Direct and Rapid Identification of Vibrio Cholerae Serogroup and Toxigenicity by a Novel Multiplex Real-Time Assay
Abstract
1. Introduction
2. Results
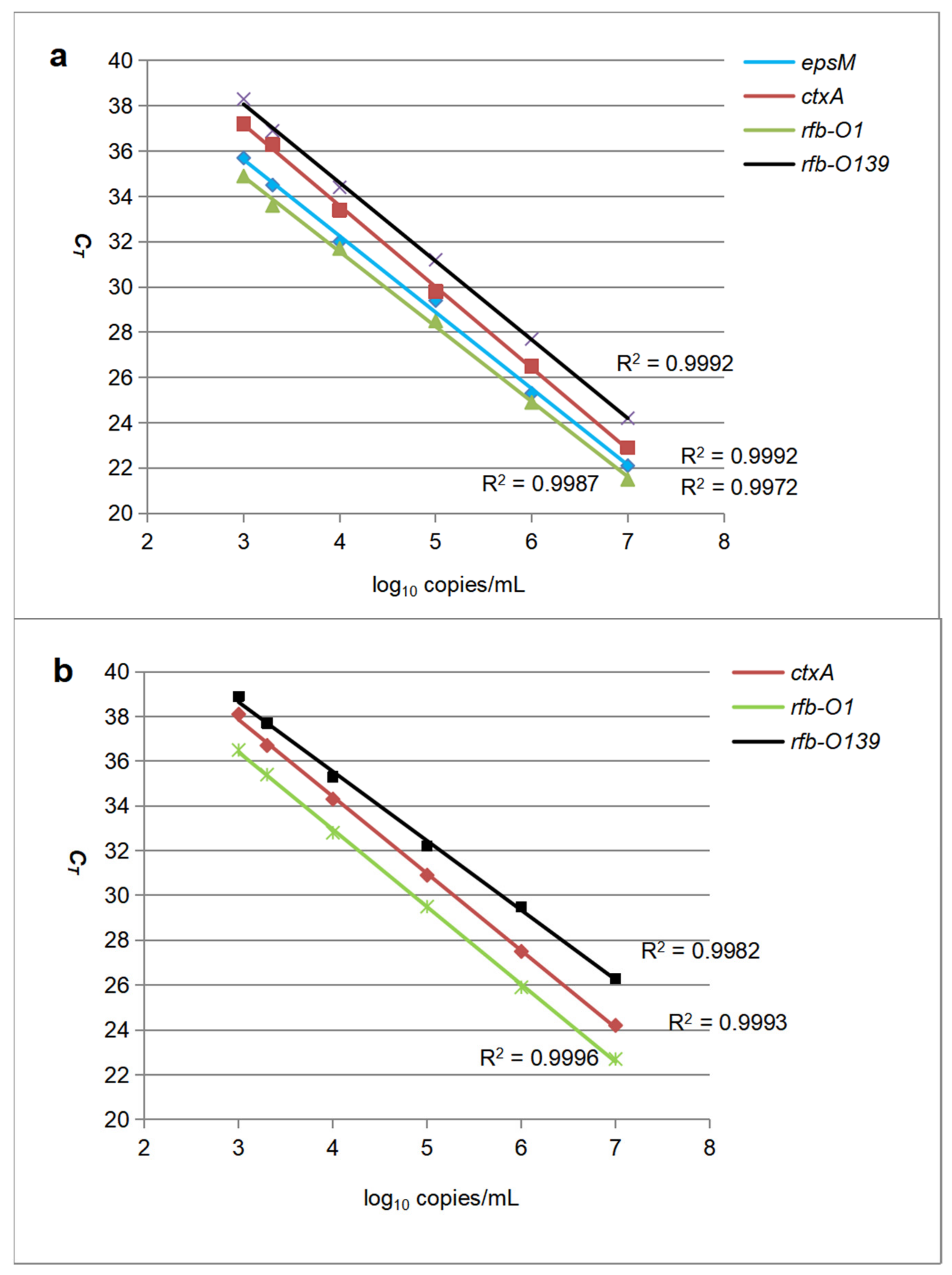
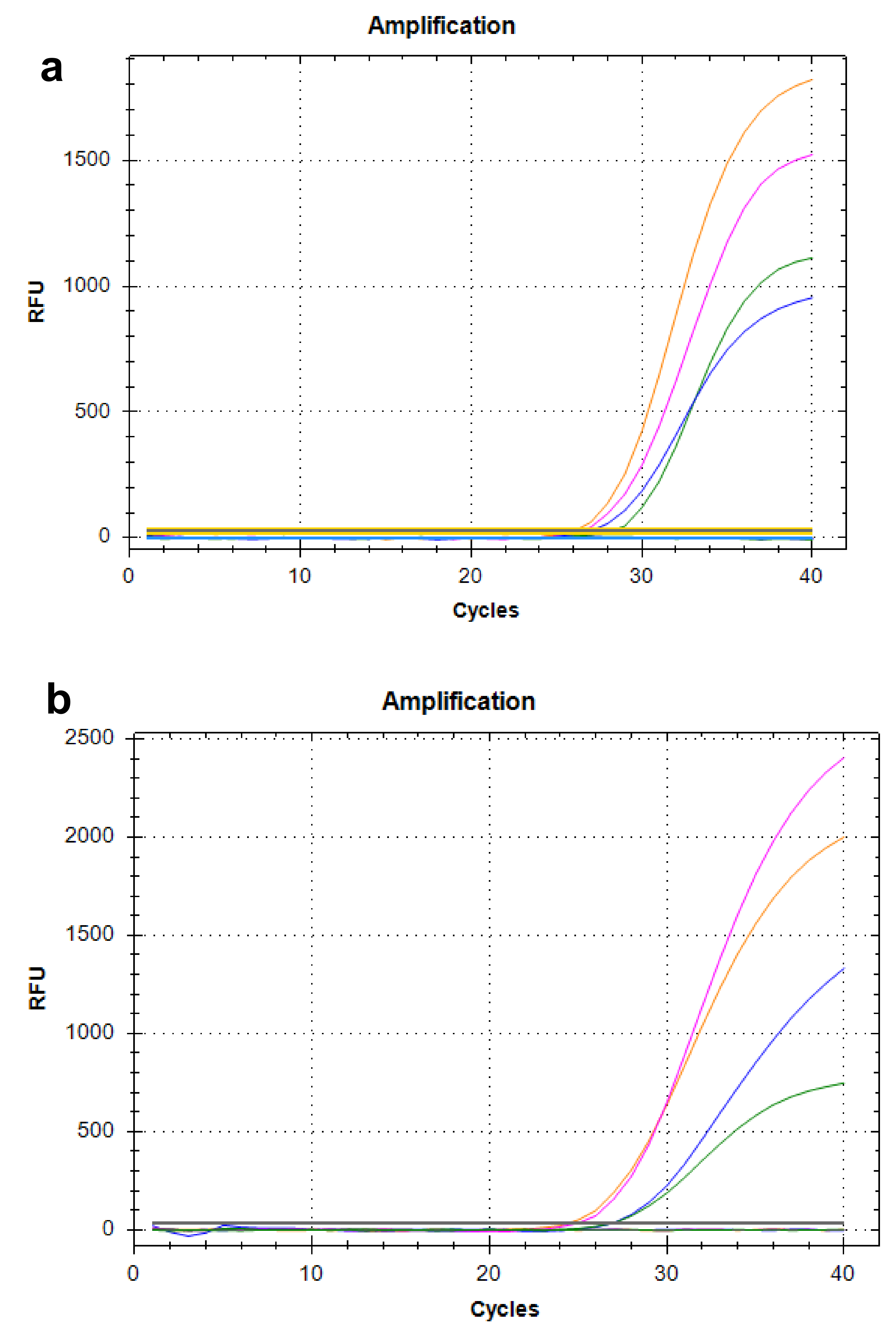
2.1. Analysis of Analytical Performance
2.2. Analysis of Diagnostic Performance
2.3. Analysis of Cost-Effectiveness
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Specimens and Positive Controls
4.3. Bacterial Nucleic Acid Extraction
4.4. Primers and Probes
4.5. Previous Monoplex Real-Time Assay
4.6. New Multiplex Real-Time Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Islam, M.T.; Rashed, S.M.; Johura, F.T.; Bhuiyan, N.A.; Delgado, G.; Morales, R.; Mendez, J.L.; Navarro, A.; Watanabe, H.; et al. Vibrio cholerae classical biotype strains reveal distinct signatures in Mexico. J. Clin. Microbiol. 2012, 50, 2212–2216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, M.; Lopez, A.L.; You, Y.A.; Kim, Y.E.; Sah, B.; Maskery, B.; Clemens, J. The global burden of cholera. Bull. World Health Organ. 2012, 90, 209–218A. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- World Health Organization. Cholera 30 March 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cholera (accessed on 30 June 2022).
