Retrospective Survival Analysis of Cats with Feline Infectious Peritonitis Treated with Polyprenyl Immunostimulant That Survived over 365 Days
Abstract
1. Introduction
2. Results
2.1. Signalment and Clinical Signs
2.2. Diagnosis
2.3. Treatment
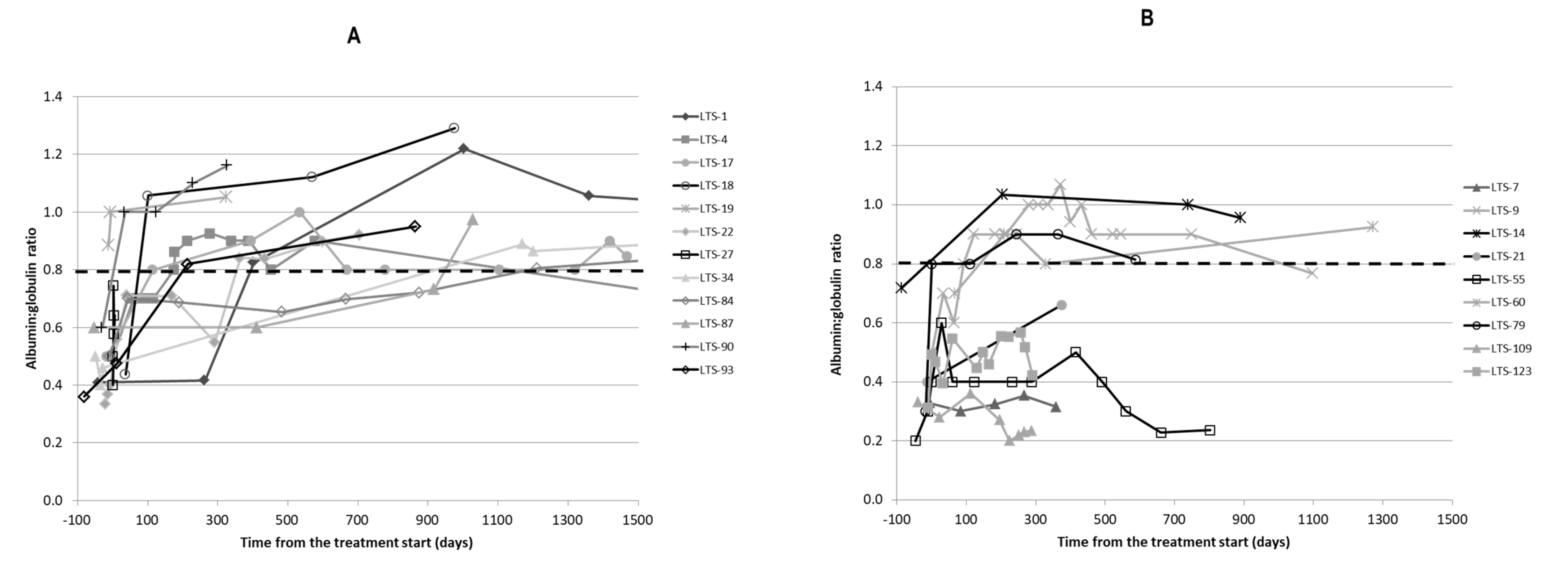
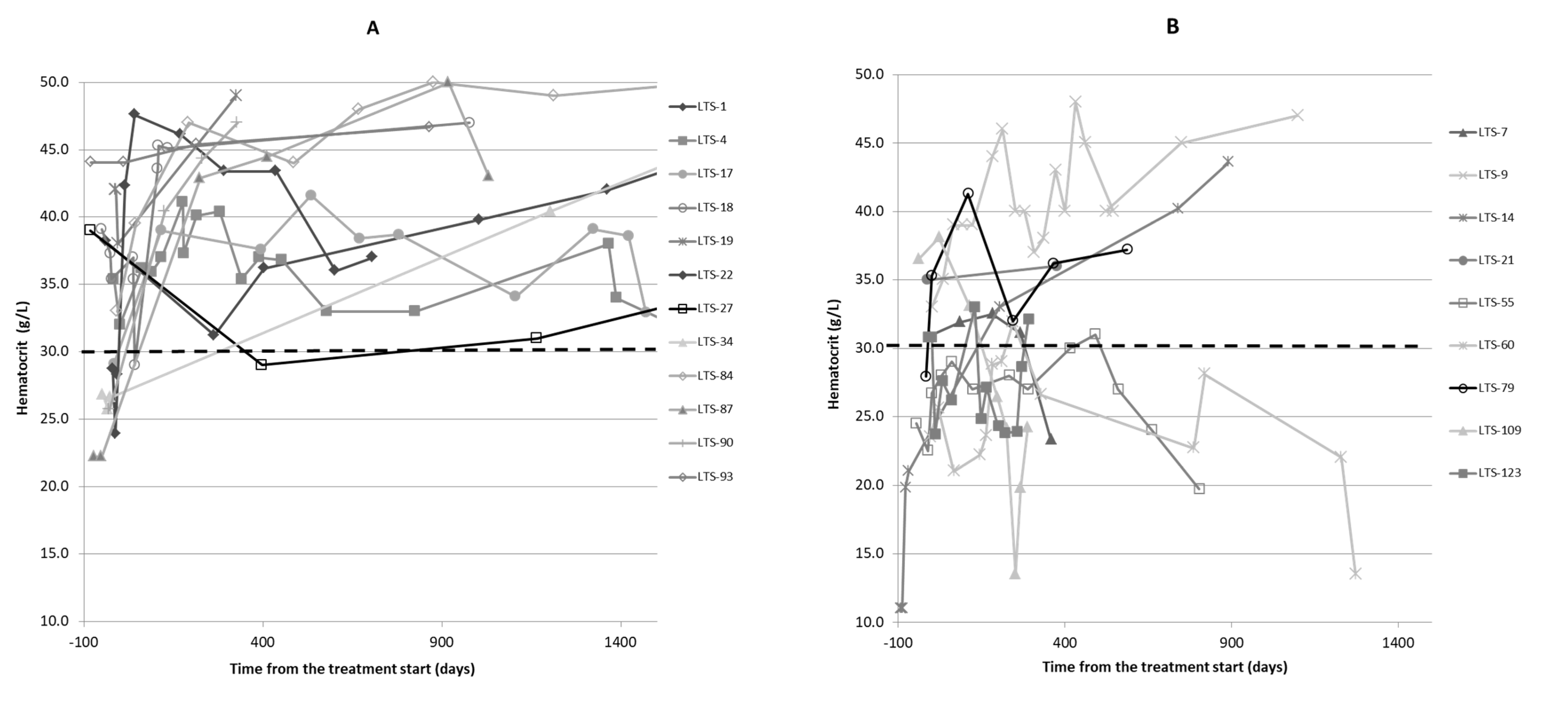
2.4. Clinicopathological Findings
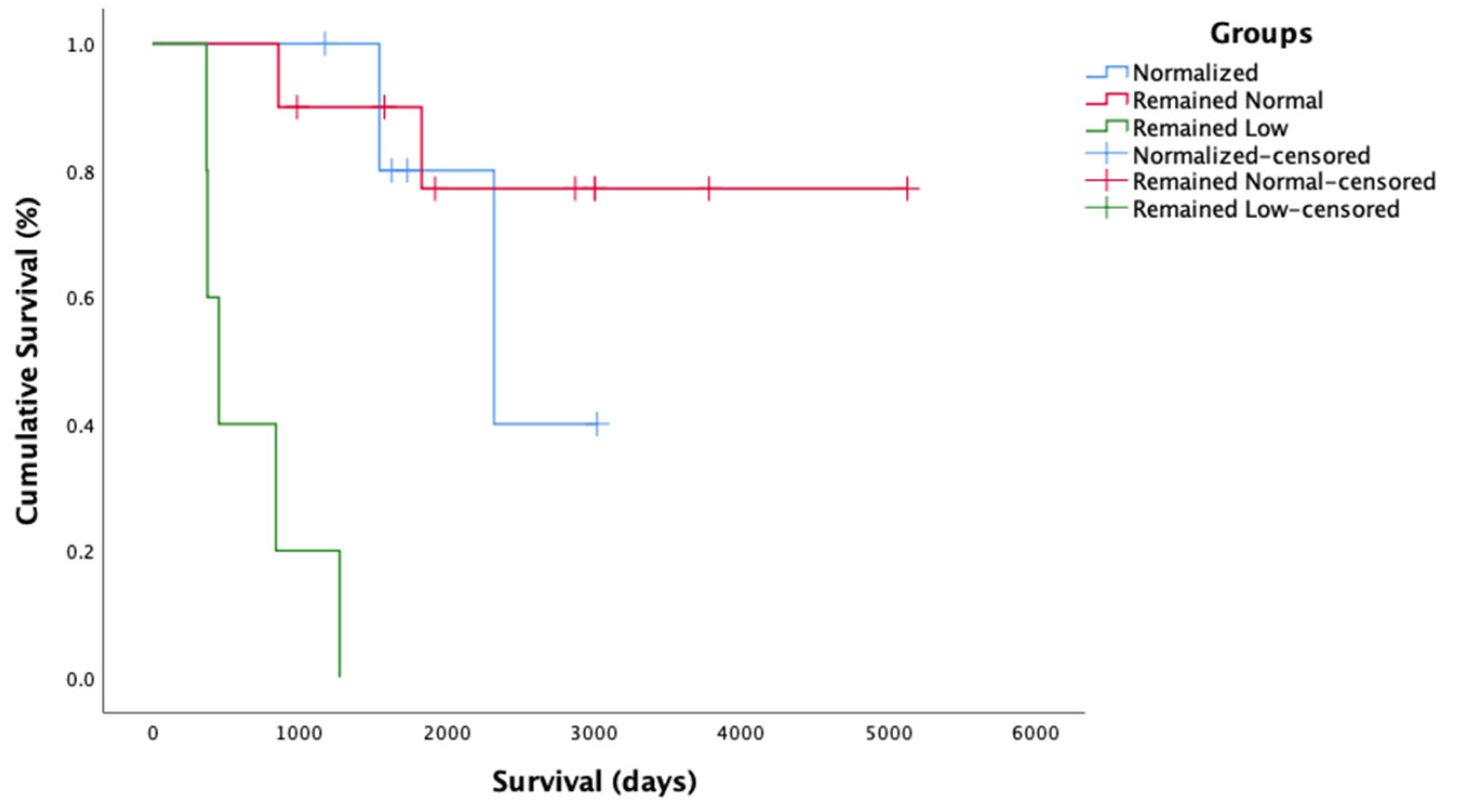
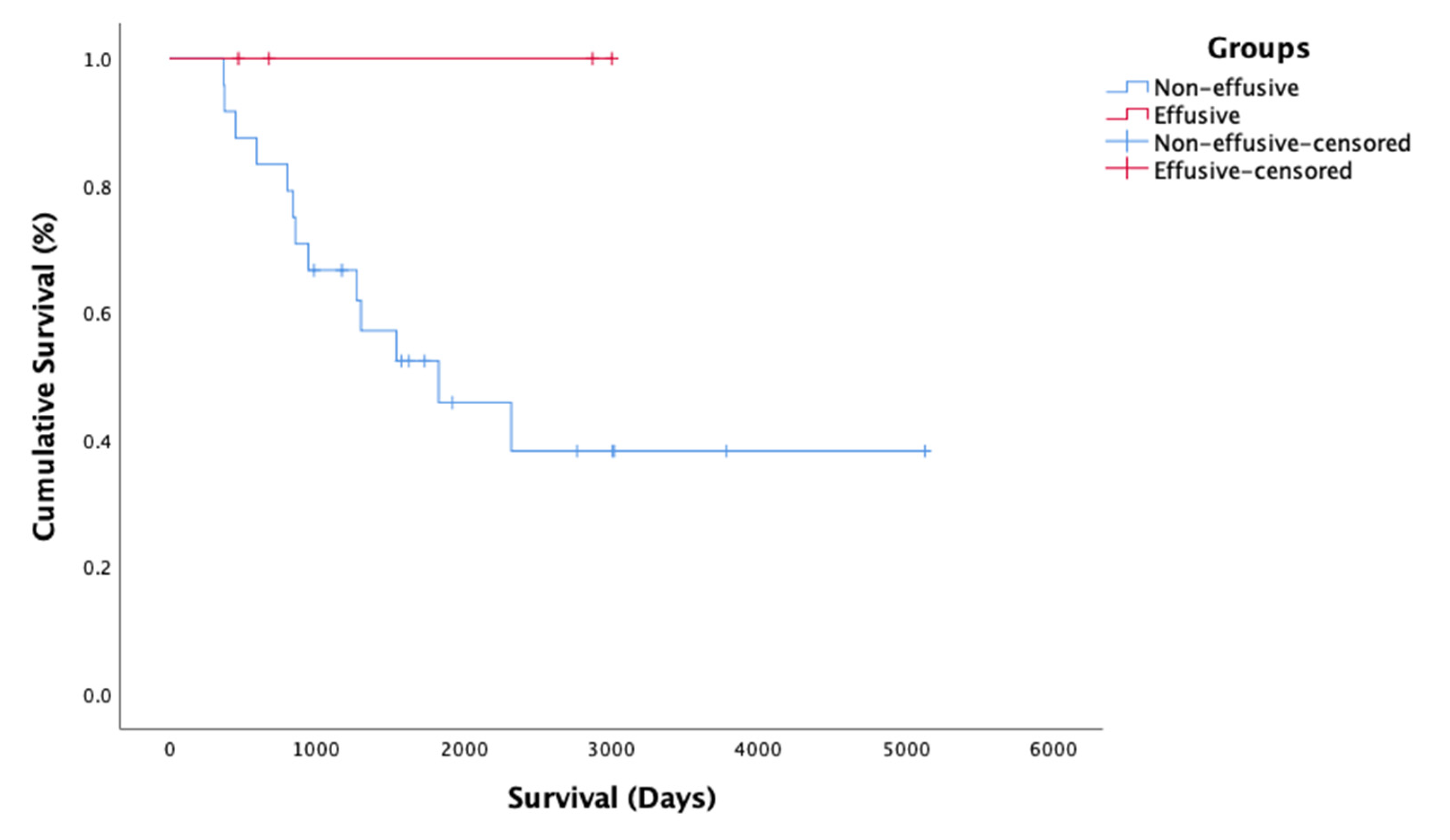
2.5. Survival Time
3. Discussion
4. Materials and Methods
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holzworth, J. Some important disorders of cats. Cornell Vet. 1963, 53, 157–160. [Google Scholar] [PubMed]
- Pedersen, N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009, 11, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sherding, R.G. Feline Infectious Peritonitis (Feline Coronavirus). In Saunders Manual of Small Animal Practice; Elsevier: Amsterdam, The Netherlands, 2006; p. 132. [Google Scholar]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis—A retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.; Gruffydd-Jones, T.; Harbour, D. Feline infectious peritonitis: A review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Vet. Rec. 1991, 129, 209–212. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chueh, L.-L.; Lin, C.-N.; Su, B.-L. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J. Feline Med. Surg. 2011, 13, 74–80. [Google Scholar] [CrossRef]
- Norris, J.; Bosward, K.; White, J.; Baral, R.; Catt, M.; Malik, R. Clinicopathological findings associated with feline infectious peritonitis in Sydney, Australia: 42 cases (1990–2002). Aust. Vet. J. 2005, 83, 666–673. