Anthracnose of Onion (Allium cepa L.): A Twister Disease
Abstract
:1. Introduction
2. Symptomatology
2.1. Our Initiatives and Research Findings
2.1.1. Anthracnose Symptom Development
2.1.2. Twister—Anthracnose Complex Development
2.1.3. Disease Scoring and Assessment
3. Aetiology
| Pathogen | Host | Reference |
|---|---|---|
| Colletotrichum gloeosporioides | Allium cepa | [9,54,55] |
| C. gloeosporioides + Fusarium fujikuroi/Gibberella moniliformis | Allium cepa | [32,56] |
| C. gloeosporioides + Fusarium oxysporum | Allium cepa | [43,57,58] |
| C. gloeosporioides + F. oxysporum + Meloidogyne graminicola | Allium cepa | [31] |
| C. truncatum | Allium cepa, A. fistulosum, and A. angulosum | [59,60,61] |
| C. dematium f circinans | Allium cepa | [62,63] |
| C. spaethianum | A. ledebourianum and A. fistulosum | [52,53] |
| C. coccodes | Allium cepa | [14,64] |
| C. siamense | Allium cepa | [59] |
| C. chardonianum | Aliium cepa | [65] |
| C. theobromicola | Allium fistulosum | [60] |
| C. acutatum | Allium cepa | [13] |
| C. scovillei and C. nymphaeae | Allium cepa | [66] |
3.1. Classification
3.2. Identification and Characterisation of Colletotrichum spp. and Fusarium spp.
4. Epidemiology
4.1. Our Initiatives and Research Findings
4.2. Genetic Diversity and Spatial Distribution of Anthracnose/Anthracnose-Twister Disease
4.3. Infection Process in Onion by Colletotrichum
5. Disease Management
5.1. Cultural Management
5.2. Botanicals and Biological Management
Our Initiatives and Research Findings
5.3. Host Plant Resistance
Our Initiatives and Research Findings
5.4. Chemical Management
| Fungicides with Concentration | Disease | Reference |
|---|---|---|
| Benomyl at 0.2% | Onion anthracnose | [135] |
| Carbendazim and captafol at 10 or 15 g/20 litres | Onion anthracnose | [136] |
| Thiophanate methyl | Onion twister | [58] |
| Mancozeb at 0.25% | Onion anthracnose | [127] |
| Thiophanate methyl 50% + thiram 30% WP and thiophanate methyl 70% wp, and chlorothalonil 70% WP | Onion leaf twister | [45,137,138] |
| Seed treatment with thiram and spray with zineb 0.25% | Onion twister | [139] |
| Mancozeb, carbendazim, propiconazole, and thiophanate methyl at 0.1% | Onion twister-anthracnose | [126] |
| Hexaconazole at 0.1% | Onion twister | [52] |
| Captan, mancozeb/benomyl Mancozeb or difenoconazole/propiconazole | Onion anthracnose | [140] |
| Triazoles with gibberellin inhibitor | Onion anthracnose | [141] |
| Thiophanate methyl | Onion anthracnose | [142] |
| Trifloxystrobin + tebuconazole, pyraclostrobin + metiram and fluzinam 500 g/L sc. | Onion anthracnose | [143] |
| Dithane or mancozeb or chlorothalanil or strobilurin fungicides, quadris and cabrio | Onion anthracnose | [144] |
| Propiconazole at 0.1% and iprobenfos at 0.15% | Onion anthracnose | [145] |
| Mancozeb 0.25% + tricyclazole 0.1% + hexaconazole 0.1% | Onion twister | [127] |
| Captan and carbendazim + paclobutrazol | Onion anthracnose-twister | [33] |
| Carbendazim + mancozeb 0.25%, propiconazole 0.1% | Onion twister | [116] |
5.5. Integrated Disease Management
Our Initiatives and Research Findings
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vohra, S.B.; Rizman, M.; Khan, J.A. Medicinal uses of common Indian vegetables. Planta Med. 1974, 23, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.A.; Hussein, M.A.M. New Disease Report; Plant Pathology Department, Faculty of Agriculture, Assiut University: Asyut, Egypt, 2007. [Google Scholar]
- Vavilov, N.I. The Origin, Variation, Immunity and Breeding of Cultivated Plants; Lippincot Williams Wilkins: Philadelphia, PA, USA, 1951; Volume 72, 482p. [Google Scholar]
- Jaggi, R.C. Sulphur levels and sources affecting yield and yield attributes in onion (Allium cepa). Indian J. Agric. Sci. 2005, 75, 154–156. [Google Scholar]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- FAOSTAT. 2015. Available online: http://faostat.fao.org/faostat/collectionssubset=agriculture (accessed on 5 June 2022).
- Paibomesai, M.; Celetti, M.; Tesfaendrias, M. Update on Stemphylium leaf blight in onions in Ontario. Hort Matters OMAFRA Spec. Hortic. Spec. Crops 2012, 12, 11–12. [Google Scholar]
- Nisha, H.A.C. Cultural, morphological and molecular characterization of Stemphylium vesicarium causing white blotch of onion. Ph.D. Dissertation, Shere-Bangla Agricultural University, Dhaka, Bangladesh, 2013. [Google Scholar]
- Ebenebe, A.C. Onion twister disease caused by Glomerella cingulata in northern Nigeria. Plant Dis. 1980, 64, 1030–1032. [Google Scholar] [CrossRef]
- Wiyono, S. Climate change and pests and diseases explosion. Pro. Biodiversity in the middle of global warming. Kheti Found. Jkt. Indones. 2007. [Google Scholar]
- Hill, J.P. Compendium of Onion and Garlic Diseases, 2nd ed.; The American Phytopathological Society: Saint Paul, MN, USA, 2008. [Google Scholar]
- Farr, D.F.; Rossman, A.Y. Fungal Databases. Systematic Mycology and Microbiology Laboratory, ARS, USDA. Available online: http://nt.ars-grin.gov/fungaldatabases/ (accessed on 6 August 2010).
