Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs
Abstract
1. Introduction
2. Results
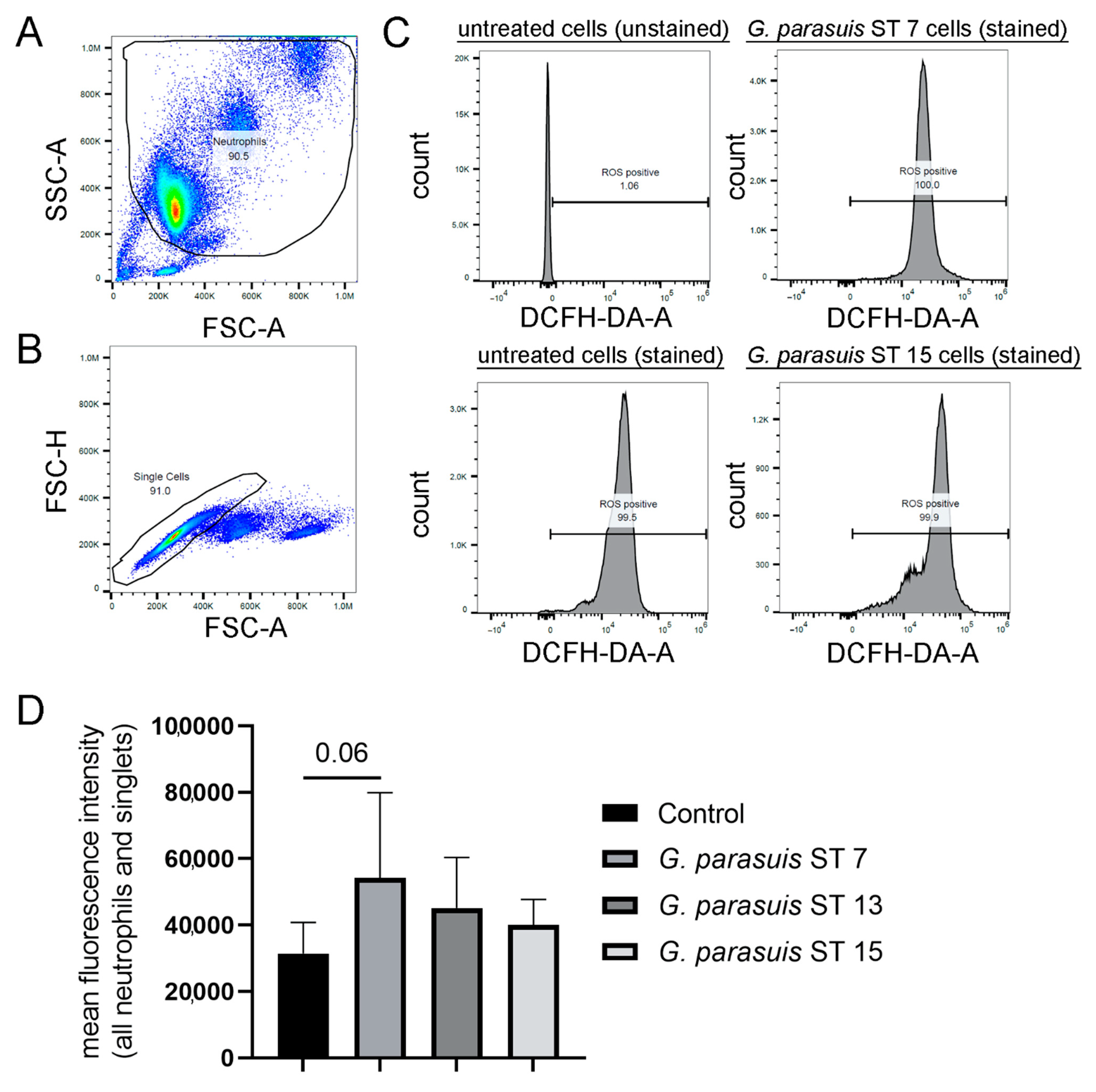
2.1. G. parasuis Serotypes Show a Tendency to Induce ROS Production
2.2. G. parasuis Stimulates Neutrophils to Release the Maximum Amount of NET Activated Cells within One Hour of Infection
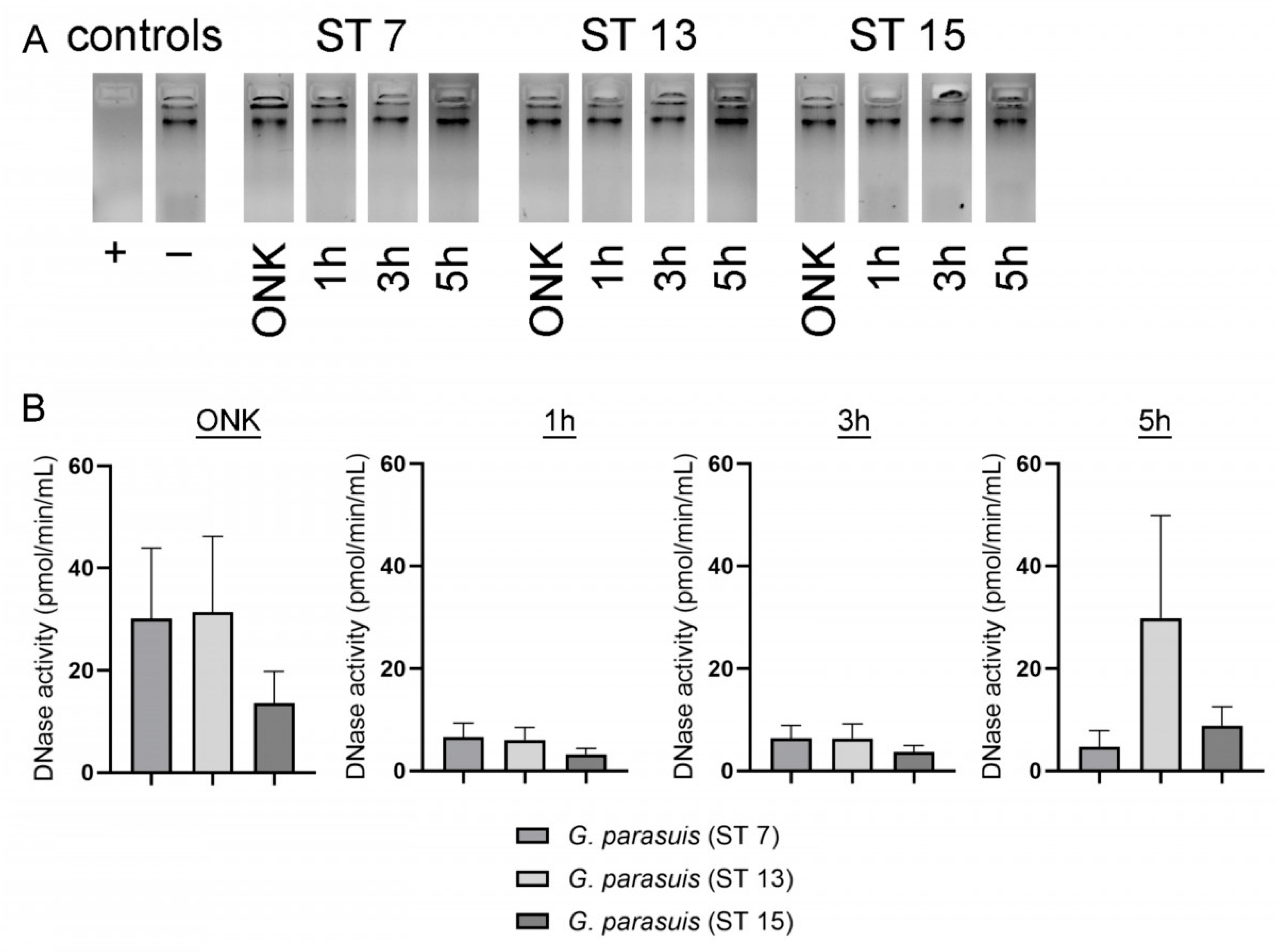
2.3. BLAST Analysis Revealed No Hint for DNase(s) in G. parasuis. However, Dnase Activity Was Detected in Supernatants of G. parasuis Serotypes by a Sensitive DNase Activity Test
2.4. External DNase, Neutrophils and Degraded NETs Enhance Growth of Different G. parasuis Serotypes
2.5. Enhancement of the Growth of G. parasuis Serotype 15 Dependents on the 5′-Nucleotidase
3. Discussion
4. Materials and Methods
4.1. PPLO Medium
4.2. Glaesserella (G.) parasuis Strains and Growth Conditions
Cryostocks for NET Induction and Survival Assay
4.3. BLAST Analysis
4.4. DNase Activity Assays
4.5. Purification of Porcine Neutrophils
4.6. NET Induction G. parasuis
4.7. Visualization and Quantification of NETs
4.8. G. parasuis ST 15 Survival Assay
4.9. G. parasuis Survival Assays
4.10. Measurement of ROS
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glässer’s disease: How to control the disease under restrictive use of antimicrobials. Vet. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef] [PubMed]
- Aragon, V.; Segalés, J.; Oliveira, S. Diseases of swine-Glässer’s disease. In Disease of Swine, 10th ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 760–769. [Google Scholar]
- Barasuol, B.M.; Guizzo, J.A.; Fegan, J.E.; Martínez-Martínez, S.; Rodríguez-Ferri, E.F.; Gutiérrez-Martín, C.B.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. New insights about functional and cross-reactive properties of antibodies generated against recombinant TbpBs of Haemophilus parasuis. Sci. Rep. 2017, 7, 10377. [Google Scholar] [CrossRef] [PubMed]
- Bello-Ortí, B.; Deslandes, V.; Tremblay, Y.D.; Labrie, J.; Howell, K.J.; Tucker, A.W.; Maskell, D.J.; Aragon, V.; Jacques, M. Biofilm formation by virulent and non-virulent strains of Haemophilus parasuis. Vet. Res. 2014, 45, 104. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, J.I.; Desrosiers, R. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can. J. Vet. Res. 1999, 63, 83–89. [Google Scholar]