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Cammarata, M.P.; Cammarata, G.; Comazzi, S. Some aspects of humoral and cellular immunity in naturally occuring feline infectious peritonitis. Vet. Immunol. Immunopathol. 1998, 65, 205–220. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Harbour, D.A. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. Am. Anim. Hosp. Assoc. (USA) 1994, 30, 345–350. [Google Scholar]
- Rottier, P.J.; Nakamura, K.; Schellen, P.; Volders, H.; Haijema, B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005, 79, 14122–14130. [Google Scholar] [CrossRef]
- Dewerchin, H.; Cornelissen, E.; Nauwynck, H. Replication of feline coronaviruses in peripheral blood monocytes. Arch. Virol. 2005, 150, 2483–2500. [Google Scholar] [CrossRef]
- Pedersen, N.C. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet. J. 2014, 201, 123–132. [Google Scholar] [CrossRef]
- Kipar, A.; Meli, M. Feline infectious peritonitis: Still an enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.; Van Dun, J.M.; Van Der Most, R.G.; De Groot, R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 2005, 79, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Malbon, A.; Meli, M.L.; Barker, E.; Davidson, A.; Tasker, S.; Kipar, A. Inflammatory mediators in the mesenteric lymph nodes, site of a possible intermediate phase in the immune response to feline coronavirus and the pathogenesis of feline infectious peritonitis? J. Comp. Pathol. 2019, 166, 69–86. [Google Scholar] [CrossRef]
- Watanabe, R.; Eckstrand, C.; Liu, H.; Pedersen, N.C. Characterization of peritoneal cells from cats with experimentally-induced feline infectious peritonitis (FIP) using RNA-seq. Vet. Res. 2018, 49, 81. [Google Scholar] [CrossRef]
- Kuritz, T.K. Methods and Compositions for Modulation of Innate Immunity. U.S. Patent 9,872,867, 23 January 2018. [Google Scholar]