- Alberto, R.T.; Aquino, V.M. Characterization of Colletotrichum gloeosporioides (Penzig) Penzig and Sacc. (anthracnose) and Gibberella moniliformis Wineland (twister) infecting onions in the Philippines. Asia Life Sci. 2010, 19, 23–58. [Google Scholar]
- Rodriguez-Salamanca, L.M.; Enzenbacher, T.B.; Derie, M.L.; du Toit, L.J.; Feng, C.; Correll, J.C.; Hausbeck, M.K. First report of Colletotrichum coccodes causing leaf and neck anthracnose on onions (Allium cepa) in Michigan and the United States. Plant Dis. 2012, 96, 769. [Google Scholar] [CrossRef]
- Qadri, S.M.H.; Srivastava, K.J. Leaf blight of onion (Allium cepa L.) caused by Colletotrichum gloesporioides Penz. and Sacc. hitherto unrecorded from India. In Proceedings of the 72nd Indian Science Congress Association, Lucknow, India, 1985; pp. 74–75. [Google Scholar]
- Qadri, S.M.H. Two onion diseases new to Andhra Pradesh and Karnataka. Indian Phytopathol. 1988, 41, 293. [Google Scholar]
- Sinha, J.N.; Singh, A.P. Severe curls or twister disease of onion in India. Indian Phytopathol. 1994, 47, 214. [Google Scholar]
- Guarro, J.; Svidzinski, T.E.; Zaror, L.; Forjaz, M.H.; Gené, J.; Fischman, O. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. J. Clin. Microbiol. 1998, 36, 3060–3065. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Walker, J.C. Histology of watermelon anthracnose. Phytopathology 1961, 52, 650–654. [Google Scholar]
- Brown, G.D. Ultrastructure of penetration of ethylene-degree Robinson tangerines by Colletotrichum gloeosporioides. Phytopathology 1977, 67, 315–320. [Google Scholar] [CrossRef]
- Chau, K.F.; Alvarez, A.M. A histology study of anthracnose on Carica papaya. Phytopathology 1983, 73, 1113–1116. [Google Scholar] [CrossRef]
- Dickman, M.B.; Alvarez, A.M. Latent infection of papaya caused by Colletotrichum gloeosporioides. Plant Dis. 1983, 67, 748–750. [Google Scholar] [CrossRef]
- Bergstrom, G.C.; Nicholson, R.L. The biology of corn anthracnose. Plant Dis. 1999, 63, 596–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, H.F.; Mohan, K.S. Compendium of Onion and Garlic Diseases and Pests; American Phytopathological Society: Saint Paul, MN, USA, 1995. [Google Scholar]
- Nischwitz, C.; Langston, D.; Sanders, H.; Torrance, R.; Lewis, K.; Gitaitis, R. First report of Colletotrichum gloeosporioides causing ‘twister disease’of onion (Allium cepa L.) in Georgia, USA. In Proceedings of the 2008 National Allium Research Conference, Savannah, GA, USA, 10–13 December 2008. [Google Scholar]
- Galvan, G. Screening onions and related species for resistance to anthracnose (C. gloeosporoides). In Mass Screening Techniques for Selecting Crop Resistance to Disease; International Atomic Energy Agency: Vienna, Austria, 2010; pp. 309–319. [Google Scholar]
- Haddad, F.; Maffia, L.A.; Mizubuti, E.S.G. Evaluation of fungicides to control Colletotrichum gloeosporioides on onion. Fitopatol. Bras. 2003, 28, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, H.F.; du Toit, L.J.; Coutinho, T. Common Names of Plant Diseases: Diseases of Onion and Garlic (Allium cepa L. and A. sativum L., Respectively); American Phytopathological Society: Saint Paul, MN, USA, 2015. [Google Scholar]
- Offei, S.K.; Cornelius, E.W.; Sakyi-Dawson, O. Crop Diseases in Ghana and Their Management; Smartline Publishing Ltd.: Tema, Ghana, 2008; p. 104. [Google Scholar]
- Alberto, R.T. Pathological response and biochemical changes in Allium cepa L. (bulb onions) infected with anthracnose-twister disease. Plant Pathol. Quar. 2014, 4, 23–31. [Google Scholar] [CrossRef]
- Patil, S.; Nargund, V.B.; Hariprasad, K.; Hegde, G.; Lingaraju, S.; Benagi, V.I. Etiology of Twister Disease Complex in Onion. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3644–3657. [Google Scholar]
- Perez, P.M.; Alberto, R.T. Chemical Management of anthracnose—Twister (Colletotrichum gloeosporioides and Fusarium fujikuroi) disease of onion (Allium cepa) Plant Pathol. Quar. 2020, 10, 198–216. [Google Scholar]
- Alberto, R.T.; Duca, M.S.V.; Santiago, S.E.; Miller, S.E.; Black, L.L. First report of anthracnose of onion (Allium cepa L.) caused by Colletotrichum gloeosporioides (Penzig) Penzig and Sacc., in the Philippines. J. Trop. Plant Pathol. 2004, 37, 46–51. [Google Scholar]
- Gyempeh, N. Aetiology and control of the onion twister disease in the Eastern Region of Ghana. Master’s Thesis, University of Ghana, Legon, Accra, Ghana, 2013; p. 93. [Google Scholar]
- Isaac, S. Fungal-Plant Interactions; Springer Science and Business Media: Berlin, Germany, 1991. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.A.; Jeger, M.J. Colletotrichum: Biology, Pathology and Control; CABI: Wallingford, UK, 1992. [Google Scholar]
