Blocking Serum Amyloid-P Component from Binding to Macrophages and Augmenting Fungal Functional Amyloid Increases Macrophage Phagocytosis of Candida albicans
Abstract
:1. Introduction
2. Methods
2.1. Macrophages
2.2. Fungi
2.3. Macrophage–Yeast Interactions
2.4. SAP and Other Additives
2.5. The SAP-Competitor Compounds
2.6. Pro-Amyloid and Anti-Amyloid Peptides
2.7. Flow Cytometry
2.8. Statistics
3. Results
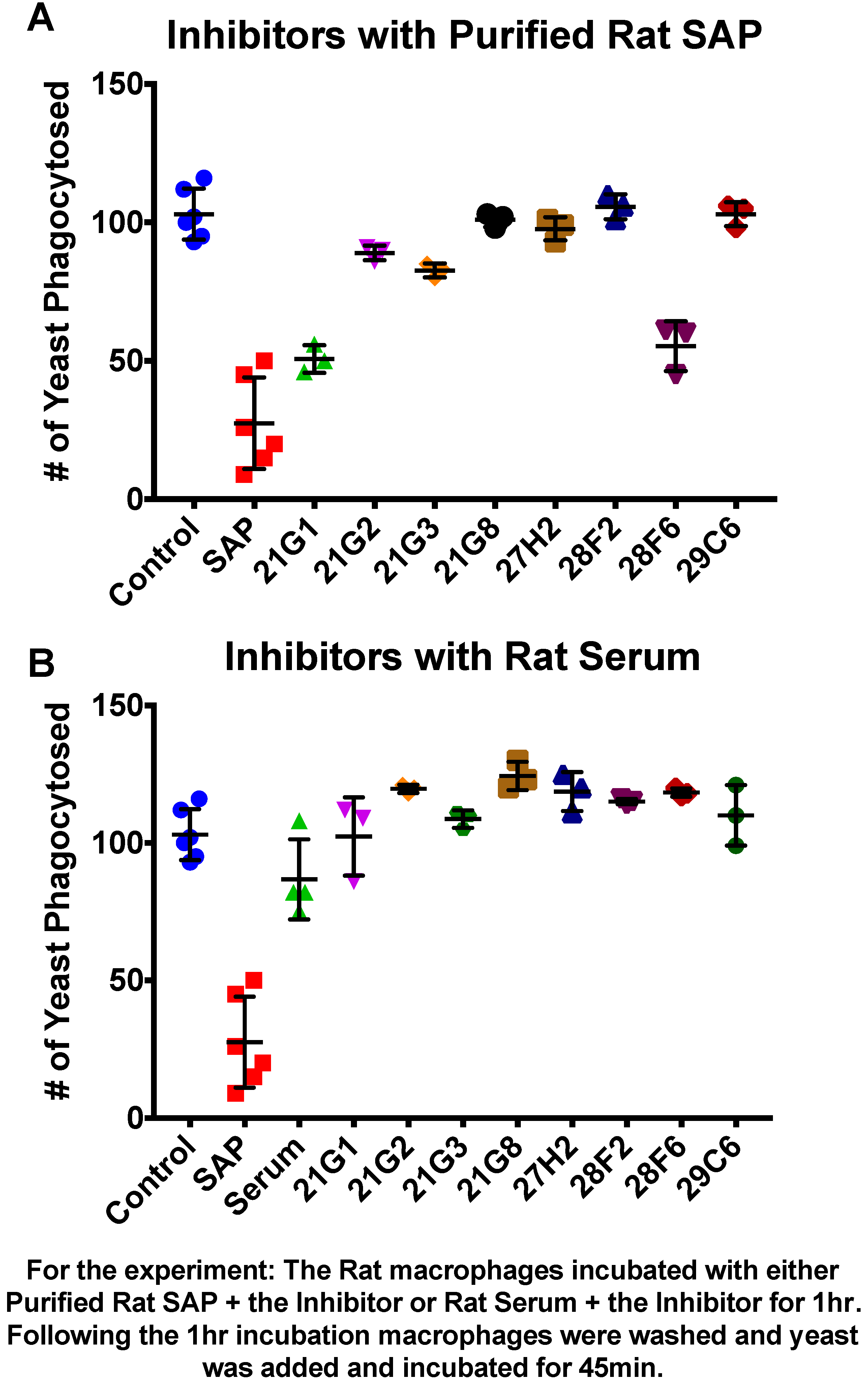
3.1. Compounds That Inhibit SAP Binding to Macrophages Enhance Phagocytosis
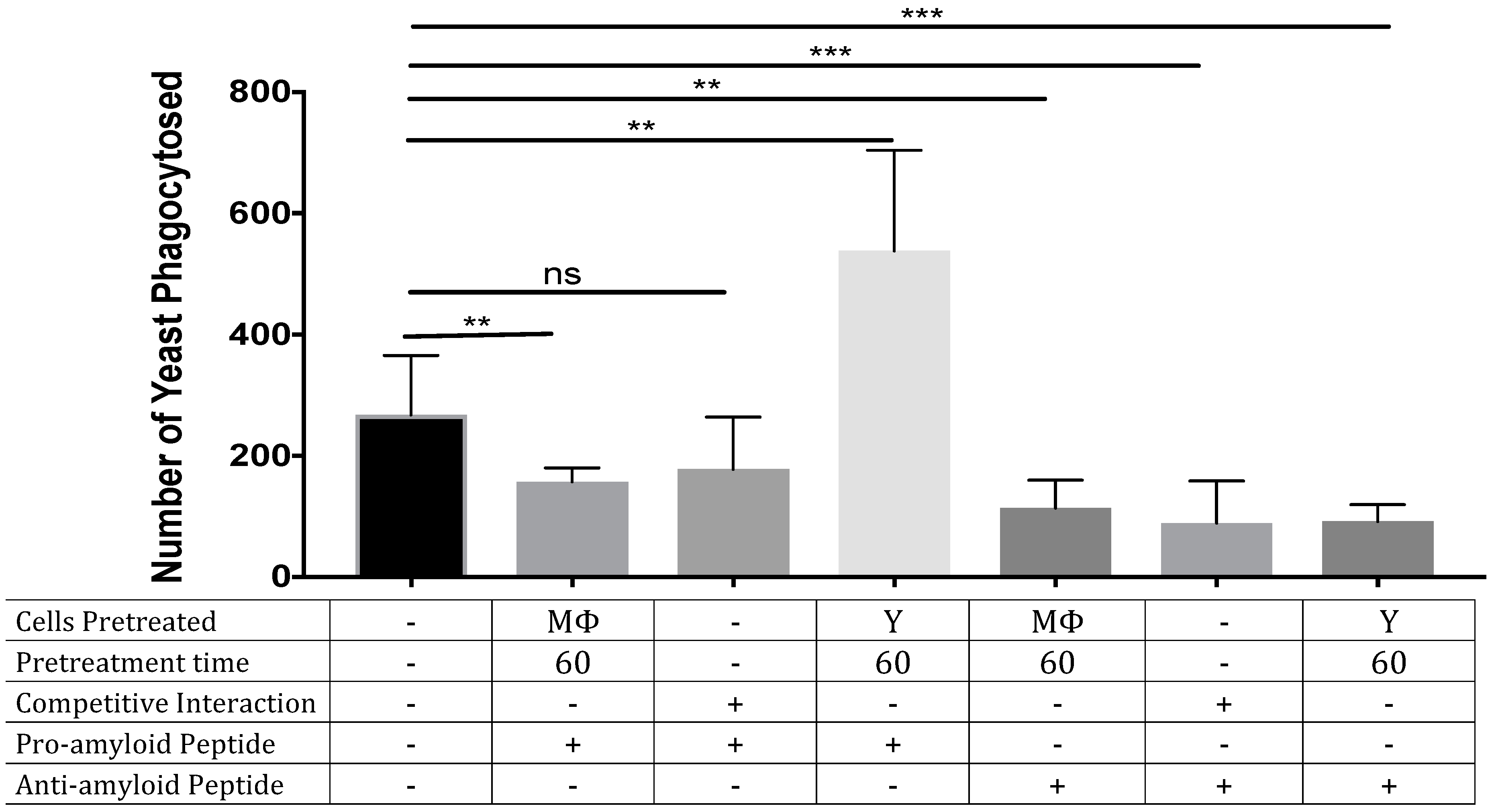
3.2. ‘Seeding’ Amyloid on the Yeast Surface Enhances Phagocytosis of Yeasts
3.3. Miridesap Successfully Removes SAP from the Yeast Cell Surface
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilchrist, K.B.; Garcia, M.C.; Lipke, P.N.; Klotz, S.A. New features of invasive candidiasis in humans: Amyloid formation by fungi and deposition of serum amyloid P component by the host. J. Infect. Dis. 2012, 206, 1473–1478. [Google Scholar] [CrossRef]
- Balistreri, A.; Goetzler, E.; Chapman, M. Functional Amyloids Are the Rule Rather Than the Exception in Cellular Biology. Microorganisms 2020, 8, 1951. [Google Scholar] [CrossRef]
- Otzen, D. Functional amyloid: Turning swords into plowshares. Prion 2010, 4, 256–264. [Google Scholar] [CrossRef]
- Pepys, M.B. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos. Trans. R. Soc. Lond B Biol. Sci. 2001, 356, 203–210. [Google Scholar] [CrossRef]
- Behrens, N.E.; Lipke, P.N.; Pilling, D.; Gomer, R.H.; Klotz, S.A. Serum Amyloid P Component Binds Fungal Surface Amyloid and Decreases Human Macrophage Phagocytosis and Secretion of Inflammatory Cytokines. mBio 2019, 10, e00218–e00219. [Google Scholar] [CrossRef]
