cannon, D.; Maradiaga, A.; Tran, A.; et al. Domestically Acquired Seoul Virus Causing Hemophagocytic Lymphohistiocytosis-Washington, DC, 2018. Open Forum Infect. Dis. 2019, 6, ofz404. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, J.; Yamanouchi, T.; Dohmae, K.; Miyamoto, H.; Takahaski, M.; Yamanishi, K.; Kurata, T.; Lee, H.W. Control of laboratory acquired hemorrhagic fever with renal syndrome (HFRS) in Japan. Lab. Anim. Sci. 1987, 37, 431–436. [Google Scholar] [PubMed]
- Lloyd, G.; Jones, N. Infection of laboratory workers with hantavirus acquired from immunocytomas propagated in laboratory rats. J. Infect. 1986, 12, 117–125. [Google Scholar] [CrossRef]
- Swanink, C.; Reimerink, J.; Gisolf, J.; de Vries, A.; Claassen, M.; Martens, L.; Waegemaekers, T.; Rozendaal, H.; Valkenburgh, S.; Hoornweg, T.; et al. Autochthonous Human Case of Seoul Virus Infection, the Netherlands. Emerg. Infect. Dis. 2018, 24, 2158–2163. [Google Scholar] [CrossRef]
- Kerins, J.L.; Koske, S.E.; Kazmierczak, J.; Austin, C.; Gowdy, K.; Dibernardo, A.; Group, C.S.V.I.; Group, C.S.V.I.; Group, S.V.W. Outbreak of Seoul virus among rats and rat owners—United States and Canada, 2017. Morb. Mortal. Wkly. Rep. 2018, 67, 131. [Google Scholar] [CrossRef]
- Jameson, L.J.; Logue, C.H.; Atkinson, B.; Baker, N.; Galbraith, S.E.; Carroll, M.W.; Brooks, T.; Hewson, R. The continued emergence of hantaviruses: Isolation of a Seoul virus implicated in human disease, United Kingdom, October 2012. Euro Surveill. 2013, 18, 4–7. [Google Scholar] [CrossRef]
- Mace, G.; Feyeux, C.; Mollard, N.; Chantegret, C.; Audia, S.; Rebibou, J.M.; Spagnolo, G.; Bour, J.B.; Denoyel, G.A.; Sagot, P.; et al. Severe Seoul hantavirus infection in a pregnant woman, France, October 2012. Euro Surveill. 2013, 18, 20464. [Google Scholar] [CrossRef]
- Li, Y.; Cazelles, B.; Yang, G.; Laine, M.; Huang, Z.X.Y.; Cai, J.; Tan, H.; Stenseth, N.C.; Tian, H. Intrinsic and extrinsic drivers of transmission dynamics of hemorrhagic fever with renal syndrome caused by Seoul hantavirus. PLoS Negl. Trop. Dis. 2019, 13, e0007757. [Google Scholar] [CrossRef]
- Marx, V. PCR heads into the field. Nat. Methods 2015, 12, 393–397. [Google Scholar] [CrossRef]
- Thomas, A.C.; Tank, S.; Nguyen, P.L.; Ponce, J.; Sinnesael, M.; Goldberg, C.S. A system for rapid eDNA detection of aquatic invasive species. Environ. DNA 2020, 2, 261–270. [Google Scholar] [CrossRef]