- Legendre, A.M.; Kuritz, T.; Heidel, R.E.; Baylor, V.M. Polyprenyl immunostimulant in Feline rhinotracheitis: Randomized Placebo-controlled experimental and Field safety studies. Front. Vet. Sci. 2017, 4, 24. [Google Scholar] [CrossRef]
- Legendre, A.M.; Bartges, J.W. Effect of Polyprenyl Immunostimulant on the survival times of three cats with the dry form of feline infectious peritonitis. J. Feline Med. Surg. 2009, 11, 624–626. [Google Scholar] [CrossRef]
- Legendre, A.M.; Kuritz, T.; Galyon, G.; Baylor, V.M.; Heidel, R.E. Polyprenyl immunostimulant treatment of cats with presumptive non-effusive feline infectious peritonitis in a field study. Front. Vet. Sci. 2017, 4, 7. [Google Scholar] [CrossRef]
- Marioni-Henry, K.; Vite, C.H.; Newton, A.L.; Van Winkle, T.J. Prevalence of diseases of the spinal cord of cats. J. Vet. Intern. Med. 2004, 18, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Spagnolo, V.; Colombo, A.; Paltrinieri, S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet. J. 2004, 167, 38–44. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L.; Baptiste, K.E.; Bowker, L.J.; Lutz, H. Sites of feline coronavirus persistence in healthy cats. J. Gen. Virol. 2010, 91, 1698–1707. [Google Scholar] [CrossRef]
- Dewerchin, H.L.; Cornelissen, E.; Nauwynck, H.J. Feline infectious peritonitis virus-infected monocytes internalize viral membrane-bound proteins upon antibody addition. J. Gen. Virol. 2006, 87, 1685–1690. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.-O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, J.; Kemp, M.; Yawei, N.; Carpenter, R.; Shannon, W.; McAnalley, B. In vitro evaluation of the synergistic antiviral effects of acemannan in combination with azidothymidine and acyclovir. Mol. Biother. 1991, 3, 214–223. [Google Scholar]