- Macedo, N.; Rovira, A.; Torremorell, M. Haemophilus parasuis: Infection, immunity and enrofloxacin. Vet. Res. 2015, 46, 128. [Google Scholar] [CrossRef]
- Olvera, A.; Ballester, M.; Nofrarias, M.; Sibila, M.; Aragon, V. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet. Res. 2009, 40, 12. [Google Scholar] [CrossRef]
- Costa-Hurtado, M.; Aragon, V. Advances in the quest for virulence factors of Haemophilus parasuis. Vet. J. 2013, 198, 571–576. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Chen, P.; Li, Z.; Meng, X.; Liu, M.; Li, S.; Shi, D.; Xiao, Y.; Wang, X.; et al. Haemophilus parasuis infection activates the NF-κB pathway in PK-15 cells through IκB degradation. Vet. Microbiol. 2012, 160, 259–263. [Google Scholar] [CrossRef]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 155, 1428–1433. [Google Scholar]
- Amano, H.; Shibata, M.; Kajio, N.; Morozumi, T. Pathologic Observations of Pigs Intranasally Inoculated with Serovar 1, 4 and 5 of Haemophilus parasuis Using Immunoperoxidase Method. J. Vet. Med. Sci. 1994, 56, 639–644. [Google Scholar] [CrossRef]
- Ježek, J.; Starič, J.; Nemec, M.; Plut, J.; Oven, I.G.; Klinkon, M.; Štukelj, M. The influence of age, farm, and physiological status on pig hematological profiles. J. Swine Health Prod. 2018, 26, 72–78. [Google Scholar]
- Fingerhut, L.; Dolz, G.; de Buhr, N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? Int. J. Mol. Sci. 2020, 21, 4523. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J. Neutrophil Extracellular Traps Go Viral. Front. Immunol. 2016, 7, 11–14. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte-Derived Extracellular Traps Act Efficiently against Protozoan Parasites In Vitro and In Vivo. Mediat. Inflamm. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- de Buhr, N.; Bonilla, M.C.; Pfeiffer, J.; Akhdar, S.; Schwennen, C.; Kahl, B.C.; Waldmann, K.; Valentin-Weigand, P.; Hennig-Pauka, I.; von Köckritz-Blickwede, M. Degraded neutrophil extracellular traps promote the growth of Actinobacillus pleuropneumoniae. Cell Death Dis. 2019, 10, 657. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. 5′-Nucleotidases and their new roles in NAD+ and phosphate metabolism. New J. Chem. 2010, 34, 845. [Google Scholar] [CrossRef]
