Leptospira interrogans Sensu Lato in Wild Small Mammals in Three Moravian Localities of the Czech Republic
Abstract
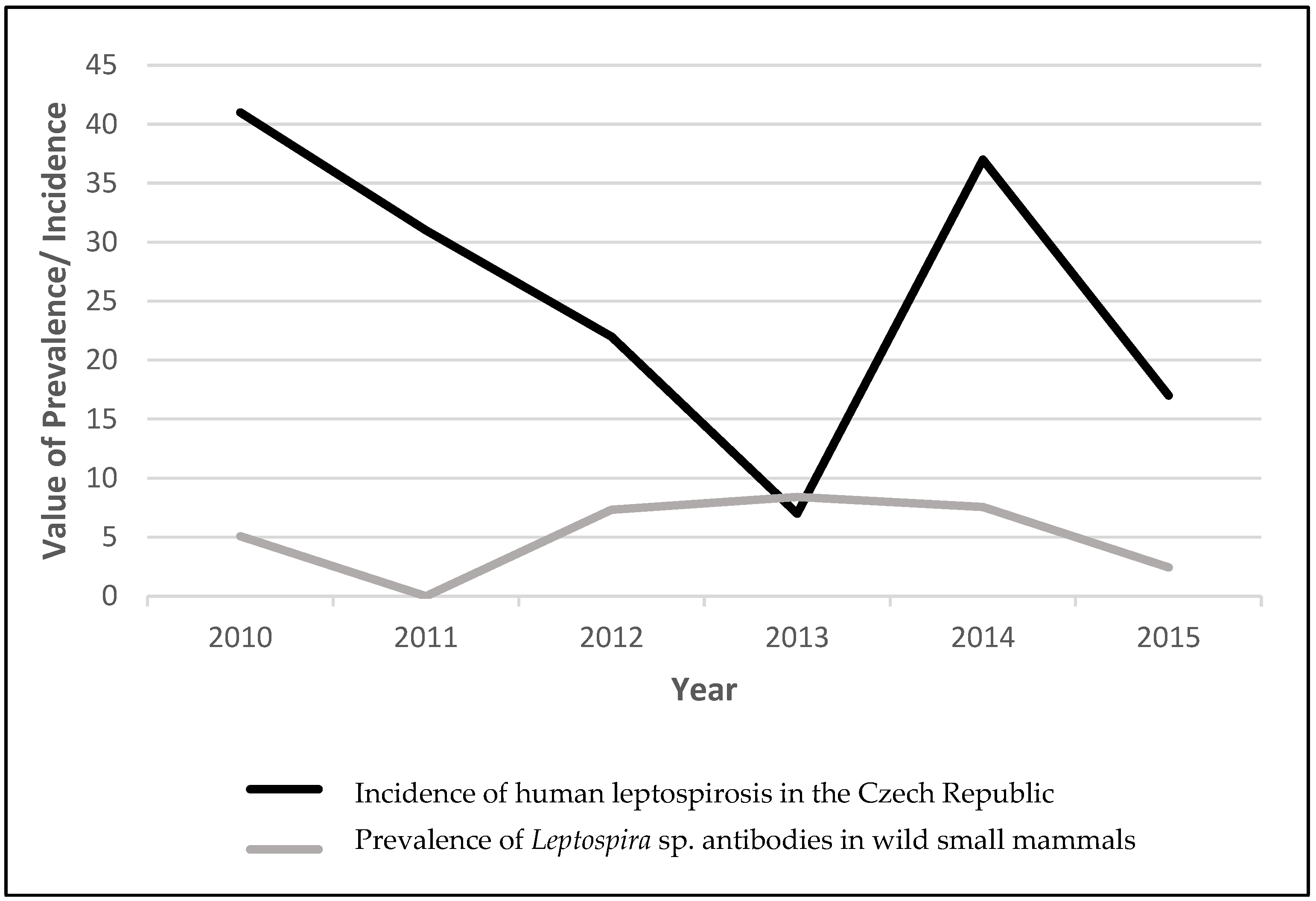
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Trapping of Wild Small Mammals
4.2. Detection of Antibodies to Leptospira by Microscopic Agglutination Test
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira infection in rats: A literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Sedlák, K.; Tomšíčkova, M. Nebezpečné Infekce Zvířat a Člověka (Dangerous Infections of Animals and Humans); Scientia: Prague, Czech Republic, 2006; ISBN 80-86960-07-2. [Google Scholar]
- Smetana, J.; Čermáková, Z.; Boštíková, V.; Kučerová, P.; Prášil, P.; Pavliš, O.; Chlíbek, R. Leptospirosis in the Czech Republic and potential for laboratory diagnosis. Epidemiol. Mikrobiol. Imunol. 2010, 59, 159–167. [Google Scholar]
- Zítek, K.; Beneš, C. Dlouhodobá epidemiologie leptospirózy (1963–2003) v České republice (Longitudinal epidemiology of leptospirosis in the Czech Republic (1963–2003). Epidemiol. Mikrobiol. Imunol. 2005, 54, 21–26. [Google Scholar]
- Treml, F.; Pikula, J.; Holešovská, Z. Prevalence of antibodies against leptospires in the wild boar (Sus scrofa L-1758). Vet. Med. 2003, 48, 66–70. [Google Scholar] [CrossRef]
- Ghasemzadeh, I.; Namazi, S.H. Review of bacterial and viral zoonotic infections transmitted by dogs. J. Med. Life. 2015, 8, 1–5. [Google Scholar]
- Treml, F.; Pejčoch, M.; Holešovska, Z. Small mammals—Natural reservoir of pathogenic leptospires. Vet. Med. 2002, 47, 309–314. [Google Scholar] [CrossRef]
- Treml, F.; Pikula, J.; Holešovska, Z. Prevalence of antibodies against leptospires in the European hare (Lepus europaeus Pall.). Acta Vet. Brno 2003, 72, 377–381. [Google Scholar] [CrossRef][Green Version]
- URL 1: Statní zdravotní Ústav (The National Institute of Public Health). Available online: www.szu.cz (accessed on 20 January 2022).
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A Systematic Review. PLoS Negl. Trop Dis. 2015, 17, e0003898. [Google Scholar] [CrossRef]
- Villanueva, S.Y.; Ezoe, H.; Baterna, R.A.; Yanagihara, Y.; Muto, M.; Koizumi, N.; Fukui, T.; Okamoto, Y.; Masuzawa, T.; Cavinta, L.L.; et al. Serologic and molecular studies of Leptospira and leptospirosis among rats in the Philippines. Am. J. Trop. Med. Hyg. 2010, 82, 889–898. [Google Scholar] [CrossRef]