- Lanford, R.E.; Guerra, B.; Lee, H.; Averett, D.R.; Pfeiffer, B.; Chavez, D.; Notvall, L.; Bigger, C. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly (i)-poly (c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 2003, 77, 1092–1104. [Google Scholar] [CrossRef][Green Version]
- Zheng, B.-J.; Chan, K.-W.; Lin, Y.-P.; Zhao, G.-Y.; Chan, C.; Zhang, H.-J.; Chen, H.-L.; Wong, S.S.; Lau, S.K.; Woo, P.C. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc. Natl. Acad. Sci. USA 2008, 105, 8091–8096. [Google Scholar] [CrossRef]
- Addie, D.D.; Silveira, C.; Aston, C.; Brauckmann, P.; Covell-Ritchie, J.; Felstead, C.; Fosbery, M.; Gibbins, C.; Macaulay, K.; McMurrough, J. Alpha-1 Acid Glycoprotein Reduction Differentiated Recovery from Remission in a Small Cohort of Cats Treated for Feline Infectious Peritonitis. Viruses 2022, 14, 744. [Google Scholar] [CrossRef]


| # | Breed | Sex | Age at Diagnosis (Years) | Survival Days * | Clinical Signs at Presentation | Diagnosis Confirmed by | Cause of Death (Where Known) |
|---|---|---|---|---|---|---|---|
| LTS-1 | Domestic Shorthair | MC | 3.8 | Alive 5126 | Weight loss | IHC (mesenteric LN) | |
| LTS-4 | Domestic Shorthair | MC | 0.4 | Alive 3780 | Diarrhea | IHC (intestines) | |
| LTS-6 | Domestic Shorthair | FS | 3.8 | Dead 802 | Weight loss, inappetence | IHC (abdominal mass) | Lymphoma |
| LTS-7 | Domestic Longhair | FS | 11.8 | Dead 374 | Weight loss, vomiting, diarrhea | IHC (intestinal mass) | Anemia |
| LTS-9 | Domestic Longhair | MC | 3.3 | Dead 2035 | Weight loss | IHC (abdominal mass | Renal failure |