- Garcia-Sherman, M.C.; Lundberg, T.; Sobonya, R.E.; Lipke, P.N.; Klotz, S.A. A unique biofilm in human deep mycoses: Fungal amyloid is bound by host serum amyloid P component. NPJ Biofilms Microbiomes 2015, 1, 15009. [Google Scholar] [CrossRef]
- Noursadeghi, M.; Bickerstaff, M.C.; Gallimore, J.R.; Herbert, J.; Cohen, J.; Pepys, M.B. Role of serum amyloid P component in bacterial infection: Protection of the host or protection of the pathogen. Proc. Natl. Acad. Sci. USA 2000, 97, 14584–14589. [Google Scholar] [CrossRef]
- Klotz, S.A.; Sobonya, R.E.; Lipke, P.N.; Garcia-Sherman, M.C. Serum Amyloid P Component and Systemic Fungal Infection: Does It Protect the Host or Is It a Trojan Horse? Open Forum Infect. Dis. 2016, 3, ofw166. [Google Scholar] [CrossRef]
- Pepys, M.B. The Pentraxins 1975–2018: Serendipity, Diagnostics and Drugs. Front. Immunol. 2018, 9, 2382. [Google Scholar] [CrossRef]
- Tennent, G.A.; Lovat, L.B.; Pepys, M.B. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc. Natl. Acad. Sci. USA 1995, 92, 4299–4303. [Google Scholar] [CrossRef] [Green Version]
- Kolstoe, S.E.; Jenvey, M.C.; Purvis, A.; Light, M.E.; Thompson, D.; Hughes, P.; Pepys, M.B.; Wood, S.P. Interaction of serum amyloid P component with hexanoyl bis(D-proline) (CPHPC). Acta Cryst. D Biol. Crystallogr. 2014, 70, 2232–2240. [Google Scholar] [CrossRef]
- Richards, D.B.; Cookson, L.M.; Barton, S.V.; Liefaard, L.; Lane, T.; Hutt, D.F.; Ritter, J.M.; Fontana, M.; Moon, J.C.; Gillmore, J.D.; et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci. Transl. Med. 2018, 10, eaan3128. [Google Scholar] [CrossRef]
- Du, C.; Calderone, R.A. Phagocytosis and killing assays for Candida species. Methods Mol. Biol. 2009, 499, 17–26. [Google Scholar] [CrossRef]