- Daigle, J.; Onyilagha, C.; Truong, T.; Le, V.P.; Nga, B.T.T.; Nguyen, T.L.; Clavijo, A.; Ambagala, A. Rapid and highly sensitive portable detection of African swine fever virus. Transbound Emerg. Dis. 2021, 68, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.; Nfon, C. Foot-and-mouth disease virus detection on a handheld real-time polymerase chain reaction platform. Transbound Emerg. Dis. 2019, 66, 1789–1795. [Google Scholar] [CrossRef]
- Figueroa, D.M.; Kuisma, E.; Matson, M.J.; Ondzie, A.U.; Bushmaker, T.; Seifert, S.N.; Ntoumi, F.; Escudero-Pérez, B.; Muñoz-Fontela, C.; Walzer, C. Development and validation of portable, field-deployable Ebola virus point-of-encounter diagnostic assay for wildlife surveillance. One Health Outlook 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Onyilagha, C.; Mistry, H.; Marszal, P.; Pinette, M.; Kobasa, D.; Tailor, N.; Berhane, Y.; Nfon, C.; Pickering, B.; Mubareka, S. Evaluation of mobile real-time polymerase chain reaction tests for the detection of severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Ettinger, J.; Hofmann, J.; Enders, M.; Tewald, F.; Oehme, R.M.; Rosenfeld, U.M.; Ali, H.S.; Schlegel, M.; Essbauer, S.; Osterberg, A.; et al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg Infect. Dis. 2012, 18, 1461–1464. [Google Scholar] [CrossRef]
- Song, J.W.; Moon, S.S.; Gu, S.H.; Song, K.J.; Baek, L.J.; Kim, H.C.; Kijek, T.; O’Guinn, M.L.; Lee, J.S.; Turell, M.J.; et al. Hemorrhagic fever with renal syndrome in 4 US soldiers, South Korea, 2005. Emerg Infect. Dis. 2009, 15, 1833–1836. [Google Scholar] [CrossRef]
- Kim, H.-C.; Kim, W.-K.; No, J.S.; Lee, S.-H.; Gu, S.H.; Chong, S.-T.; Klein, T.A.; Song, J.-W. Urban Rodent Surveillance, Climatic Association, and Genomic Characterization of Seoul Virus Collected at US Army Garrison, Seoul, Republic of Korea, 2006–2010. Am. J. Trop. Med. Hyg. 2018, 99, 470. [Google Scholar] [CrossRef]