| LTS-14 | Maine Coon | MC | 4.3 | Dead 2320 | Weight loss | IHC (mediastinal mass) | HCM |
| LTS-15 | Domestic Shorthair | MC | 3.1 | Dead 943 | Weight loss, lethargy | IHC (mesenteric LN) | Unknown |
| LTS-17 | Sacred Birman | FS | 0.5 | Alive 3020 | Straining to urinate, stunted growth | IHC (abdominal mass) | |
| LTS-18 | Domestic Shorthair | MC | 3.3 | Alive 3002 | Inappetence, lethargy, pyrexia, cough | IHC (LN) | |
| LTS-19 | Domestic Longhair | FS | 12.8 | Alive 3007 | Inappetence, vomiting | IHC (abdominal mass) | |
| LTS-21 | Domestic Shorthair | MC | 1.8 | Dead 856 | Inappetence, weight loss, pyrexia, diarrhea | IHC (mesenteric LN) | FIP |
| LTS-22 | Domestic Shorthair | MC | 2.2 | Alive 1171 | Inappetence, weight loss | IHC (LN) | |
| LTS-27 | Persian | FS | 4.4 | Alive 2870 | Vomiting | IHC (liver and LN) | |
| LTS-29 | Siamese | MC | 11.3 | Dead 1301 | Inappetence, weight loss, uveitis, diarrhea, pyrexia | IHC (colon) | FIP |
| LTS-34 | Sacred Birman | MC | 0.5 | Alive 2767 | Pyrexia | IHC (mesenteric LN) | |
| LTS-55 | Domestic Shorthair | MC | 6.2 | Dead 839 | Weight loss | IHC (abdominal LN) | Anemia |
| LTS-60 | Persian | FS | 6.9 | Dead 1272 | Inappetence, weight loss, lethargy, lameness, pyrexia | IHC (popliteal LN) | Renal failure |
| LTS-79 | Siamese | MC | 11.2 | Dead 1541 | Inappetence, weight loss, diarrhea, vomiting | IHC (Ileocolic mass) | Unknown |
| LTS-83 | Sacred Birman | MC | 5.8 | Alive 2023 | Weight loss | IHC (LN) | |
| LTS-84 | Abyssinian | FS | 6.7 | Alive 1920 | Respiratory signs, lethargy | RT-PCR (BAL fluid) | |
| LTS-87 | Domestic Shorthair | MC | 0.3 | Alive 1731 | Ataxia | RT-PCR (LN) | |
| LTS-90 | Domestic Shorthair | MC | 0.5 | Alive 1625 | Pyrexia | RT-PCR (LN) | |