- Gautam, A.K.; Avasthi, S.; Bhadauria, R. New Plant Fungal Diseases during 21st Century in India: An Update for 2000–2011. In New Plant Fungal Diseases During 21st Century in India: An Update for 2000–2011; Lambert Academic Publishin: Saarbrücken, Germany, 2012. [Google Scholar]
- Wikee, S.; Cai, L.; Pairin, N.; McKenzie, E.H.C.; Su, Y.-Y.; Chukeatirote, E.; Thi, H.N.; Bahkali, A.H.; Moslem, M.A.; Abd-Elsalam, K.; et al. Colletotrichum species from Jasmine (Jasminum sambac). Fungal Divers. 2011, 46, 171–182. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanlong, N.; Inchun, P.; Srisomsuk, P. Colletorichum gloeosporioides, the causal agent of onion twister disease. Warasan Wichakankaset 1987, 5, 49–53. [Google Scholar]
- Panday, S.S.; Alberto, R.T.; Labe, M.S. Ultrastructural characterization of infection and colonization of Colletotrichum gloeosporioides in onion. Plant Pathol. Quar. 2012, 2, 168–177. [Google Scholar] [CrossRef]
- Weeraratne, G.W.A.P. Leaf twister disease of onion (Allium cepa L.). Trop. Agric. 1997, 151, 25–33. [Google Scholar]
- Rajapaksha, R.G.A.S.; Edirimanne, S.P.; Weeraratne, G.; Yapa, D.R. Management of leaf twister disease of shallots in Kolonna area (IM2 Agro ecological region). Ann. Sri Lanka Dep. Agric. 2001, 3, 201–210. [Google Scholar]
- Nugaliyadda, M.M.; Ekanayake, H.M.R.K. Present situftion of nematode problem on onion. Krushi 1986, 9, 7–8. [Google Scholar]
- Mithrasena, A.H.J. Screening of fungicides against red onion leaf curl disease. In Seasonal Report (Maha 1993/94) RARDC; Department of Agriculture: Angunakolapalassa, Sri Lanka, 1994; p. 24. [Google Scholar]
- Hegde, G.M.; Rajkumar, G.R.; Jaware, G. Integrated Disease Management of Twister Disease of Onion; Universities for Agricultural Sciences: Dharwad, India, 2012. [Google Scholar]
- Patil, S.; Nargund, V.B.; Balol, G.; Machenahalli, S.; Byadgi, A.S.; Ravichandra, S. Molecular variability of causal agents of twister disease of onion. J. Pure Appl. Microbiol. 2016, 10, 3043–3049. [Google Scholar] [CrossRef]
- Agron, E.B. New anthracnose pathogen attacking onion found-study [conducted in the Philippines]. Agriculture 2009, 10, 98. [Google Scholar]
- Herath, I.S.; Udayanga, D.; Miriyagalla, S.; Castlebury, L.A.; Manamgoda, D.S. Colletotrichum siamense causing anthracnose-twister disease of onion (Allium cepa) in Sri Lanka. Australas. Plant Dis. Notes 2021, 16, 1–6. [Google Scholar]
- Anonymous. Investigations on Bulb Rot and Twisting of Onion Leaves and It Management; Report Submitted by Dr. S. T. Yenjerappa, Krishi Vigyan Kendra, Hagari; Bellary To Dept. of Horticulture, GOK: Bangalore, India, 2005. [Google Scholar]
- Santana, K.F.A.; Garcia, C.B.; Matos, K.S.; Hanada, R.E. First Report of Anthracnose Caused by Colletotrichum spaethianum on Allium fistulosum in Brazil. Plant Dis. 2016, 100, 1–3. [Google Scholar] [CrossRef]
- Salunkhe, V.N.; Anandhan, S.; Gawande, S.J.; Ikkar, R.B.; Bhagat, Y.S.; Mahajan, V.; Singh, M. First Report of Anthracnose Caused by Colletotrichum spaethianum on Allium ledebourianum in India. Plant Dis. 2018, 102, 1–3. [Google Scholar] [CrossRef]
- Bajad, A.R.; Patil, A.C.; Jadhav, R.R.; Shendge, V.S. Bioefficacy of botanicals against Colletotrichum gloeosporioides of onion. J. Pharmacogn. Phytochem. 2018, 7, 1086–1088. [Google Scholar]
- Rajasab, A.H.; Chawda, H.T. Dispersal of the conidia of Colletotrichum gloeosporioides by rain and the development of anthracnose on onion. Grana 1994, 33, 162–165. [Google Scholar] [CrossRef]
- Alberto, R.T.; Isip, M.F.; Biagtan, A.R.; Tagaca, R.C. Disease risk map of anthracnose-twister of onion based on previous disease locations as a future predictors. Spat. Inf. Res. 2019, 27, 259–265. [Google Scholar] [CrossRef]
- Kuruppu, P.U. First Report of Fusarium oxysporum causing a leaf twisting disease on Allium cepa var. ascalonicum in Sri Lanka, Disease Notes Louisiana State University, Baton Rouge. Plant Dis. 1999, 83, 695. [Google Scholar] [CrossRef]
- Vengadaramana, A.; Costa, D.M.D. Molecular and pathogenic diversity of the causal agent of Onion Leaf Twister Disease in Batticaloa District of Sri Lanka. Univers. J. Plant Sci. 2014, 2, 121–127. [Google Scholar]
- Chowdappa, P.; Chethana, C.S.; Pavani, K.V. Colletotrichum siamense and C. truncatum are responsible for severe outbreaks of anthracnose on onion in southwest India. J. Plant Pathol. 2015, 1, 77–86. [Google Scholar]