- Kim, W.K.; No, J.S.; Lee, S.H.; Song, D.H.; Lee, D.; Kim, J.A.; Gu, S.H.; Park, S.; Jeong, S.T.; Kim, H.C.; et al. Multiplex PCR-Based Next-Generation Sequencing and Global Diversity of Seoul Virus in Humans and Rats. Emerg Infect. Dis. 2018, 24, 249–257. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, W.K.; Park, K.; Lee, S.H.; Hwang, J.; No, J.S.; Cho, S.; Lee, D.; Song, D.H.; Gu, S.H.; et al. Phylogeographic diversity and hybrid zone of Hantaan orthohantavirus collected in Gangwon Province, Republic of Korea. PLoS Negl. Trop. Dis. 2020, 14, e0008714. [Google Scholar] [CrossRef]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Kim, W.-K.; Klein, T.A.; Chong, S.-T.; Nunn, P.V.; Kim, J.-A.; Lee, S.-H.; No, J.S.; Song, J.-W. Hantavirus surveillance and genetic diversity targeting small mammals at Camp Humphreys, a US military installation and new expansion site, Republic of Korea. PLoS ONE 2017, 12, e0176514. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Yasuda, S.P.; Yoshimatsu, K.; Koma, T.; Shimizu, K.; Endo, R.; Isozumi, R.; Arikawa, J. Application of truncated nucleocapsid protein (N) for serotyping ELISA of murinae-associated hantavirus infection in rats. J. Vet. Med. Sci. 2012, 74, 215–219. [Google Scholar] [CrossRef]
- Lee, H.W.; Baek, L.J.; Johnson, K.M. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever, from wild urban rats. J. Infect. Dis. 1982, 146, 638–644. [Google Scholar] [CrossRef]
- Dupinay, T.; Pounder, K.C.; Ayral, F.; Laaberki, M.H.; Marston, D.A.; Lacote, S.; Rey, C.; Barbet, F.; Voller, K.; Nazaret, N.; et al. Detection and genetic characterization of Seoul virus from commensal brown rats in France. Virol. J. 2014, 11, 32. [Google Scholar] [CrossRef]
- Hu, D.; Hao, L.; Zhang, J.; Yao, P.; Zhang, Q.; Lv, H.; Gong, X.; Pan, X.; Cao, M.; Zhu, J. Development of reverse transcription loop-mediated isothermal amplification assays to detect Hantaan virus and Seoul virus. J. Virol. Methods 2015, 221, 68–73. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Liu, H.; Lin, F.; Cai, X.; Sun, T.; Chang, G.; Zhu, Q.; Yang, Y. A duplex real-time reverse transcriptase polymerase chain reaction assay for detecting western equine and eastern equine encephalitis viruses. Virol. J. 2010, 7, 284. [Google Scholar] [CrossRef]