- Xiang, W.; Cox, N.; Gomer, R.H. Identification of compounds that decrease numbers of Mycobacteria in human macrophages in the presence of serum amyloid P. J. Leukoc. Biol. 2017, 102, 857–869. [Google Scholar] [CrossRef]
- Garcia-Sherman, M.C.; Lysak, N.; Filonenko, A.; Richards, H.; Sobonya, R.E.; Klotz, S.A.; Lipke, P.N. Peptide detection of fungal functional amyloids in infected tissue. PLoS ONE 2014, 9, e86067. [Google Scholar] [CrossRef]
- Dehullu, J.; Valotteau, C.; Herman-Bausier, P.; Garcia-Sherman, M.; Mittelviefhaus, M.; Vorholt, J.A.; Lipke, P.N.; Dufrêne, Y.F. Fluidic Force Microscopy Demonstrates That Homophilic Adhesion by Candida albicans Als Proteins Is Mediated by Amyloid Bonds between Cells. Nano Lett. 2019, 19, 3846–3853. [Google Scholar] [CrossRef]
- Lipke, P.N.; Klotz, S.A.; Dufrene, Y.F.; Jackson, D.N.; Garcia-Sherman, M.C. Amyloid-Like β-Aggregates as Force-Sensitive Switches in Fungal Biofilms and Infections. Microbiol. Mol. Biol. Rev. 2017, 82, e00035-17. [Google Scholar] [CrossRef]
- Klotz, S.A. Fungal adherence to the vascular compartment: A critical step in the pathogenesis of disseminated candidiasis. Clin. Infect. Dis. 1992, 14, 340–347. [Google Scholar] [CrossRef]
- Singh, P.P.; Kaur, S. Serum amyloid P-component in murine tuberculosis: Induction kinetics and intramacrophage Mycobacterium tuberculosis growth inhibition in vitro. Microbes Infect. 2006, 8, 541–551. [Google Scholar] [CrossRef]
- Raghu, G.; van den Blink, B.; Hamblin, M.J.; Brown, A.W.; Golden, J.A.; Ho, L.A.; Wijsenbeek, M.S.; Vasakova, M.; Pesci, A.; Antin-Ozerkis, D.E.; et al. Long-term treatment with recombinant human pentraxin 2 protein in patients with idiopathic pulmonary fibrosis: An open-label extension study. Lancet Respir. Med. 2019, 7, 657–664. [Google Scholar] [CrossRef]