- Harris, J.B.; LaRocque, R.C.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476. [Google Scholar] [CrossRef]
- Singh, D.V.; Matte, M.H.; Matte, G.R.; Jiang, S.; Sabeena, F.; Shukla, B.N.; Sanyal, S.C.; Huq, A.; Colwell, R.R. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: Clonal relationships between clinical and environmental isolates. Appl. Environ. Microbiol. 2001, 67, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Ochi, S.; Mizuno, T.; Morita, D.; Morita, M.; Ohnishi, M.; Koley, H.; Dutta, M.; Chowdhury, G.; Mukhopadhyay, A.K.; et al. Virulence of Cholera Toxin Gene-Positive Vibrio cholerae Non-O1/non-O139 Strains Isolated From Environmental Water in Kolkata, India. Front. Microbiol. 2021, 12, 726273. [Google Scholar] [CrossRef]
- Diep, T.T.; Nguyen, N.T.; Nguyen, T.N.; An, H.K.; Nguyen, T.Q.; Nguyen, V.H.; Nguyen, T.V.; Nguyen, T.N.; Izumiya, H.; Ohnishi, M.; et al. Isolation of New Delhi metallo-β-lactamase 1-producing Vibrio cholerae non-O1, non-O139 strain carrying ctxA, st and hly genes in southern Vietnam. Microbiol. Immunol. 2015, 59, 262–267. [Google Scholar] [CrossRef]
- Yamai, S.; Okitsu, T.; Shimada, T.; Katsube, Y. Distribution of serogroups of Vibrio cholerae non-O1 non-O139 with specific reference to their ability to produce cholera toxin, and addition of novel serogroups. Kansenshogaku zasshi. J. Jpn. Assoc. Infect. Dis. 1997, 71, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Gubala, A.J. Multiplex real-time PCR detection of Vibrio cholerae. J. Microbiol. Methods 2006, 65, 278–293. [Google Scholar] [CrossRef]
- Greig, D.R.; Hickey, T.J.; Boxall, M.D.; Begum, H.; Gentle, A.; Jenkins, C.; Chattaway, M.A. A real-time multiplex PCR for the identification and typing of Vibrio cholerae. Diagn. Microbiol. Infect. Dis. 2018, 90, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gubala, A.J.; Proll, D.F. Molecular-beacon multiplex real-time PCR assay for detection of Vibrio cholerae. Appl. Environ. Microbiol. 2006, 72, 6424–6428. [Google Scholar] [CrossRef] [PubMed]
- Mantri, C.K.; Mohapatra, S.S.; Ramamurthy, T.; Ghosh, R.; Colwell, R.R.; Singh, D.V. Septaplex PCR assay for rapid identification of Vibrio cholerae including detection of virulence and int SXT genes. FEMS Microbiol. Lett. 2006, 265, 208–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tebbs, R.S.; Brzoska, P.M.; Furtado, M.R.; Petrauskene, O.V. Design and validation of a novel multiplex real-time PCR assay for Vibrio pathogen detection. J. Food Prot. 2011, 74, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Lyon, W.J. TaqMan PCR for detection of Vibrio cholerae O1, O139, non-O1, and non-O139 in pure cultures, raw oysters, and synthetic seawater. Appl. Environ. Microbiol. 2001, 67, 4685–4693. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D. Cholera Prevention Manual, 6th ed.; People’s Medical Publishing House (PMPH): Beijing, China, 2013; pp. 93–95. [Google Scholar]
- Sandkvist, M.; Keith, J.M.; Bagdasarian, M.; Howard, S.P. Two regions of EpsL involved in species-specific protein-protein interactions with EpsE and EpsM of the general secretion pathway in Vibrio cholerae. J. Bacteriol. 2000, 182, 742–748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandkvist, M.; Hough, L.P.; Bagdasarian, M.M.; Bagdasarian, M. Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J. Bacteriol. 1999, 181, 3129–3135. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, H.; Dehkordi, F.S.; Rahimi, E.; Asgarifar, A. Detection of Escherichia coli, Salmonella species, and Vibrio cholerae in tap water and bottled drinking water in Isfahan, Iran. BMC Public Health 2013, 13, 556. [Google Scholar] [CrossRef]