- Huhtamo, E.; Hasu, E.; Uzcategui, N.Y.; Erra, E.; Nikkari, S.; Kantele, A.; Vapalahti, O.; Piiparinen, H. Early diagnosis of dengue in travelers: Comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J. Clin. Virol. 2010, 47, 49–53. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol Infect. 2004, 10, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.; Kerner, A.; Drobni, P.; Bucht, G.; Evander, M.; Ahlm, C. Detection of Puumala and Rift Valley Fever virus by quantitative RT-PCR and virus viability tests in samples of blood dried and stored on filter paper. J. Virol. Methods 2011, 178, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewicz Brown, A.; McAloose, D.; Calle, P.P.; Auer, A.; Posautz, A.; Slavinski, S.; Brennan, R.; Walzer, C.; Seimon, T.A. Development and validation of a portable, point-of-care canine distemper virus qPCR test. PLoS ONE 2020, 15, e0232044. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef]
- Houldcroft, C.J.; Beale, M.A.; Breuer, J. Clinical and biological insights from viral genome sequencing. Nat. Rev. Microbiol. 2017, 15, 183–192. [Google Scholar] [CrossRef]
- Carroll, M.W.; Matthews, D.A.; Hiscox, J.A.; Elmore, M.J.; Pollakis, G.; Rambaut, A.; Hewson, R.; Garcia-Dorival, I.; Bore, J.A.; Koundouno, R.; et al. Temporal and spatial analysis of the 2014-2015 Ebola virus outbreak in West Africa. Nature 2015, 524, 97–101. [Google Scholar] [CrossRef]
- De Jesus, J.G.; Giovanetti, M.; Rodrigues Faria, N.; Alcantara, L.C.J. Acute Vector-Borne Viral Infection: Zika and MinION Surveillance. Microbiol. Spectr. 2019, 7, 4. [Google Scholar] [CrossRef]
- Perez-Sautu, U.; Gu, S.H.; Caviness, K.; Song, D.H.; Kim, Y.J.; Paola, N.D.; Lee, D.; Klein, T.A.; Chitty, J.A.; Nagle, E.; et al. A Model for the Production of Regulatory Grade Viral Hemorrhagic Fever Exposure Stocks: From Field Surveillance to Advanced Characterization of SFTSV. Viruses 2020, 12, 958. [Google Scholar] [CrossRef]
- Page, A.J.; Mather, A.E.; Le-Viet, T.; Meader, E.J.; Alikhan, N.F.; Kay, G.L.; de Oliveira Martins, L.; Aydin, A.; Baker, D.J.; Trotter, A.J.; et al. Large-scale sequencing of SARS-CoV-2 genomes from one region allows detailed epidemiology and enables local outbreak management. Microb. Genom. 2021, 7, 6. [Google Scholar] [CrossRef]
- Kim, W.-K.; Kim, J.-A.; Song, D.H.; Lee, D.; Kim, Y.C.; Lee, S.-Y.; Lee, S.-H.; No, J.S.; Kim, J.H.; Kho, J.H. Phylogeographic analysis of hemorrhagic fever with renal syndrome patients using multiplex PCR-based next generation sequencing. Sci. Rep. 2016, 6, 1–8. [Google Scholar]