- Karhadkar, T.R.; Pilling, D.; Gomer, R.H. Serum Amyloid P inhibits single stranded RNA-induced lung inflammation, lung damage, and cytokine storm in mice. PLoS ONE 2021, 16, e0245924. [Google Scholar] [CrossRef] [PubMed]
- Pilling, D.; Gomer, R.H. The Development of Serum Amyloid P as a Possible Therapeutic. Front. Immunol. 2018, 9, 2328. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B. Invasive candidiasis: New insights presaging new therapeutic approaches? J. Infect. Dis. 2012, 206, 1339–1341. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotz, S.A.; Bradley, N.; Lipke, P.N. Blocking Serum Amyloid-P Component from Binding to Macrophages and Augmenting Fungal Functional Amyloid Increases Macrophage Phagocytosis of Candida albicans. Pathogens 2022, 11, 1000. https://doi.org/10.3390/pathogens11091000
Klotz SA, Bradley N, Lipke PN. Blocking Serum Amyloid-P Component from Binding to Macrophages and Augmenting Fungal Functional Amyloid Increases Macrophage Phagocytosis of Candida albicans. Pathogens. 2022; 11(9):1000. https://doi.org/10.3390/pathogens11091000
Chicago/Turabian StyleKlotz, Stephen A., Nicole Bradley, and Peter N. Lipke. 2022. "Blocking Serum Amyloid-P Component from Binding to Macrophages and Augmenting Fungal Functional Amyloid Increases Macrophage Phagocytosis of Candida albicans" Pathogens 11, no. 9: 1000. https://doi.org/10.3390/pathogens11091000
APA StyleKlotz, S. A., Bradley, N., & Lipke, P. N. (2022). Blocking Serum Amyloid-P Component from Binding to Macrophages and Augmenting Fungal Functional Amyloid Increases Macrophage Phagocytosis of Candida albicans. Pathogens, 11(9), 1000. https://doi.org/10.3390/pathogens11091000