- Matos, K.S.; Santana, K.F.A.; Catarino, A.M.; Hanada, R.E. First report of anthracnose on welsh onion (Allium fistulosum) in Brazil caused by Colletotrichum theobromicola and C. truncatum. Plant Dis. 2017, 101, 1055–1056. [Google Scholar] [CrossRef]
- Salunkhe, V.N.; Anandhan, S.; Gawande, S.J.; Ikkar, R.B.; Bhagat, Y.S.; Mahajan, V. First Report of Colletotrichum truncatum Causing Anthracnose of Mouse Garlic (Allium angulosum) in India. Plant Dis. 2018, 102, 240. [Google Scholar] [CrossRef]
- Shumway, C.; Russo, V.M.; Pappelis, A.J. Germination and post-germination development of Colletotrichum dematium f. circinans on Allium cepa. Mycopathologia 1983, 82, 125–127. [Google Scholar] [CrossRef]
- John, H.K.; Pappelis, A.J.; Russo, V.M. Visualization of halos in the epidermal cell wall of Allium cepa caused by Colletotrichum dematium f. circinans and Botrytis alli using fluorochromes. Mycopathologia 1987, 97, 137–141. [Google Scholar]
- Baysal-Gurel, F.; Subedi, N.; Mamiro, D.P.; Miller, S.A. First report of Anthracnose of onion caused by Colletotrichum coccodes in Ohio. Plant Dis. 2014, 98, 1271. [Google Scholar] [CrossRef]
- Nolla, J.A.B. Onion-leaf anthracnose. J. Agric. Univers. Puerto Rico 1926, 10, 245–256. [Google Scholar] [CrossRef]
- Lopes, L.H.R.; Boiteux, L.S.; Rossato, M.; Aguiar, F.M.; Fonseca, M.E.N.; Oliveira, V.R.; Reis, A. Diversity of Colletotrichum species causing onion anthracnose in Brazil. Eur. J. Plant Pathol. 2021, 59, 339–357. [Google Scholar] [CrossRef]
- Zhang, N.; Blackwell, M. Population structure of dogwood anthracnose fungus. Phytopathology 2002, 92, 1276–1283. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.S.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Huhndorf, S.M.; Miller, A.N.; Fernańdez, F.A. Molecular systematics of the Sordariales: The order and the family Lasiosphaeriaceae redefined. Mycologia 2004, 96, 368–387. [Google Scholar] [CrossRef]
- Von Arx, J.V. Die Arten der Gattung Colletotrichum Corda. Phytopathol. Zeitschrift 1957, 29, 413–468. [Google Scholar]
- Sutton, B.C. Te Genus Glomerella and Its anamorph Colletotrichum; Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 1–26. [Google Scholar]
- Xie, L.; Zhang, J.Z.; Wan, Y.; Hu, D.W. Identification of Colletotrichum spp. Isolated from strawberry in Zhejiang Province and Shanghai City, China. J. Zhejiang Univ. Sci. B Biomed. Biotechnol. 2010, 11, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Malik, S.A. Some studies on anthracnose of mango caused by Glomerella cingulata (Stonem.) Spaulding and von Schrenk, (Colletotrichum gloeosporioides Penz.) in Punjab. Indian J. Agric. Sci. 1939, 9, 511–521. [Google Scholar]
- Bose, S.K.; Sindhan, G.S.; Pandey, B.H. Studies on dieback disease of mango in Tarai region of Kumaon. Prog. Hortic. 1973, 70, 557–584. [Google Scholar]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, A.; Tiwari, K.K.; Chaudhary, K.; Pratap, D. Genic molecular markers in fungi: Availability and utility for bioprospection. In Molecular markers in Mycology; Springer: Cham, Switzerland, 2017; pp. 151–176. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Chethana, B.S.; Girija, G.; Archana, S.R.; Bellishree, K. Morphological and molecular characterization of Alternaria isolates causing purple blotch disease of onion. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3478–3493. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. Te Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, T.B.; Correll, J.C. Clarifcation of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology 2006, 96, 982–992. [Google Scholar]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.; Waller, J.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Galván, G.A.; Koning-Boucoiran, C.F.; Koopman, W.J.; Burger-Meijer, K.; González, P.H.; Waalwijk, C.; Scholten, O.E. Genetic variation among Fusarium isolates from onion, and resistance to Fusarium basal rot in related Allium species. Eur. J. Plant Pathol. 2008, 121, 499–512. [Google Scholar] [CrossRef]
- Dissanayake, M.L.M.C.; Kashima, R.; Tanaka, S.; Ito, S.I. Genetic diversity and pathogenicity of Fusarium oxysporum isolated from wilted Welsh onion in Japan. J. General Plant Pathol. 2009, 75, 125–130. [Google Scholar] [CrossRef]
- Schoch, C.L. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Udayanga, D.; Manamgoda, D.S.; Tedersoo, L.; Larsson, E.; Abarenkov, K.; Bertrand, Y.J.; Oxelman, B.; Hartmann, M.; Kauserud, H.; et al. Incorporating molecular data in fungal systematics: A guide for aspiring researchers. Curr. Res. Environ. Appl. Mycol. 2013, 3, 1–32. [Google Scholar] [CrossRef]