- Cornett, J.B.; Shockman, G.D. Cellular lysis of Streptococcus faecalis induced with triton X-100. J. Bacteriol. 1978, 135, 153–160. [Google Scholar] [CrossRef]
- Ma, J.; Su, C.; Hu, S.; Chen, Y.; Shu, Y.; Yue, D.; Zhang, B.; Qi, Z.; Li, S.; Wang, X.; et al. The Effect of Residual Triton X-100 on Structural Stability and Infection Activity of Adenovirus Particles. Mol. Ther. Methods Clin. Dev. 2020, 19, 35–46. [Google Scholar] [CrossRef]
- Varadharajan, B.; Parani, M. DMSO and betaine significantly enhance the PCR amplification of ITS2 DNA barcodes from plants. Genome 2021, 64, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ralser, M.; Querfurth, R.; Warnatz, H.J.; Lehrach, H.; Yaspo, M.L.; Krobitsch, S. An efficient and economic enhancer mix for PCR. Biochem. Biophys. Res. Commun. 2006, 347, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Polymerase Chain Reaction (PCR) Amplification of GC-Rich Templates. Cold Spring Harb. Protoc. 2019, 2019, pdb-prot095141. [Google Scholar] [CrossRef] [PubMed]
- Lajin, B.; Alachkar, A.; Alhaj Sakur, A. Betaine significantly improves multiplex tetra-primer ARMS-PCR methods. Mol. Biotechnol. 2013, 54, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Schalasta, G.; Arents, A.; Schmid, M.; Braun, R.W.; Enders, G. Fast and type-specific analysis of herpes simplex virus types 1 and 2 by rapid PCR and fluorescence melting-curve-analysis. Infection 2000, 28, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Schalasta, G.; Roth, B.; Enders, G. Rapid typing of the codon 129 polymorphism of the human prion protein gene by combined real-time PCR and melting curve analysis. Clin. Lab. 2002, 48, 25–30. [Google Scholar]
- Yan, Y.; Luo, J.Y.; Chen, Y.; Wang, H.H.; Zhu, G.Y.; He, P.Y.; Guo, J.L.; Lei, Y.L.; Chen, Z.W. A multiplex liquid-chip assay based on Luminex xMAP technology for simultaneous detection of six common respiratory viruses. Oncotarget 2017, 8, 96913–96923. [Google Scholar] [CrossRef][Green Version]
- Sharman, M.; Thomas, J.E.; Dietzgen, R.G. Development of a multiplex immunocapture PCR with colourimetric detection for viruses of banana. J. Virol. Methods 2000, 89, 75–88. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.H.; Gao, L.; Ji, J.M.; Ge, Z.J.; Zhu, X.Q.; He, P.Y.; Chen, Z.W. A one-step multiplex real-time RT-PCR assay for rapid and simultaneous detection of human norovirus genogroup I, II and IV. J. Virol. Methods 2013, 189, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Li, F.; Wu, C.; Shi, B.; Zhai, K. The G-BHQ synergistic effect: Improved double quenching molecular beacons based on guanine and Black Hole Quencher for sensitive simultaneous detection of two DNAs. Talanta 2017, 174, 289–294. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Mochizuki, H.; Omata, M. Double-quencher probes improve detection sensitivity toward Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a reverse-transcription polymerase chain reaction (RT-PCR) assay. J. Virol. Methods 2020, 284, 113926. [Google Scholar] [CrossRef]
- Sangon Biotech (Shanghai) Co., Ltd. Sangon DBQ Series Double-Quenched Fluorescent Probes Are Launched. Available online: https://www.sangon.com/sales2021003.html (accessed on 30 June 2022).
- Integrated DNA Technologies, Inc. (IDT). Reduce qPCR Background and Improve qPCR Signal, ZEN™ and TAO™ Double-Quenched Probes. Available online: https://sg.idtdna.com/pages/education/decoded/article/zen (accessed on 30 June 2022).