- Nielsen, R. Pathogenicity and Immunity Studies of Haemophilus Parasuis Serotypes. Acta Vet. Scand. 1993, 34, 193–198. [Google Scholar] [CrossRef]
- Schuwerk, L.; Hoeltig, D.; Waldmann, K.-H.; Strutzberg-Minder, K.; Valentin-Weigand, P.; Rohde, J. Serotyping and pathotyping of Glaesserella parasuis isolated 2012–2019 in Germany comparing different PCR-based methods. Vet. Res. 2020, 51, 137. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Register, K.B.; Kuehn, J.S.; Nicholson, T.L.; Loving, C.L.; Bayles, D.O.; Shore, S.M.; Phillips, G.J. Virulence and Draft Genome Sequence Overview of Multiple Strains of the Swine Pathogen Haemophilus parasuis. PLoS ONE 2014, 9, e103787. [Google Scholar] [CrossRef]
- Macedo, N.; Gottschalk, M.; Strutzberg-Minder, K.; Van, C.N.; Zhang, L.; Zou, G.; Zhou, R.; Marostica, T.; Clavijo, M.J.; Tucker, A.; et al. Molecular characterization of Glaesserella parasuis strains isolated from North America, Europe and Asia by serotyping PCR and LS-PCR. Vet. Res. 2021, 52, 68. [Google Scholar] [CrossRef]
- de Buhr, N.; Stehr, M.; Neumann, A.; Naim, H.Y.; Valentin-Weigand, P.; von Köckritz-Blickwede, M.; Baums, C.G. Identification of a novel DNase of Streptococcus suis (EndAsuis) important for neutrophil extracellular trap degradation during exponential growth. Microbiology 2015, 161, 838–850. [Google Scholar] [CrossRef]
- de Buhr, N.; Neumann, A.; Jerjomiceva, N.; von Köckritz-Blickwede, M.; Baums, C.G. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology 2014, 160, 385–395. [Google Scholar] [CrossRef]
- Blokesch, M.; Schoolnik, G.K. The Extracellular Nuclease Dns and Its Role in Natural Transformation of Vibrio cholerae. J. Bacteriol. 2008, 190, 7232–7240. [Google Scholar] [CrossRef]
- Seper, A.; Hosseinzadeh, A.; Gorkiewicz, G.; Lichtenegger, S.; Roier, S.; Leitner, D.R.; Röhm, M.; Grutsch, A.; Reidl, J.; Urban, C.F.; et al. Vibrio cholerae Evades Neutrophil Extracellular Traps by the Activity of Two Extracellular Nucleases. PLoS Pathog. 2013, 9, e1003614. [Google Scholar] [CrossRef]
- Juneau, R.A.; Stevens, J.S.; Apicella, M.A.; Criss, A.K. A Thermonuclease of Neisseria gonorrhoeae Enhances Bacterial Escape From Killing by Neutrophil Extracellular Traps. J. Infect. Dis. 2015, 212, 316–324. [Google Scholar] [CrossRef]