- Gazis, R.; Rehner, S.; Chaverri, P. Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Mol. Ecol. 2011, 20, 3001–3013. [Google Scholar] [CrossRef]
- Parka, M.S.; Kimb, B.R.; Parkb, I.H.; Hahmb, S.S. First report of two Colletotrichum species associated with bitter rot on apple fruit in Korea—C. fructicola and C. siamense. Mycobiology 2018, 46, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005; p. 922. [Google Scholar]
- Than, P.P.; Jeewon, R.; Hyde, K.D.; Pongsupasamit, S.; Mongkolporn, O.; Taylor, P.W.J. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol. 2008, 57, 562–572. [Google Scholar] [CrossRef]
- Dodd, J.C.; Estrada, A.; Jeger, M.J. Epidemiology of Colletotrichum gloeosporioides in the Tropics. In Colletotrichum: Biology, Pathology and Control; CAB International: Wallingford, UK, 1992; pp. 308–325. [Google Scholar]
- Alberto, R.T.; Tiedeman, A.V.; Wolf, G.; Danzinger, H.L. Light microscopy study on the pathological histology of Colletotrichum gloeosporioides (Penzig) Penzig and Sacc. in onion. J. Trop. Plant Pathol. 2003, 38, 47–51. [Google Scholar]
- Roberts, P.D.; Pernezny, K.; Kucharek, T.A. Anthracnose caused by Colletotrichum sp. on pepper. J. Univ. Florida/Inst. Food Agric. Sci. 2001. [Google Scholar]
- Royle, D.; Butler, D. Liquid water in crop canopies and its role in disease forecasting. Water Fungi Plants 1986, 11, 139. [Google Scholar]
- Senechal, Y.; Sanier, C.; Gohet, E.; Dauzac, J. Different ways of penetration of Colletotrichum gloeosporioides into the leaves of Hevea brasiliensis. Phytopathology 1987, 305, 537–542. [Google Scholar]
- Bailey, J.A.; O’Connell, R.J.; Pring, R.J.; Nash, C. Infection strategies of Colletotrichum species. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CABI: Wallingford, UK, 1992; pp. 88–120. [Google Scholar]
- Gadge, S.S.; Lawande, K.E. Crop Damage Due to Climatic Change: A Major Constraint in Onion Farming. Indian Res. J. Ext. Educ. 2012, 2, 38–41. [Google Scholar]
- Alberto, R.T.; Perez, P.M. Development of integrated disease management program against Anthracnose-Twister (Colletotrichum gloeosporioides-Gibberella moniliformis) disease of onion (Allium cepa). Plant Pathol. Quar. 2020, 10, 111–119. [Google Scholar] [CrossRef]
- Bajwa, R.; Khalid, A.; Cheema, T.S. Antifungal activity of allelopathic plant extracts III: Growth response of some pathogenic fungi to aqueous extract of Parthenium hysterophorus. Pakistan J. Plant Pathol. 2003, 2, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Ghewande, M.P. Management of foliar diseases of groundnut (Arachis hypogaea) using plant extracts. Indian J. Agric. Sci. 1989, 59, 133–134. [Google Scholar]
- Mishra, R.K.; Jaiswal, R.K.; Kumar, D.; Saabale, P.R.; Singh, A. Management of major diseases and insect pests of onion and garlic: A comprehensive review. J. Plant Breed. Crop Sci. 2014, 6, 160–170. [Google Scholar]
- Singh; Mandvi; Singh, R.P. Management of mushroom pathogens through botanicals. Indian Phytopathol. 2005, 58, 189–193. [Google Scholar]
- Rana, U.; Sugha, S.K.; Rana, S.K. Integrated management of Colocasia esculenta blight. Indian Phytopathol. 2007, 60, 457–461. [Google Scholar]
- Singh, S.R.; Prajapati, R.K.; Srivastava, S.S.L.; Pandey, R.K.; Gupta, P.K. Evaluation of different botanicals and non-target pesticides against Scterotium rolfsii causing collar rot of lentil. Indian Phytopathol. 2007, 60, 499–501. [Google Scholar]
- Meena, L.; Verma, A. Fungal Diseases of Onion and Their Biological Management: A Review. Int. J. Recent. Sci. Res. 2017, 8, 19441–19445. [Google Scholar]
- Svetlana, Z.; Stojanovic, S.; Ivanovic, Z.; Gavrilovic, V.; Popovic, T.; Jelica, B. Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gleosporioides. Arch. Biol. Sci. 2010, 62, 611–623. [Google Scholar]
- Streptomyces sp.MJM5763. J. App. Microbio. 2011, 111, 443–455.
- Soytong, K.; Srinon, W.; Rattanacherdchai, K.; Kanokmedhakul, S.; Kanokmedhakul, K. Application of antagonistic fungi to control anthracnose disease of grape. J. Agric. Biotechnol. 2005, 1, 33–41. [Google Scholar]
- Gyempeh, N.; Offei, S.K.; Cornelius, E.W.; Honger, J.O. Importance of the onion leaf twister disease in Ghana and the effect of Trichoderma asperellium on the mycelial growth and sporulation of the causal agent. Ghana J. Sci. 2015, 55, 51–65. [Google Scholar]
- Thongprom, N. Biological Control of Anthracnose on Green Shallot Using Antagonistic Bacillus Subtilis. Ph.D. Dissertation, School of Crop Production Technology Institute of Agricultural Technology, Suranaree University of Technology, Nakhon Ratchasima, Thailand.