- Kim, W.K.; No, J.S.; Lee, D.; Jung, J.; Park, H.; Yi, Y.; Kim, J.A.; Lee, S.H.; Kim, Y.; Park, S.; et al. Active Targeted Surveillance to Identify Sites of Emergence of Hantavirus. J. Infect. Dis. 2020, 70, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Cho, S.; Lee, S.H.; No, J.S.; Lee, G.Y.; Park, K.; Lee, D.; Jeong, S.T.; Song, J.W. Genomic Epidemiology and Active Surveillance to Investigate Outbreaks of Hantaviruses. Front. Cell Infect. Microbiol. 2020, 10, 532388. [Google Scholar] [CrossRef] [PubMed]
- Solà-Riera, C.; Gupta, S.; Ljunggren, H.-G.; Klingström, J. Orthohantaviruses belonging to three phylogroups all inhibit apoptosis in infected target cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Vulin, J.; Murri, S.; Madrieres, S.; Galan, M.; Tatard, C.; Piry, S.; Vaccari, G.; D’Agostino, C.; Charbonnel, N.; Castel, G.; et al. Isolation and Genetic Characterization of Puumala Orthohantavirus Strains from France. Pathogens 2021, 10, 349. [Google Scholar] [CrossRef]
- Song, G.; Hang, C.; Liao, H.; Qiu, X.; Gao, G.; Du, Y.; Zhao, J.; Xu, J.; Kong, B. Isolation of EHF-related agent from Rattus norvegicus captured from patients’ home in endemic areas of the mild type of hemorrhagic fever. Acta Microbiol. Sin. 1982, 22, 372–377. [Google Scholar]
- KITAMURA, T.; KOMATSU, T.; SUGIYAMA, K.; MORITA, C.; IMAIZUMI, K.; SHIGA, S.; AKAO, Y.; OYA, A.; TAKEDA, H.; ARIKAWA, J. Isolation of virus causing hemorrhagic fever with renal syndrome (HFRS) through a cell culture system. Jpn. J. Med. Sci. Biol. 1983, 36, 17–25. [Google Scholar] [CrossRef]
- Lee, H.W. Global distribution and molecular biological characteristics of Hantaviruses. J. Bacteriol. Virol. 1986, 16, 1–5. [Google Scholar]
- Pilaski, J.; Ellerich, C.; Kreutzer, T.; Lang, A.; Benik, W.; Pohl-Koppe, A.; Bode, L.; Vanek, E.; Autenrieth, I.B.; Bigos, K.; et al. Haemorrhagic fever with renal syndrome in Germany. Lancet 1991, 337, 111. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Smith, G.A.; Johnson, K.M. Hantaan-like viruses from domestic rats captured in the United States. Am. J. Trop. Med. Hyg. 1984, 33, 992–998. [Google Scholar] [CrossRef]
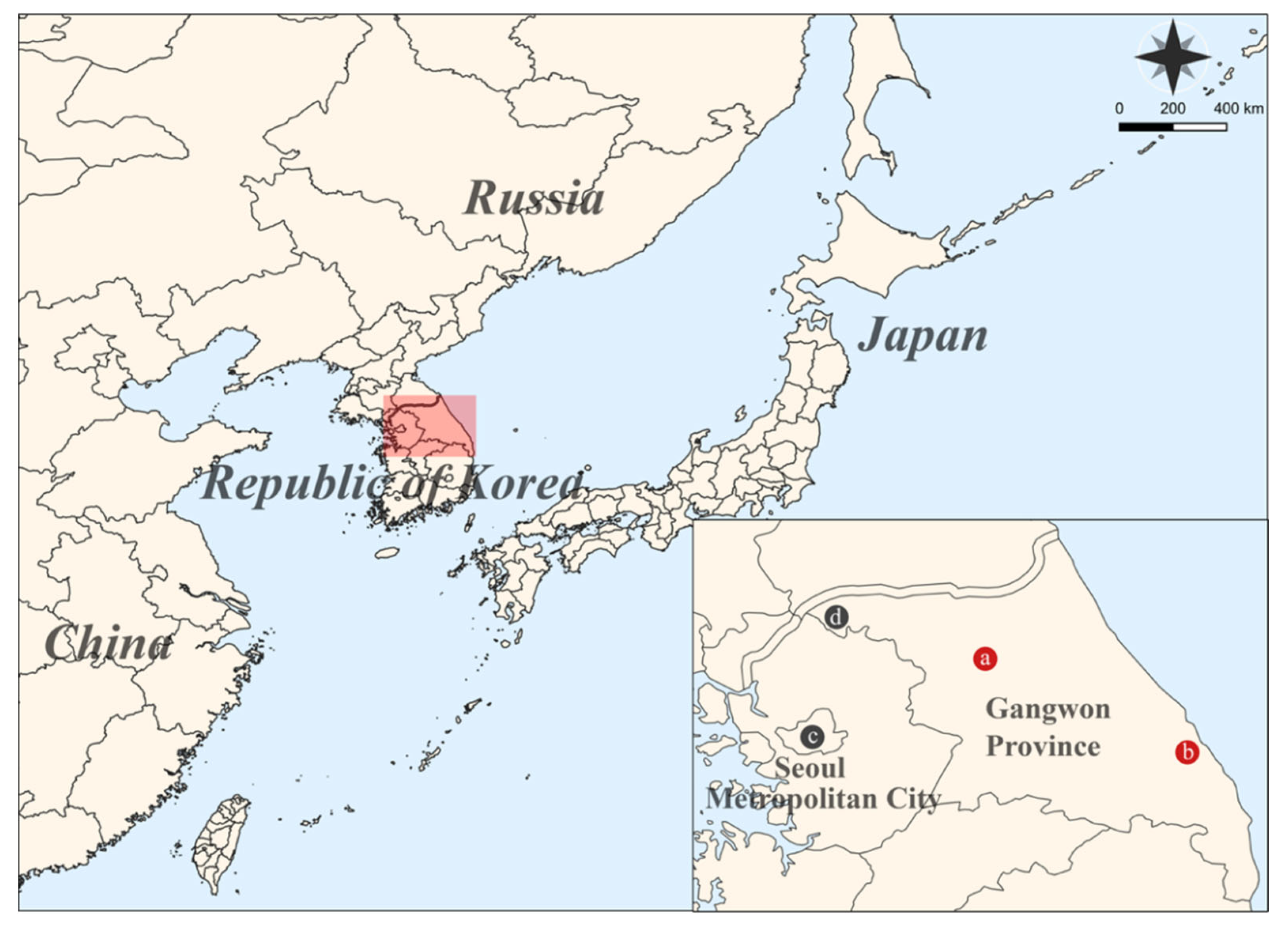
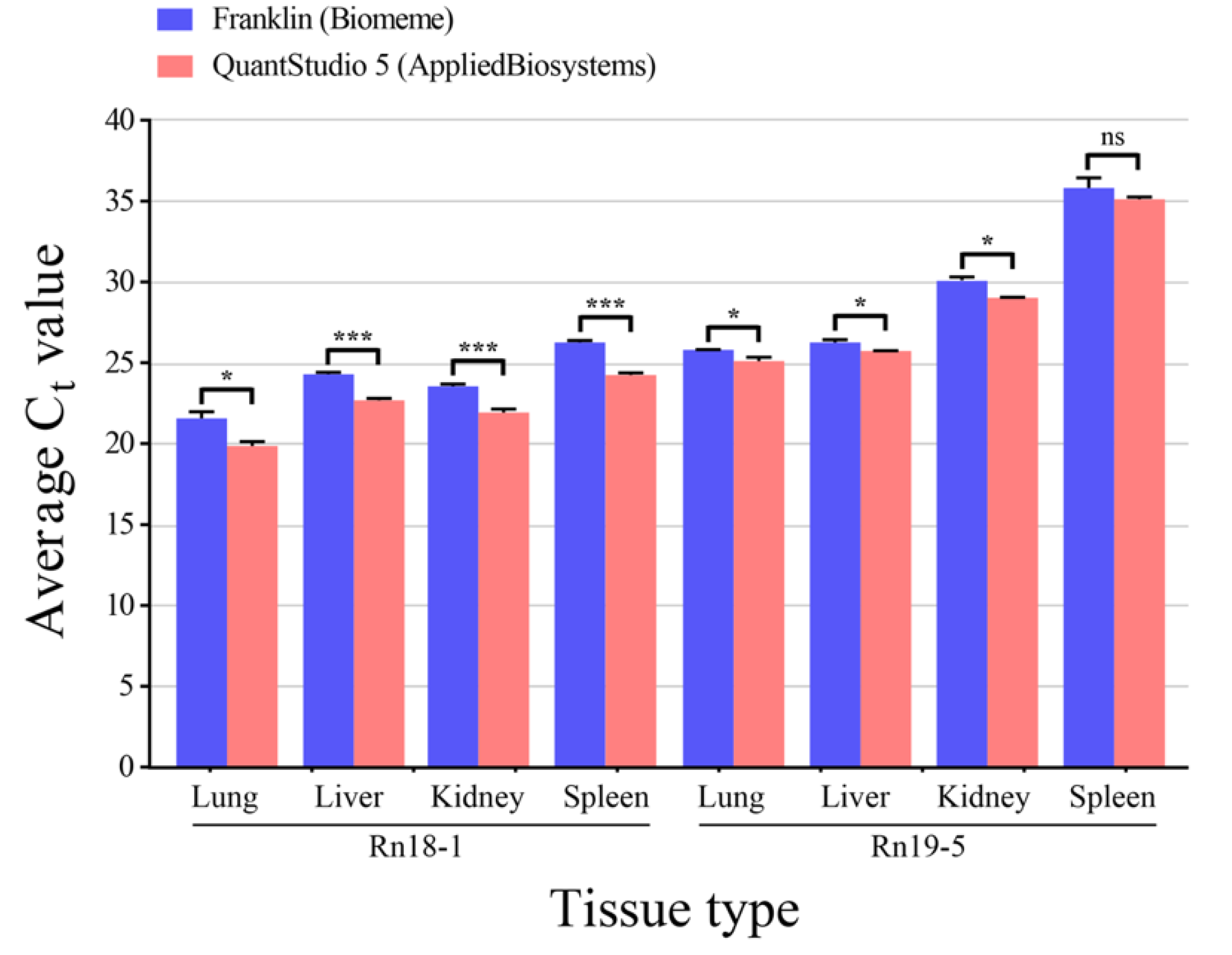

| Characteristic | Number of Captured R. norvegicus | Seropositivity for Anti-SEOV IgG (%) | SEOV RNA Positivity (%) |
|---|---|---|---|