- Stokdyk, J.P.; Firnstahl, A.D.; Spencer, S.K.; Burch, T.R.; Borchardt, M.A. Determining the 95% limit of detection for waterborne pathogen analyses from primary concentration to qPCR. Water Res. 2016, 96, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vaks, J.E.; Hemyari, P.; Rullkoetter, M.; Santulli, M.J.; Schoenbrunner, N. Verification of Claimed Limit of Detection in Molecular Diagnostics. J. Appl. Lab. Med. 2016, 1, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Faruk Hossain, M.; Slaughter, G. Flexible electrochemical uric acid and glucose biosensor. Bioelectrochemistry 2021, 141, 107870. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Dhakal, G.; Nguyen, V.Q.; Shim, J.J. Molecularly imprinted hornlike polymer@electrochemically reduced graphene oxide electrode for the highly selective determination of an antiemetic drug. Anal. Chim. Acta 2021, 1141, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Perumal, V.; Gopinath, S.; Raja, P.B.; Ibrahim, M.; Jantan, I.N.; Suhaimi, N.; Liu, W.W. Laser-scribed graphene nanofiber decorated with oil palm lignin capped silver nanoparticles: A green biosensor. Sci. Rep. 2021, 11, 5475. [Google Scholar] [CrossRef] [PubMed]
| Samples &. No. | Monoplex Assay | Multiplex Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| CtxA | Rfb-O1 | Rfb-O139 | EpsM | CtxA | Rfb-O1 | Rfb-O139 | ||
| Feces of patientsa | FC1 | + c | − c | + | + | + | − | + |
| FC2 | + | − | + | + | + | − | + | |
| FC3 | + | − | + | + | + | − | + | |
| Anal swabs of patients a | AS1 | + | − | + | + | + | − | + |
| AS2 | + | − | + | + | + | − | + | |
| AS3 | + | − | + | + | + | − | + | |
| Foods from outbreaks a | FD1 | + | − | + | + | + | − | + |
| FD2 | + | − | + | + | + | − | + | |
| FD3 | + | − | + | + | + | − | + | |
| Waters from outbreaks a | WT1 | + | − | + | + | + | − | + |
| WT2 | + | − | + | + | + | − | + | |
| WT3 | + | − | + | + | + | − | + | |
| Waters from an epidemic spot in a 2013 outbreak | WA1 | + | + | − | + | + | + | − |
| WA2 | + | + | − | + | + | + | − | |
| WA5 | + | + | − | + | + | + | − | |
| WA6 | + | + | − | + | + | + | − | |
| Collected bacteria strains b | ||||||||
| V. cholerae O1,Ogawa serotype | ATCC14035 | + | + | − | + | + | + | − |
| V. cholerae O1,Ogawa serotype | 130605 | + | + | − | + | + | + | − |
| V. cholerae O1,Ogawa, ctx-negative | 200907 | − | + | − | + | − | + | − |
| V. cholerae O1,Inaba serotype | 200806 | + | + | − | + | + | + | - |
| V. cholerae O1,Inaba, ctx-negative | 190625 | − | + | − | + | − | + | − |
| V. cholerae O139(0511) | 180511 | + | − | + | + | + | − | + |
| V. cholerae O139(0618) | 180618 | + | − | + | + | + | − | + |
| V. cholerae O139, ctx-negative | 200513 | − | − | + | + | − | − | + |
| V. cholerae non-O1/non-O139,ctx-postive | 190524 | + | − | − | + | + | − | − |
| V. cholerae non-O1/non-O139 | 090717-203 | − | − | − | + | − | − | − |
| V. cholerae non-O1/non-O139 | 090717-207 | − | − | − | + | − | − | − |
| V. cholerae non-O1/non-O139 | 190626 | − | − | − | + | − | − | − |