| LTS-93 | Norwegian Forrest Cat | MC | 1.4 | Alive 1575 | Inappetence | RT-PCR (LN) | |
| LTS-98 | Domestic Shorthair | FS | 4.3 | Dead 591 | Inappetence, weight loss, uveitis | IHC (eye globe) | Unknown |
| LTS-108 | Persian | FS | 0.6 | Alive 982 | Vomiting | IHC (mesenteric LN) | |
| LTS-109 | Somali | FS | 9.7 | Dead 368 | Inappetence, vomiting | RT-PCR (LN) | Anemia |
| LTS-123 | Domestic Shorthair | MC | 0.6 | Dead 451 | Inappetence, weight loss | IHC (abdominal mass) | Anemia |
| LTS-146 | Persian | MC | 3.0 | Alive 677 | Weight loss, dyspnea | RT-PCR (pleural effusion) | |
| LTS-149 | Domestic Shorthair | MC | 0.3 | Alive 469 | Lethargy, dyspnea | RT-PCR (pleural effusion) |
| # | PI Dose | Note |
|---|---|---|
| LTS-1 | Decreased to maintenance dose * | |
| LTS-4 | Decreased to maintenance dose * | |
| LTS-7 | Remained on full dose | |
| LTS-9 | Decreased to maintenance dose * | |
| LTS-14 | Discontinued | Alive off PI for over 1 year |
| LTS-15 | Discontinued | Alive off PI for over 1 year |
| LTS-17 | Decreased to maintenance dose * | |
| LTS-18 | Full dose again after relapse | Relapsed when PI stopped or decreased |
| LTS-19 | Decreased to maintenance dose * | |
| LTS-21 | Remained on full dose | Concurrent treatment with prednisolone (from 5 mg every 12 h to 2.5 mg every over day) |
| LTS-27 | Discontinued | Alive off PI for over 1 year |
| LTS-34 | Discontinued | Alive off PI for over 1 year |
| LTS-55 | Remained on full dose | |
| LTS-60 | Remained on full dose | Concurrent treatment with prednisolone (1.25 mg every 24 h) |
| LTS-83 | Remained on full dose | |
| LTS-84 | Decreased to maintenance dose * | |
| LTS-87 | Discontinued | Alive off PI for over 1 year |
| LTS-90 | Discontinued | Alive off PI for over 1 year |