| Region (n = 27) | |||
| Seoul Metropolitan City | 23 | 3/23 (13.0) | 0/23 |
| Cheorwon-gun | 1 | 0/1 | 0/1 |
| Chuncheon-si | 1 | 1/1 (100) | 1/1 (100) |
| Gangneung-si | 2 | 1/2 (50) | 1/2 (50) |
| Sex (n = 27) | |||
| Male | 19 | 4/19 (21.1) | 2/19 (10.5) |
| Female | 8 | 1/8 (12.5) | 0/8 |
| Weight (n = 27) | |||
| <50 | 10 | 1/10 (10) | 0/10 |
| 51–100 | 11 | 2/11 (18.2) | 0/11 |
| 101–150 | 3 | 0/3 | 0/3 |
| 151–200 | 3 | 2/3 (66.7) | 2/3 (66.7) |
| Total | 27 | 5/27 (18.5) | 2/27 (7.4) |
| Sample | Region | Origin | Anti-SEOV IgG Titer | Ct Value a | SEOV Genomes, % Coverage b | ||
|---|---|---|---|---|---|---|---|
| L Segment | M Segment | S Segment | |||||
| Rn18-1 | Chuncheon-si | Lung | 1:256 | 21.6 | 97.3 | 96.5 | 90.0 |
| Rn19-5 | Gangneung-si | Lung | 1:256 | 25.8 | 99.5 | 98.9 | 97.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Lee, S.-H.; Kim, J.; Lee, J.; Lee, G.-Y.; Cho, S.; Noh, J.; Choi, J.; Park, J.; Song, D.-H.; et al. A Portable Diagnostic Assay, Genetic Diversity, and Isolation of Seoul Virus from Rattus norvegicus Collected in Gangwon Province, Republic of Korea. Pathogens 2022, 11, 1047. https://doi.org/10.3390/pathogens11091047
Park K, Lee S-H, Kim J, Lee J, Lee G-Y, Cho S, Noh J, Choi J, Park J, Song D-H, et al. A Portable Diagnostic Assay, Genetic Diversity, and Isolation of Seoul Virus from Rattus norvegicus Collected in Gangwon Province, Republic of Korea. Pathogens. 2022; 11(9):1047. https://doi.org/10.3390/pathogens11091047
Chicago/Turabian StylePark, Kyungmin, Seung-Ho Lee, Jongwoo Kim, Jingyeong Lee, Geum-Young Lee, Seungchan Cho, Juyoung Noh, Jeewan Choi, Juwon Park, Dong-Hyun Song, and et al. 2022. "A Portable Diagnostic Assay, Genetic Diversity, and Isolation of Seoul Virus from Rattus norvegicus Collected in Gangwon Province, Republic of Korea" Pathogens 11, no. 9: 1047. https://doi.org/10.3390/pathogens11091047
APA StylePark, K., Lee, S.-H., Kim, J., Lee, J., Lee, G.-Y., Cho, S., Noh, J., Choi, J., Park, J., Song, D.-H., Gu, S. H., Yun, H., Kim, J.-E., Lee, D., Hwang, I.-U., Kim, W.-K., & Song, J.-W. (2022). A Portable Diagnostic Assay, Genetic Diversity, and Isolation of Seoul Virus from Rattus norvegicus Collected in Gangwon Province, Republic of Korea. Pathogens, 11(9), 1047. https://doi.org/10.3390/pathogens11091047