| V. cholerae non-O1/non-O139 | 160517 | − | − | − | + | − | − | − |
| V. cholerae non-O1/non-O139 | 170626 | − | − | − | + | − | − | − |
| V. cholerae non-O1/non-O139 | 191016 | − | − | − | + | − | − | − |
| Vibrio mimicus | ATCC33653 | − | − | − | − | − | − | − |
| Vibrio parahaemolyticus | ATCC33847 | − | − | − | − | − | − | − |
| Aeromonas hydrophila | 8315 | − | − | − | − | − | − | − |
| Staphylococcus aureus | ATCC25923 | − | − | − | − | − | − | − |
| Escherichia coli | ATCC25922 | − | − | − | − | − | − | − |
| Salmonella anatum | ATCC50083 | − | − | − | − | − | − | − |
| Shigella flexneri | ATCC51573 | − | − | − | − | − | − | − |
| Positive Total | 22 | 9 | 15 | 31 | 22 | 9 | 15 | |
| Target | Monoplex Assay | Multiplex Assay | Accordance Rate (%) | Kappa | χ2 | p | |||
|---|---|---|---|---|---|---|---|---|---|
| + | − | Sensitivity (%) | Specificity (%) | ||||||
| V. Choleraea | + | 124 | 0 | 100.00 | 17.29 | 47.63 | 0.133 | 175.006 | <0.001 |
| − | 177 | 37 | |||||||
| ctxA | + | 124 | 0 | 100.00 | 99.53 | 99.70 | 0.994 | 0.000 | 0.990 |
| − | 1 | 212 | |||||||
| rfb-O1 | + | 79 | 0 | 100.00 | 97.70 | 98.24 | 0.952 | 4.167 | <0.05 |
| − | 6 | 255 | |||||||
| rfb-O139 | + | 46 | 1 | 97.87 | 99.31 | 99.11 | 0.963 | 0.000 | 0.990 |
| − | 2 | 288 | |||||||
| Assays | Time /Plate | Time (2 Plates) | Cost /Reaction a | Cost (2 Plates) a |
|---|---|---|---|---|
| Previous 3-panel monoplex assay | ||||
| DNA and RNA extraction | 30 min | 30 min | ¥30.0 | ¥5760.0 |
| First real-time PCR Panel 1(25 μL) for ctxA | 50 min | 50 min | ¥10.0 | ¥1920.0 |
| Vazyme qPCR reagents (UDG+) | ¥4.5 | |||
| Primers (forward and reverse, 1 target) | ¥0.5 | |||
| TaqMan fluorescent probe (1 target) | ¥5.0 | |||
| First real-time PCR Panel 2 (25 μL) for O1 target | 50 min | ¥10.0 | ¥1920.0 | |
| First real-time PCR Panel 3 (25 μL) for O139 target | 50 min | ¥10.0 | ¥1920.0 | |
| Second real-time PCR Panel 1–3 (25 μL) for 3 targets | 150 min | ¥30.0 | ¥5760.0 | |
| Total | 80 min | 330 min | ¥90.0 | ¥17,280.0 |
| New one-tube multiplex assay | ||||
| DNA and RNA extraction | 0 min | 0 min | ¥0.0 | ¥0.0 |
| Real-time RT-PCR (25 μL volume) | 40 min | 80 min | ¥30.0 | ¥5760.0 |
| Vazyme One Step qRT-PCR reagents (UDG+) | ¥8.0 | |||
| Primers (forward and reverse, 4 targets) | ¥2.0 | |||
| TaqMan fluorescent probe (4 targets) | ¥20.0 | |||
| Total | 40 min | 80 min | ¥30.0 | ¥5760.0 |
| Positive Standards a | Sequence (5′-3′) | Reference Seq ID and Position(bp) b |
|---|---|---|
| epsM-PC | CGCAATTGGTCTCATGGATTGCGTATTTGCAAGAGCGCCAAGGGGTGAGCGTGGATGCGATTGATATTGACCGTGGTAAAGTGAACGGCGTTGTGGAAGTCAAACGTCTGCAACTGAAGC | DQ775328.1: 371–490 |
| ctxA-PC | AGTTCATTTTGGGGTGCTTGATGAACAATTACATCGTAATAGGGGCTACAGAGATAGATATTACAGTAACTTAGATATTGCTCCAGCAGCAGATGGTTATGGATTGGCAGGTTTCCCTCCGGAGCATAGAGCTTGGAGGGAAGAGCCGTG | AB699245.1: 441–590 |
| O1-PC | CGTTGGGAATAACTCAAGGCGATGAAGTGATTGTACCAACATTCACTTATGTTGCCTCGGTTAATACCATAGTCCAGTGTGGTGCGTTACCCGTTTTTGCTGAAATCGAAGGTGAGTCTCTACAAGTGAGCGTAGAGGAC | X59554.1: 5741–5880 |