| LTS-93 | Decreased to maintenance dose * | |
| LTS-98 | Remained on full dose | |
| LTS-108 | Decreased to maintenance dose * | |
| LTS-109 | Remained on full dose | |
| LTS-123 | Remained on full dose | |
| LTS-146 | Decreased to maintenance dose * | |
| LTS-149 | Decreased to maintenance dose * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černá, P.; Ayoob, A.; Baylor, C.; Champagne, E.; Hazanow, S.; Heidel, R.E.; Wirth, K.; Legendre, A.M.; Gunn-Moore, D.A. Retrospective Survival Analysis of Cats with Feline Infectious Peritonitis Treated with Polyprenyl Immunostimulant That Survived over 365 Days. Pathogens 2022, 11, 881. https://doi.org/10.3390/pathogens11080881
Černá P, Ayoob A, Baylor C, Champagne E, Hazanow S, Heidel RE, Wirth K, Legendre AM, Gunn-Moore DA. Retrospective Survival Analysis of Cats with Feline Infectious Peritonitis Treated with Polyprenyl Immunostimulant That Survived over 365 Days. Pathogens. 2022; 11(8):881. https://doi.org/10.3390/pathogens11080881
Chicago/Turabian StyleČerná, Petra, Ashley Ayoob, Caroline Baylor, Erin Champagne, Sandra Hazanow, Robert E. Heidel, Kimberly Wirth, Alfred M. Legendre, and Danièlle A. Gunn-Moore. 2022. "Retrospective Survival Analysis of Cats with Feline Infectious Peritonitis Treated with Polyprenyl Immunostimulant That Survived over 365 Days" Pathogens 11, no. 8: 881. https://doi.org/10.3390/pathogens11080881
APA StyleČerná, P., Ayoob, A., Baylor, C., Champagne, E., Hazanow, S., Heidel, R. E., Wirth, K., Legendre, A. M., & Gunn-Moore, D. A. (2022). Retrospective Survival Analysis of Cats with Feline Infectious Peritonitis Treated with Polyprenyl Immunostimulant That Survived over 365 Days. Pathogens, 11(8), 881. https://doi.org/10.3390/pathogens11080881