- Murtado, A.; Mubarik, N.R.; Tjahjoleksono, A. Isolation and characterization endophytic bacteria as biological control of fungus Colletotrichum sp. on onion plants (Allium cepa L.). In Proceedings of the 3rd International Conference on Biosciences. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 20–43. [Google Scholar] [CrossRef]
- Dutta, R.; Jayalakshmi, K.; Sharath, M.N.; Vishal, S.G.; Singh, M. Biocontrol potential of native Trichoderma spp. against the anthracnose-twister disease of onion. In Proceedings of the National Symposium on Sustainable Plant Health Management Amidst Covid Pandemic: Challenges and Strategies, Virtual, 1–3 December 2021. 93p. [Google Scholar]
- Ademe, A.; Ayalew, A.; Woldetsadik, K. Evaluation of antifungal activity of plant extracts against papaya anthracnose (Colletotrichum gloeosporioides). J. Plant Pathol. Microbiol. 2013, 4, 207. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S.; Ahmad, M.Z.; Salam, F. An Update Review on Polyherbal Formulation: A Global Perspective. Syst. Rev. Pharm. 2016, 7, 35–41. [Google Scholar] [CrossRef]
- Naguleswaran, V.; Pakeerathan, K.; Mikunthan, G. Biological control: A promising tool for bulb-rot and leaf twisting fungal diseases in red onion (Allium cepa L.) in Jaffna district. World Appl. Sci. J. 2014, 31, 1090–1095. [Google Scholar]
- Manthesha, H.D.; Mallikarjun; Kenganal; Yenjerappa, S.T.; Aswathanarayana, D.S. Vikas Kulkarni Management of onion twister disease under field condition. Pharma. Innov. J. 2022, 11, 551–555. [Google Scholar]
- Russell, G.S. A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields. Agronomy 2013, 3, 757–793. [Google Scholar]
- Massimo, M.; Raffaella, C. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, H.R.; Gupta, M. Screening of Cucumber Germplasm against Economically Important Diseases under Open Field and Mid-Hill Conditions of Himachal Pradesh. Environ. Ecol. 2017, 35, 1484–1486. [Google Scholar]
- Suhardi, H.A. Epidemiological Studies of Anthracnose (Colletotrichum gloeosporioides) on Shallot (Allium cepa var. aggregatum). Ph.D. Thesis, Universitas Gadjah Mada, Yokyakarta, Indonesia, 1994. [Google Scholar]
- Suhardi, H.A. Anthracnose on shallot (Allium cepa group aggregatum) in Java. Onion Newsl. Trop. 1993, 5, 48–50. [Google Scholar]
- Melo, I.G.; Costa, C.P. Selec¸aomassalemcebola (Allium cepa L.) populac¸ao Pira Ouro para resistencia a Colletotrichum gloeosporioides Penz. Summa. Phytopathol. 1983, 9, 214–218. [Google Scholar]
- Galván, G.A.; Wietsma, W.A.; Putrasemedja, S.; Permadi, A.H.; Kik, C. Screening for resistance to anthracnose (Colletotrichum gloeosporioides Penz.) in Allium cepa and its wild relatives. Euphytica 1997, 95, 173–178. [Google Scholar] [CrossRef]
- Assuncao, I.P.; Coclho, R.S.B.; Lima, G.S.; de-A.; Lima, J.A.S.; Tavares, S.C.C. Reaction of onion cultivators to isolates of Collectotrichum gloeosporioides from sub medio San Francisco Region, Brazil. Summa. Phytopathol. 1999, 25, 205–209. [Google Scholar]
- Alberto, R.T. Integrated management of onion anthracnose [Colletotrichum gloeosporioides (Penzig) Penzig and Sacc.]. Trop. Plant Pathol. 2003, 39, 43–48. [Google Scholar]
- Patil, S. Onion Twister Disease: Etiology, Their Characterization, Epidemiology and Integrated Management. Ph.D. Dissertation, University of Agricultural Sciences, Dharwad, India, 2013. [Google Scholar]
- Directorate of Onion and Garlic Research. Annual Report 2012–2013; ICAR-DOGR: Rajgurunagar, India, 2013; p. iv.
- Directorate of Onion and Garlic Research. Annual Report 2017–2018; ICAR-DOGR: Rajgurunagar, India, 2018; p. 25.
- Directorate of Onion and Garlic Research. Annual Report 2018–2019; ICAR-DOGR: Rajgurunagar, India, 2019; p. 35.
- Directorate of Onion and Garlic Research. Annual Report 2019–2020; ICAR-DOGR: Rajgurunagar, India, 2020; p. 41.
- Directorate of Onion and Garlic Research. Annual Report 2020–2021; ICAR-DOGR: Rajgurunagar, India, 2021; pp. 46–48.