- de Faria, M.T.; Calderwood, M.S.; Athanazio, D.A.; McBride, A.J.A.; Hartskeerl, R.A.; Pereira, M.M.; Ko, A.I.; Reis, M.G. Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Trop. 2008, 108, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scialfa, E.; Bolpe, J.; Bardon, J.C.; Ridao, G.; Gentile, J.; Gallicchio, O. Isolation of Leptospira interrogans from suburban rats in Tandil, Buenos Aires, Argentina. Rev. Argent. Microbiol. 2010, 42, 126–128. [Google Scholar] [PubMed]
- Foronda, P.; Martin-Alonso, A.; del Castillo-Figueruelo, B.; Feliu, C.; Gil, H.; Valladares, B. Pathogenic Leptospira spp. in wild rodents, Canary Islands, Spain. Emerg. Infect. Dis. 2011, 17, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J.F.; Picardeau, M.; Mielcarek, M.; Tatard, C.; Chaval, Y.; Suputtamongkol, Y.; Buchy, P.; Jittapalapong, S.; Herbreteau, V.; Moran, D.S. Epidemiology of Leptospira transmitted by rodents in Southeast Asia. PLoS Negl. Trop. Dis. 2014, 8, e2902. [Google Scholar] [CrossRef] [PubMed]
- Vanasco, N.B.; Schmeling, M.F.; Lottersberger, J.; Costa, F.; Ko, A.I.; Tarabla, H.D. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999–2005). Acta Trop. 2008, 107, 255–258. [Google Scholar] [CrossRef]
- Stanko, M.; Prokopcakova, H.; Fricova, J.; Petko, B. Occurence of antibodies to leptospira in small mammals in Eastern Slovakia. Vet. Med. 1996, 41, 373–377. [Google Scholar]
- Tadin, A.; Turk, N.; Korva, M.; Margaletic, J.; Beck, R.; Vucelja, M.; Habus, J.; Svoboda, P.; Zupanc, T.A.; Henttonen, H.; et al. Multiple co-infections of rodents with hantaviruses, Leptospira, and Babesia in Croatia. Vector Borne Zoonotic Dis. 2012, 12, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Cvetnic, Z.; Margaletic, J.; Toncic, J.; Turk, N.; Milas, Z.; Spicic, S.; Lojkic, M.; Terzic, S.; Jemersic, L.; Humski, A.; et al. A serological survey and isolation of leptospires from small rodents and wild boars in the Republic of Croatia. Vet. Med. 2003, 48, 321–329. [Google Scholar] [CrossRef]