| O139-PC | GATCGTGCTACGATGGCGTGTTCATTAGAAGGGCGGGTTCCCTTGTTAGACCACCGCATTGCTGAGTTTGCTGCCAGTTTGCCGATCCATTTGAAATACCGAGGTGGAAAGGGAAAGTGGCTTTTACGAGAAGTACTGTATCGTTATGTACCTAAAAAAT | AB012956.1: 34141–34300 |
| Target | Primer or Probe a | Sequence (5′-3′) | Reference Seq and Position(bp) b | Reaction Conc.(uM) |
|---|---|---|---|---|
| epsM | epsM-F | GGTCTCATGGATTGCGTATTTG | DQ775328.1:378–399 | 0.4 |
| epsM-R | GTTGCAGACGTTTGACTTCC | DQ775328.1:465–484(-) | 0.4 | |
| epsM-P c | ACGGTCAATATCAATCGCATCCACGCT | DQ775328.1:418–444(-) | 0.2 | |
| ctxA | ctxA-F | GGGTGCTTGATGAACAATTACA | AB699245.1:452–473 | 0.5 |
| ctxA-R | TTCCCTCCAAGCTCTATGC | AB699245.1:564–582(-) | 0.5 | |
| ctxA-P d | ACCTGCCAATCCATAACCATCTGCTGC | AB699245.1:526–552(-) | 0.25 | |
| m-ctxA-F | CTTCCCTCCAAGCTCTATGCTC | AB699245.1:562–583(-) | 0.2 | |
| m-ctxA-R | TACATCGTAATAGGGGCTACAGAG | AB699245.1:470–493 | 0.2 | |
| m-ctxA-P g | ACCTGCCAATCCATAACCATCTGCTGCTG | AB699245.1:524–552(-) | 0.2 | |
| rfb-O1 | O1-F | CAAGGCGATGAAGTGATTGTA | X59554.1:5755–5775 | 0.4 |
| O1-R | CGCTCACTTGTAGAGACTCA | X59554.1:5853–5872(-) | 0.4 | |
| O1-P e | ACGGGTAACGCACCACACTGGACTATG | X59554.1:5808–5834(-) | 0.2 | |
| m-O1-F | GGAATAACTCAAGGCGATGAAGTG | X59554.1:5746–5769 | 0.2 | |
| m-O1-R | TAGAGACTCACCTTCGATTTCAGC | X59554.1:5839–5862(-) | 0.2 | |
| m-O1-P g | AAACGGGTAACGCACCACACTGGACT | X59554.1:5811–5836(-) | 0.2 | |
| rfb-O139 | O139-F | GGTACATAACGATACAGTACTTCTC | AB012956.1:34268–34292(-) | 0.5 |
| O139-R | CGATGGCGTGTTCATTAGA | AB012956.1:34151–34169 | 0.5 | |
| O139-P f | CCTTGTTAGACCACCGCATTGCTGAGT | AB012956.1:34181–34207 | 0.25 | |
| m-O139-F | CGATGGCGTGTTCATTAGAAGG | AB012956.1:34151–34172 | 0.2 | |
| m-O139-R | TCCCTTTCCACCTCGGTATTTC | AB012956.1:34233–34252(-) | 0.2 | |
| m-O139-P g | CGGCAAACTGGCAGCAAACTCAGCA | AB012956.1:34200–34224(-) | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhan, L.; Zhu, G.; Zhang, J.; Li, P.; Chen, L.; He, P.; Luo, J.; Chen, Z. Direct and Rapid Identification of Vibrio Cholerae Serogroup and Toxigenicity by a Novel Multiplex Real-Time Assay. Pathogens 2022, 11, 865. https://doi.org/10.3390/pathogens11080865
Yan Y, Zhan L, Zhu G, Zhang J, Li P, Chen L, He P, Luo J, Chen Z. Direct and Rapid Identification of Vibrio Cholerae Serogroup and Toxigenicity by a Novel Multiplex Real-Time Assay. Pathogens. 2022; 11(8):865. https://doi.org/10.3390/pathogens11080865
Chicago/Turabian StyleYan, Yong, Li Zhan, Guoying Zhu, Junyan Zhang, Ping Li, Lixia Chen, Peiyan He, Jianyong Luo, and Zhongwen Chen. 2022. "Direct and Rapid Identification of Vibrio Cholerae Serogroup and Toxigenicity by a Novel Multiplex Real-Time Assay" Pathogens 11, no. 8: 865. https://doi.org/10.3390/pathogens11080865
APA StyleYan, Y., Zhan, L., Zhu, G., Zhang, J., Li, P., Chen, L., He, P., Luo, J., & Chen, Z. (2022). Direct and Rapid Identification of Vibrio Cholerae Serogroup and Toxigenicity by a Novel Multiplex Real-Time Assay. Pathogens, 11(8), 865. https://doi.org/10.3390/pathogens11080865