- Hedden, P.; Graebe, J.E. Inhibition of gibberellin biosynthesis by paclobutrazol in cellfree homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J. Plant Growth Regulat. 1985, 4, 111–122. [Google Scholar] [CrossRef]
- Gianfagna, T. Natural and synthetic growth regulators and their use in horticultural and agronomic crops. In Plant Hormones: Physiology, Biochemistry, and Molecular Biology; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 751–773. [Google Scholar]
- Remiro, D.; Kirmati, H. Control of seven curlsdisease of onion with benomyl. Sum. Phytopathol. 1975, 51–54. [Google Scholar]
- Kanlong, N.; Inchan, P.; Wannaphi, L. Anthracnose, onion twister disease and their control. Thai Phytopathol. 1988, 8, 97–104. [Google Scholar]
- Weeraratne, W.A.P.G.; Yapa, D.R. Fungicidal for Leaf Twister Diseases in Big Onion; Annual Report; Department of Agriculture, FCRDI: Mahailluppallama, Sri Lanka, 1999; pp. 101–102. [Google Scholar]
- Rajapaksha, R.G.A.S.; Edirimanne, E.R.S.P. Management of bulb rot of big onion during storage using fungicides. Ann. Sri Lanka Dep. Agric. 2002, 4, 319–326. [Google Scholar]
- Pandey, U.B.; Bhonde, S.R. Onion Production in India Technical Bulletin; National Horticultural Research and Development: Delhi, India, 2002; p. 9. [Google Scholar]
- Clarrdec. (Central Luzon Agriculture, Resources Research, Development Consortium) Major Diseases of Onion: A Field Guide; Pcarrddost, Clarrdec-Clsu: Los Baños, Laguna, CA, USA, 2007; p. 18. [Google Scholar]
- DeForest, L.B.; Brad, K.C.; Kao, G.J.; Lutton, J. A onion disease guide a practical guide for seedsmen, growers and agricultural advisors practical guide for seedsmen. In Growers and Agriculural Advisors Onion Disease Guide; 2008. [Google Scholar]
- Priyantha, M.G.D.L.; Jayasinghe, J.A.V.J.; Dissanayaka, D.M.K. Thiophanate methyl resistance among Colletotrichum gloeosporioides populations in Sri Lanka causing onion anthracnose. Trop. Agric. 2011, 159, 47–59. [Google Scholar]
- Priyantha, M.G.D.L.; Jayasinghe, J.A.V.J.; Dissanayaka, D.M.K. Relative efficacy of fungicides in controlling anthracnose and purple blotch diseases in onion. In Annual Report; Department of Agriculture, FCRDI: Mahailluppallama, Sri Lanka, 2010; pp. 41–42. [Google Scholar]
- Miller, S.A. Onion Anthracnose–New to Ohio; Department of Plant Pathology, The Ohio State University: Columbus, OH, USA, 2013. [Google Scholar]
- Nargund, V.B.; Gurudath, H.; Nayak, G.V.; Benagi, V.I.; Suresh, P.; Dharmatti, P.R.; Ravichandran, S. Management of twister disease in sweet onion—A strategy for livelihood improvement and welfare of mankind. In Proceedings of the Int. Symp. Human Health Effects of Fruits and Vegetables; Universities for Agricultural Sciences: Dharwad, India, 2013; p. 205. [Google Scholar]
- Jayalakshmi, K.; Dutta, R.; Sharath, M.N.; Vishal, S.; Gurav. Evaluation of effective modules for management of onion anthracnose in Maharashtra, India. In Proceedings of the 8th International Conference (hybrid mode) on “Plant Pathology: Retrospect and Prospects” at Sri Karan Narendra Agriculture University, Jobner-Jaipur, Rajasthan, India, 23–26 March 2022. [Google Scholar]