- Perra, A.; Servas, V.; Terrier, G.; Postic, D.; Baranton, G.; André-Fontaine, G.; Vaillant, V.; Capek, I. Clustered cases of leptospirosis in Rochefort, France, June 2001. Euro Surveill. 2002, 7, 131–136. [Google Scholar] [CrossRef]
- Aviat, F.; Slamti, L.; Cerqueira, G.M.; Lourdault, K.; Picardeau, M. Expanding the genetic toolbox for leptospira species by generation of fluorescent bacteria. Appl. Environ. Microbiol. 2010, 76, 8135–8142. [Google Scholar] [CrossRef][Green Version]
- Levieuge, A.; Aboubaker, M.H.; Terrier, O.; Drancourt, M.; Davoust, B. Real-time PCR detection of Leptospira sp. in rodents from Toulon harbour (France). Rev. Med. Vet. 2010, 161, 264–266. [Google Scholar]
- Krøjgaard, L.; Villumsen, S.; Markussen, M.; Jensen, J.; Leirs, H.; Heiberg, A.C. High prevalence of Leptospira spp. in sewer rats (Rattus norvegicus). Epidemiol. Infect. 2009, 137, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Ellis, W.; MacDonald, D.W. Prevalence of Leptospira spp. in Wild Brown Rats (Rattus norvegicus) on UK Farms. Epidemiol. Infect. 1995, 114, 195–201. [Google Scholar] [CrossRef]
- Pezzella, M.; Lillini, E.; Sturchio, E.; Ierardi, L.A.; Grassi, M.; Traditi, F.; Cristaldi, M. Leptospirosis survey in wild rodents living in urban areas of Rome. Ann. Ig. 2004, 16, 721–726. [Google Scholar] [PubMed]
- Mayer-Scholl, A.; Hammerl, J.A.; Schmidt, S.; Ulrich, R.G.; Pfeffer, M.; Woll, D.; Scholz, H.C.; Thomas, A.; Nöckler, K. Leptospira spp. in rodents and shrews in Germany. Int. J. Environ. Res. Public Health 2014, 11, 7562–7574. [Google Scholar]
- Desai, S.; van Treeck, U.; Lierz, M.; Espelage, W.; Zota, L.; Sarbu, A.; Czerwinski, M.; Sadkowska-Todys, M.; Avdicova, M.; Reetz, J.; et al. Resurgence of field fever in a temperate country: An epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin. Infect. Dis. 2009, 48, 691–697. [Google Scholar] [CrossRef]
- Delooz, L.; Mori, M.; Petitjean, T.; Evrard, J.; Czaplicki, G.; Saegerman, C. Congenital jaundice in bovine aborted foetuses: An emerging syndrome in Southern Belgium. Transbound. Emerg. Dis. 2015, 62, 124–126. [Google Scholar] [CrossRef]
- URL2: Leptospirosis-Annual Epidemiological Report 2016 [2014 Data]. Available online: www.ecdc.europa.eu/en/publications-data/leptospirosis-annual-epidemiological-report-2016-2014-data (accessed on 30 January 2017).