- Berends, E.T.M.; Horswill, A.R.; Haste, N.M.; Monestier, M.; Nizet, V.; von Köckritz-Blickwede, M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2010, 2, 576–586. [Google Scholar] [CrossRef]
- Lacks, S.; Greenberg, B.; Neuberger, M. Identification of a Deoxyribonuclease Implicated in Genetic Transformation of Diplococcus pneumoniae. J. Bacteriol. 1975, 123, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Beiter, K.; Wartha, F.; Albiger, B.; Normark, S.; Zychlinsky, A.; Henriques-Normark, B. An Endonuclease Allows Streptococcus pneumoniae to Escape from Neutrophil Extracellular Traps. Curr. Biol. 2006, 16, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Midon, M.; Schafer, P.; Pingoud, A.; Ghosh, M.; Moon, A.F.; Cuneo, M.J.; London, R.E.; Meiss, G. Mutational and biochemical analysis of the DNA-entry nuclease EndA from Streptococcus pneumoniae. Nucleic Acids Res. 2011, 39, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.F.; Midon, M.; Meiss, G.; Pingoud, A.; London, R.E.; Pedersen, L.C. Structural insights into catalytic and substrate binding mechanisms of the strategic EndA nuclease from Streptococcus pneumoniae. Nucleic Acids Res. 2011, 39, 2943–2953. [Google Scholar] [CrossRef]
- Bergé, M.J.; Kamgoué, A.; Martin, B.; Polard, P.; Campo, N.; Claverys, J.-P. Midcell Recruitment of the DNA Uptake and Virulence Nuclease, EndA, for Pneumococcal Transformation. PLoS Pathog. 2013, 9, e1003596. [Google Scholar] [CrossRef]
- Zhu, L.; Kuang, Z.; Wilson, B.A.; Lau, G.W. Competence-independent activity of pneumococcal enda mediates degradation of extracellular DNA and nets and is important for virulence. PLoS ONE 2013, 8, e70363. [Google Scholar]
- Aziz, R.K.; Ismail, S.A.; Park, H.-W.; Kotb, M. Post-proteomic identification of a novel phage-encoded streptodornase, Sda1, in invasive M1T1 Streptococcus pyogenes. Mol. Microbiol. 2004, 54, 184–197. [Google Scholar] [CrossRef]
- Walker, M.J.; Hollands, A.; Sanderson-Smith, M.L.; Cole, J.N.; Kirk, J.K.; Henningham, A.; McArthur, J.D.; Dinkla, K.; Aziz, R.K.; Kansal, R.G.; et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 2007, 13, 981–985. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Uchiyama, S.; Varki, A.; Nizet, V. Leukocyte Inflammatory Responses Provoked by Pneumococcal Sialidase. MBio 2012, 3, e00220-11. [Google Scholar] [CrossRef]