- Dutta, R.; Suresh, J.; Gawande; Jayalakshmi, K.; Sharath, M.N.; Vishal, S.; Gurav. Ram Bombale and Major Singh. Efficacy of Amritpani-based organic formulations for crop health and management of major fungal diseases of onion. In Proceedings of the International Conference on Vegetable Research and Innovations for Nutrition, Entrepreneurship and Environment (ICVEG-21), Varanasi, India, 14–16 December 2021. 303p. [Google Scholar]


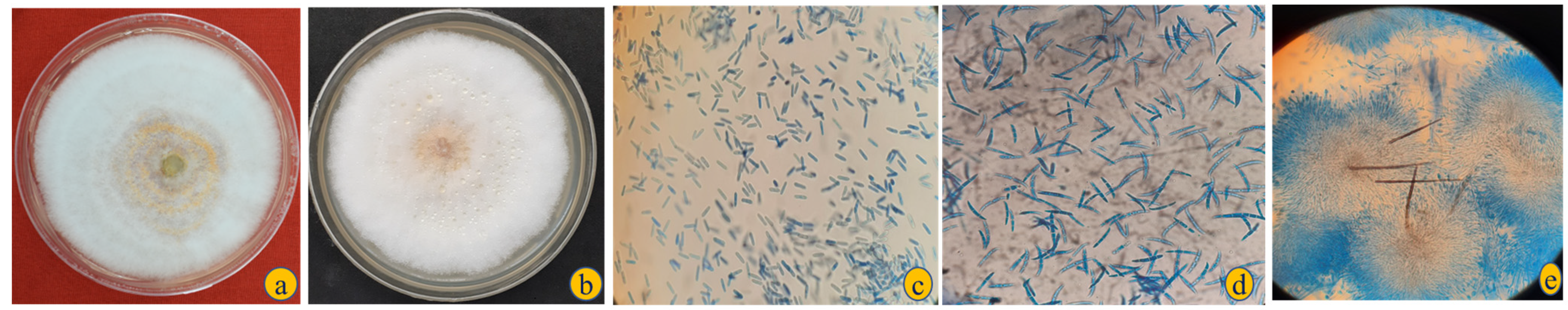
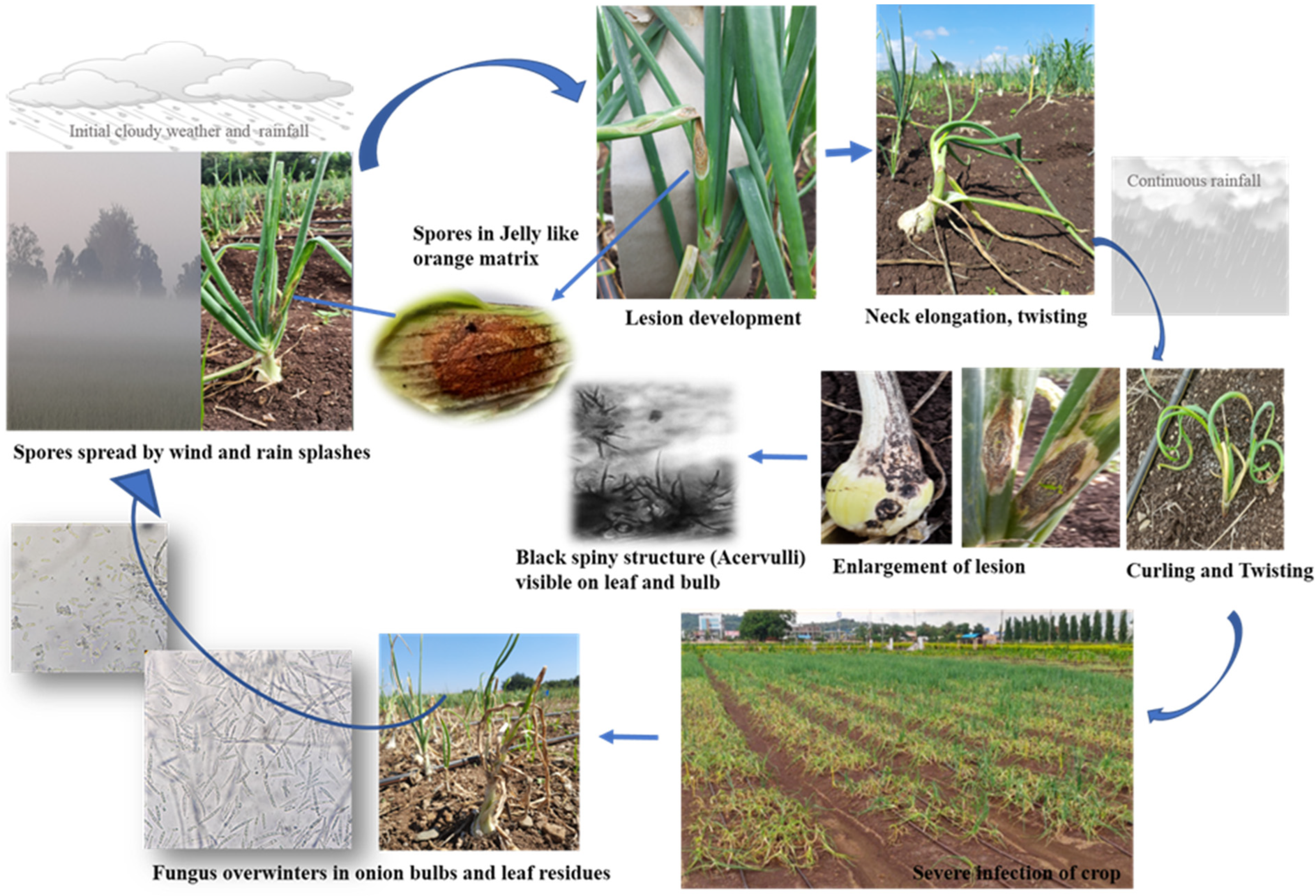

| Rating Scale | Description | Corresponding % Damage | Pictorial Representation as per Figure 1 |
|---|---|---|---|
| 1 | Small white specks | 0.1–1 | B |
| 2 | Chlorotic spots | 1.1–2 | C |
| 3 | Advancement of the lesion concentric rings | 2.1–6 | D |
| 4 | A mature lesion with salmon/orange-colored conidial mass | 3.1–11 | E |
| 5 | Lesions began to coalesce | 11.1–21 | F |
| 6 | Advanced lesion leading to death of leaf blades | 21.1–31 | G |
| 7 | Most advanced stage lesions; the dieback appearance of the plant leaving few leaf blades unaffected | 31.1–41 | H |
| 8 | Advanced lesions on the neck region | 41.1–61 | Ia,b |
| 9 | Complete infection; the death of the plant/black fruiting bodies on the entire bulb | 61.1–100 | Ja–c |
| Rating Scale | Description | Corresponding % Damage | Pictorial Representation as per Figure 2 |
|---|---|---|---|
| 1 | Slight twisting from the neck | <10 | B |
| 2 | Slight abnormal elongation of the neck and twisting | 11–20 | C |
| 3 | Twisting of the leaves with abnormal neck elongation | 21–30 | D |
| 4 | Twisting with severe neck elongation with leaf curling | 31–40 | Ea,b |
| 5 | Severe stage of twisting falling of plants on the ground and neck and foliage becoming slender | 41–50 | F |
| 6 | Leaf twisting and initial anthracnose lesions | 51–60 | G |
| 7 | Lesion advancement with fruiting body formation along with twisting | 61–70 | H |
| 8 | Twister anthracnose complex leading to death of old and young leaves | 71–80 | I |
| 9 | Twister anthracnose complex leading to severe neck and foliage drying and defoliation or wilting | >81 | Ja,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, R.; K., J.; Nadig, S.M.; Manjunathagowda, D.C.; Gurav, V.S.; Singh, M. Anthracnose of Onion (Allium cepa L.): A Twister Disease. Pathogens 2022, 11, 884. https://doi.org/10.3390/pathogens11080884
Dutta R, K. J, Nadig SM, Manjunathagowda DC, Gurav VS, Singh M. Anthracnose of Onion (Allium cepa L.): A Twister Disease. Pathogens. 2022; 11(8):884. https://doi.org/10.3390/pathogens11080884
Chicago/Turabian StyleDutta, Ram, Jayalakshmi K., Sharath M. Nadig, Dalasanuru Chandregowda Manjunathagowda, Vishal S. Gurav, and Major Singh. 2022. "Anthracnose of Onion (Allium cepa L.): A Twister Disease" Pathogens 11, no. 8: 884. https://doi.org/10.3390/pathogens11080884
APA StyleDutta, R., K., J., Nadig, S. M., Manjunathagowda, D. C., Gurav, V. S., & Singh, M. (2022). Anthracnose of Onion (Allium cepa L.): A Twister Disease. Pathogens, 11(8), 884. https://doi.org/10.3390/pathogens11080884