- Zitek, K.; Sedláček, I. Taxonomie leptospir (Taxonomy of leptospira). Remedia—Klin. Mikrobiol. 1999, 3, 232–235. [Google Scholar]
- Vostal, K.; Žákovská, A. Two-year study of examination of blood from wild rodents for the presence of antiborrelian antibodies. Ann. Agric. Environ. Med. 2003, 10, 203–206. [Google Scholar]
- Netušil, J.; Žákovská, A.; Vostal, K.; Norek, A.; Staňko, M. The occurrence of Borrelia burgdorferi sensu lato in certain ectoparasites (Mesostigmata, Siphonaptera) of Apodemus flavicollis and Myodes glareolus in chosen localities in the Czech Republic. Acta Parasitol. 2013, 58, 337–341. [Google Scholar] [CrossRef]
- Humair, P.F.; Gern, L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 1998, 69, 213–227. [Google Scholar] [CrossRef]
- Türk, N.; Milas, Z.; Margaletic, J.; Staresina, V.; Slavica, A.; Riquelme-Sertour, N.; Bellenger, E.; Baranton, G.; Postic, D. Molecular characterization of Leptospira spp. strains isolated from small rodents in Croatia. Epidemiol. Infect. 2003, 130, 159–166. [Google Scholar] [PubMed]
- Radzijevskaja, J.; Paulauskas, A.; Rosef, O. Molecular detection and characterization of Borrelia burgdorferi sensu lato in small rodents. Vet. Med. Zoot. 2011, 55, 40–46. [Google Scholar]
- Goris, M.G.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32, 12E.5. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z.; Rudolf, I. Microbial Zoonoses and Sapronoses, 1st ed.; Springer: New York, NY, USA, 2011; 176p, ISBN 978-90-481-9656-2. [Google Scholar]
- Treml, F.; Nepeřeny, J.; Jandová, E.; Banďouchová, H.; Pikula, J. Prevalence of antibodies against leptospires in small mammals in relation to age, sex and season. Acta Vet. Brno 2012, 81, 97–102. [Google Scholar] [CrossRef][Green Version]
- Nepeřený, J.; Treml, F.; Vrzal, V. Prevalence of antibodies against leptospirosis in horses in the Czech Republic. Veterinářství 2018, 68, 94–97. [Google Scholar]
- Kucerova, P.; Cermakova, Z.; Pliskova, L.; Pavlis, O.; Kubickova, P.; Kleprlikova, H.; Valenta, Z. Our experience using real-time PCR for the detection of the gene that encodes the superficial lipoprotein LipL32 of the pathogenic leptospires to confirm the acute form of human leptospirosis. Biomed Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013, 157, 387–391. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Staňková, M.; Marešová, V.; Vaništa, J. Repetitorium infekčních nemocí (Repetitorium of Infectious Diseases); Triton: Prague, Czech Republic, 2008; ISBN 8073870560. [Google Scholar]
- Shropshire, S.B.; Veir, J.K.; Morris, A.K.; Lappin, M.R. Evaluation of Leptospira species microscopic agglutination test in experimentally vaccinated cats and Leptospira species seropositivity in aged azotemic client-owned cats. J. Feline Med. Surg. 2016, 18, 768–772. [Google Scholar] [CrossRef]
- URL 3: Statsoft, Inc. 2013. STATISTICA (Data Analysis Software System), Version 12. Available online: www.Statsoft.com (accessed on 20 January 2018).
| Years | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|
| Species | |||||||
| Apodemus agrarius | 2 + 2 */14 (28.6%) | 1 + 1 */18 (11.1%) | 0 + 1 */18 (5.6%) | 3 + 4 */50 (14%) | |||
| Apodemus flavicollis | 3/59 (5.1%) | 0/43 (0%) | 7/127 (5.5%) | 2/50 (4%) | 8/174 (4.6%) | 1/44 (2.3%) | 21/497 (4.2%) |
| Apodemus sylvaticus | 0/5 (0%) | 1/12 (8.3%) | 1/2 | 3/12 (25%) | 1/26 (3.8%) | 6/57 (10.5%) | |