- Iwasaki, M.; Igarashi, H.; Yutsudo, T. Mitogenic factor secreted by Streptococcus pyogenes is a heat-stable nuclease requiring His122 for activity. Microbiology 1997, 143, 2449–2455. [Google Scholar] [CrossRef]
- Sriskandan, S.; Unnikrishnan, M.; Krausz, T.; Cohen, J. Mitogenic factor (MF) is the major DNase of serotype M89 Streptococcus pyogenes. Microbiology 2000, 146, 2785–2792. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.K.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase Expression Allows the Pathogen Group A Streptococcus to Escape Killing in Neutrophil Extracellular Traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar] [CrossRef]
- Li, G.; Niu, H.; Zhang, Y.; Li, Y.; Xie, F.; Langford, P.R.; Liu, S.; Wang, C. Haemophilus parasuis cytolethal distending toxin induces cell cycle arrest and p53-dependent apoptosis. PLoS ONE 2017, 12, e0177199. [Google Scholar] [CrossRef]
- Lewis, D.A.; Stevens, M.K.; Latimer, J.L.; Ward, C.K.; Deng, K.; Blick, R.; Lumbley, S.R.; Ison, C.A.; Hansen, E.J. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems. Infect. Immun. 2001, 69, 5626–5634. [Google Scholar] [CrossRef][Green Version]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2012, 3, 424. [Google Scholar] [CrossRef]
- DeCoursey, T.E.; Ligeti, E. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 2005, 62, 2173–2193. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Whitby, P.W.; Morton, D.J.; VanWagoner, T.M.; Seale, T.W.; Cole, B.K.; Mussa, H.J.; McGhee, P.A.; Bauer, C.Y.S.; Springer, J.M.; Stull, T.L. Haemophilus influenzae OxyR: Characterization of Its Regulation, Regulon and Role in Fitness. PLoS ONE 2012, 7, e50588. [Google Scholar] [CrossRef]
- Wen, Y.; Wen, Y.; Wen, X.; Cao, S.; Huang, X.; Wu, R.; Zhao, Q.; Liu, M.; Huang, Y.; Yan, Q.; et al. OxyR of Haemophilus parasuis is a global transcriptional regulator important in oxidative stress resistance and growth. Gene 2018, 643, 107–116. [Google Scholar] [CrossRef]
- Bonilla, M.C.; Fingerhut, L.; Alfonso-Castro, A.; Mergani, A.; Schwennen, C.; von Köckritz-Blickwede, M.; de Buhr, N. How Long Does a Neutrophil Live?—The Effect of 24 h Whole Blood Storage on Neutrophil Functions in Pigs. Biomedicines 2020, 8, 278. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Kern, J.W.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009, 206, 2417–2427. [Google Scholar] [CrossRef]