| Myodes glareolus | 0/3 | 7/105 (6.7%) | 1/10 (10%) | 6/33 (18.2%) | 0/40 (0%) | 14/191 (7.3%) | |
| Microtus arvalis | 2/6 (33.3%) | 2/6 (33.3%) | |||||
| Sorex araneus | 0/1 | 0/2 | 0/8 (0%) | 0/6 (0%) | 1/36 (2.8%) | 1/53 (1.9%) | |
| Talpa europea | 0/1 | 0/1 | |||||
| Sex | |||||||
| Female | 1/32 (3.1%) | 0/28 (0%) | 10 + 1/137 (8%) | 5/45 (11.1%) | 11/111 (9.9%) | 3/111 (2.7%) | 30 + 1 */464 (6.7%) |
| Male | 2/27 (7.4%) | 0/24 (0%) | 7 + 1 */123 (6.5%) | 2 + 1 */50 (6%) | 6/114 (5.3%) | 0 + 1 */53 (1.9%) | 17 + 3 */391 (5.1%) |
| Localities | |||||||
| Mohelno | 1/56 (1.8%) | 1/56 (1.8%) | |||||
| Moravian Karst | 0/21 (0%) | 8/161 (5%) | 1/28 (3.6%) | 14/189 (7.4%) | 2/96 (2.1%) | 25/485 (5.2%) | |
| Poodří | 3/59 (5.1%) | 0/31 (0%) | 8 + 2 */43 (23.3%) | 6 + 1 */67 (10.4%) | 3/36 (8.3%) | 1 + 1 */78 (2.6%) | 21 + 4 */314 (8%) |
| Total | 3/59 (5.1%) | 0/52 (0%) | 17 + 2 */260 (7%) | 7 + 1 */95 (8.4%) | 17/225 (7.6%) | 3 + 1 */164 (2.4%) | 47 + 4 */855 (6%) |
| Characteristic | Number | Positive | p Value |
|---|---|---|---|
| Species | 0.002 | ||
| Apodemus agrarius | 50 | 7 | |
| Apodemus flavicollis | 497 | 21 | |
| Apodemus sylvaticus | 57 | 6 | |
| Myodes glareolus | 191 | 17 | |
| Microtus arvalis | 6 | 2 | |
| Sorex araneus | 53 | 1 | |
| Talpa europea | 1 | 0 | |
| Sex | 0.3355 | ||
| Female | 464 | 31 | |
| Male | 391 | 20 | |
| Localities | 0.1031 | ||
| Mohelno | 56 | 1 | |
| Moravian Karst | 484 | 25 | |
| Poodří | 314 | 25 | |
| Year of collection | 0.0785 | ||
| 2010 | 59 | 3 | |
| 2011 | 52 | 0 | |
| 2012 | 260 | 19 | |
| 2013 | 95 | 8 | |
| 2014 | 225 | 17 | |
| 2015 | 164 | 4 | |
| Rodent versus insectivore | 0.1635 | ||
| Rodents | 801 | 53 | |
| Insectivores | 54 | 1 | |
| Total | 855 | 54 |
| Species | Apodemus agrarius | Apodemus flavicollis | Apodemus sylvaticus | Myodes glareolus | Microtus arvalis | Sorex araneus |
|---|---|---|---|---|---|---|
| Apodemus agrarius | 3.69 (1.48, 9.17) | 1.38 (0.43, 4.43) | 2.06 (0.78, 5.41) | 0.33 (0.05, 2.13) | 8.47 (1.01, 71.51) | |
| Apodemus flavicollis | 0.38 (0.14, 0.97) | 0.56 (0.28, 1.12) | 0.09 (0.02, 0.51) | 2.29 (0.30, 17.40) | ||
| Apodemus sylvaticus | 1.49 (0.54, 4.07) | 0.24 (0.04, 1.57) | 6.12 (0.71, 52.62) | |||
| Myodes glareolus | 0.16 (0.03, 0.94) | 4.11 (0.53, 32.02) | ||||
| Microtus arvalis | 26.00 (1.91, 352.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žákovská, A.; Treml, F.; Nejezchlebová, H.; Nepeřený, J.; Budíková, M.; Bártová, E. Leptospira interrogans Sensu Lato in Wild Small Mammals in Three Moravian Localities of the Czech Republic. Pathogens 2022, 11, 888. https://doi.org/10.3390/pathogens11080888
Žákovská A, Treml F, Nejezchlebová H, Nepeřený J, Budíková M, Bártová E. Leptospira interrogans Sensu Lato in Wild Small Mammals in Three Moravian Localities of the Czech Republic. Pathogens. 2022; 11(8):888. https://doi.org/10.3390/pathogens11080888
Chicago/Turabian StyleŽákovská, Alena, František Treml, Helena Nejezchlebová, Jiří Nepeřený, Marie Budíková, and Eva Bártová. 2022. "Leptospira interrogans Sensu Lato in Wild Small Mammals in Three Moravian Localities of the Czech Republic" Pathogens 11, no. 8: 888. https://doi.org/10.3390/pathogens11080888
APA StyleŽákovská, A., Treml, F., Nejezchlebová, H., Nepeřený, J., Budíková, M., & Bártová, E. (2022). Leptospira interrogans Sensu Lato in Wild Small Mammals in Three Moravian Localities of the Czech Republic. Pathogens, 11(8), 888. https://doi.org/10.3390/pathogens11080888