- Hennig-Pauka, I.; Imker, R.; Mayer, L.; Brügmann, M.; Werckenthin, C.; Weber, H.; Menrath, A.; de Buhr, N. From Stable to Lab—Investigating Key Factors for Sudden Deaths Caused by Streptococcus suis. Pathogens 2019, 8, 249. [Google Scholar] [CrossRef]
- Steffensen, N.; Imker, R.; Lassnig, S.; Fulde, M.; Rieder, J.C.; de Buhr, N. Methylprednisolone Induces Extracellular Trap Formation and Enhances Bactericidal Effect of Canine Neutrophils. Int. J. Mol. Sci. 2021, 22, 7734. [Google Scholar] [CrossRef]



| Bacteria | Dnase Name | References |
|---|---|---|
| Vibrio cholerae | Xds and Dns | [28,29] |
| Neisseria gonorrhoeae | Nuc | [30] |
| Staphylococcus aureus | Nuc | [31] |
| Streptococcus suis | SsnA | [27,32,33] |
| Streptococcus pneumoniae | EndA | [32,33,34,35,36,37] |
| Streptococcus pyogenes | Sda1 and MF | [38,39,40,41,42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, M.C.; Lassnig, S.; Obando Corella, A.; Imker, R.; Valentin-Weigand, P.; von Köckritz-Blickwede, M.; Luther, A.-M.; Hennig-Pauka, I.; de Buhr, N. Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs. Pathogens 2022, 11, 880. https://doi.org/10.3390/pathogens11080880
Bonilla MC, Lassnig S, Obando Corella A, Imker R, Valentin-Weigand P, von Köckritz-Blickwede M, Luther A-M, Hennig-Pauka I, de Buhr N. Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs. Pathogens. 2022; 11(8):880. https://doi.org/10.3390/pathogens11080880
Chicago/Turabian StyleBonilla, Marta C., Simon Lassnig, Andrea Obando Corella, Rabea Imker, Peter Valentin-Weigand, Maren von Köckritz-Blickwede, Anne-Marie Luther, Isabel Hennig-Pauka, and Nicole de Buhr. 2022. "Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs" Pathogens 11, no. 8: 880. https://doi.org/10.3390/pathogens11080880
APA StyleBonilla, M. C., Lassnig, S., Obando Corella, A., Imker, R., Valentin-Weigand, P., von Köckritz-Blickwede, M., Luther, A.-M., Hennig-Pauka, I., & de Buhr, N. (2022). Studying the Interaction of Neutrophils and Glaesserella Parasuis Indicates a Serotype Independent Benefit from Degradation of NETs. Pathogens, 11(8), 880. https://doi.org/10.3390/pathogens11080880








