Progress in Prophylactic and Therapeutic EBV Vaccine Development Based on Molecular Characteristics of EBV Target Antigens
Abstract
:1. Introduction
1.1. EBV Structure and Tropism
1.2. EBV Replication Cycle
1.3. Interaction of EBV and the Immune System
2. EBV Vaccine Development
2.1. Prophylactic Vaccines
2.2. Therapeutic Vacciness
3. Ongoing Clinical Trials and Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, T.; You, C.; Meng, S.; Lai, Z. EBV Infection and Its Regulated Metabolic Reprogramming in Nasopharyngeal Tumorigenesis. Front. Cell. Infect. Microbiol. 2022, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Bode, A.M. Targeting the signaling in Epstein–Barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal Transduct Target Ther. 2021, 6, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Snapper, C.M. Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases. Front. Immunol. 2021, 12, 734471. [Google Scholar] [CrossRef] [PubMed]
- Yates, D. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Neurosci. 2022, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Aghajanian, S.; Athar, M.M.T.; Gargari, O.K. Epstein–Barr virus and COVID-19. J. Med. Virol. 2022, 4040–4042. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Nahm, C.H.; Je, Y.S.; Lee, J.S.; Baek, J.H.; Kwon, H.Y.; Park, M.H. The effect of Epstein–Barr virus viremia on the progression to severe COVID-19. Medicine 2022, 101, e29027. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Hammerschmidt, W. Structure and role of the terminal repeats of Epstein-Barr virus in processing and packaging of virion DNA. J. Virol. 1995, 69, 3147–3155. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthy, K.; Hu, Y.X.; Möhl, B.S.; Chen, J.; Longnecker, R.; Jardetzky, T.S. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat. Commun. 2016, 7, 13557. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cohen, J.I. Epstein-Barr Virus (EBV) Tegument Protein BGLF2 Promotes EBV Reactivation through Activation of the p38 Mitogen-Activated Protein Kinase. J. Virol. 2016, 90, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Feederle, R.; Neuhierl, B.; Baldwin, G.; Bannert, H.; Hub, B.; Mautner, J.; Delecluse, H.J. Epstein-Barr Virus BNRF1 Protein Allows Efficient Transfer from the Endosomal Compartment to the Nucleus of Primary B Lymphocytes. J. Virol. 2006, 80, 9435–9443. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, W.; Sugden, B. Replication of Epstein-Barr viral DNA. Cold Spring Harb. Perspect Biol. 2013, 5, a013029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.B.; Zhang, A.; Chen, M.L.; Fang, Z.X.; Zeng, M.S. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat. Microbiol. 2018, 3, 164–171. [Google Scholar] [CrossRef]
- Wallaschek, N.; Reuter, S.; Silkenat, S.; Wolf, K.; Niklas, C.; Kayisoglu, Ö.; Bartfeld, S. Ephrin receptor A2, the epithelial receptor for Epstein-Barr virus entry, is not available for efficient infection in human gastric organoids. PLoS Pathog. 2021, 17, e1009210. [Google Scholar] [CrossRef]
- Rani, A.; Jakhmola, S.; Karnati, S.; Parmar, H.S.; Chandra Jha, H. Potential entry receptors for human γ-herpesvirus into epithelial cells: A plausible therapeutic target for viral infections. Tumour Virus Res. 2021, 12, 200227. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Chen, X.S.; Holers, V.M.; Hannan, J.P. Isolating the Epstein-Barr Virus gp350/220 Binding Site on Complement Receptor Type 2 (CR2/CD21). J. Biol. Chem. 2007, 282, 36614–36625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean-Pierre, V.; Lupo, J.; Buisson, M.; Morand, P.; Germi, R. Main Targets of Interest for the Development of a Prophylactic or Therapeutic Epstein-Barr Virus Vaccine. Front. Microbiol. 2021, 12, 701611. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, X.; Kang, Y.; Zeng, M. The Status and Prospects of Epstein—Barr Virus Prophylactic Vaccine Development. Front. Immunol. 2021, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, B.; Li, Y.; Zhu, W.; Akihisa, T.; Li, W.; Zhang, J. Prophylactic and therapeutic ebv vaccines: Major scientific obstacles, historical progress, and future direction. Vaccines 2021, 9, 1290. [Google Scholar] [CrossRef]
- Xiao, J.; Palefsky, J.M.; Herrera, R.; Berline, J.; Tugizov, S.M. The Epstein—Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology 2008, 370, 430–442. [Google Scholar] [CrossRef] [Green Version]
- Bánáti, F.; Koroknai, A.; Szenthe, K. Terminal repeat analysis of EBV genomes. Methods Mol. Biol. 2017, 1532, 169–177. [Google Scholar] [CrossRef]
- Perspectives, F. Epstein-Barr. HHS Public Access 2018, 47, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Roy, S.G.; Bose, P.; Saha, A. Role of EBNA-3 Family Proteins in EBV Associated B-cell Lymphomagenesis. Front. Microbiol. 2016, 7, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Tang, Y.; Wang, J.; Xiong, F.; Guo, C.; Wang, Y.; Zeng, Z. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer 2018, 9, 2852–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakiri, D.; Takada, K. Role of EBERs in the Pathogenesis of EBV Infection. Adv. Cancer Res. 2010, 107, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.M.; Tanizawa, H.; Caruso, L.B.; Hulse, M.; Kossenkov, A.; Madzo, J.; Tempera, I. The three-dimensional structure of Epstein-Barr virus genome varies by latency type and is regulated by PARP1 enzymatic activity. Nat. Commun. 2022, 13, 187. [Google Scholar] [CrossRef]
- Houen, G.; Trier, N.H. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front. Immunol. 2021, 11, 587380. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Sugimoto, A.; Inagaki, T.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H. Molecular basis of epstein–barr virus latency establishment and lytic reactivation. Viruses 2021, 13, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kieff, E. Epstein—Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Tan, Q.; Li, S.; Shen, L.; Zhang, J.; Liu, Y.; Lu, Z. Induction of EBV latent membrane specific T cells and construction of individualized TCR engineered T cells for EBV-associated malignancies. J. Immunother. Cancer 2021, 9, e002516. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, X.; Du, L.; Wang, H.; Tang, J.; Wang, M.; Tang, A. A study of Epstein-Barr virus infection in the Chinese tree shrew(Tupaia belangeri chinensis). Virol. J. 2017, 14, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Chen, H.; Feng, Y.; Shi, N.; Huang, Z.; Feng, Q.; Tang, A. Tree Shrew Is a Suitable Animal Model for the Study of Epstein Barr Virus. Front. Immunol. 2022, 12, 789604. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Sanada, T.; Kohara, M.; Tsukiyama-Kohara, K. Tree shrew as an emerging small animal model for human viral infection: A recent overview. Viruses 2021, 13, 1641. [Google Scholar] [CrossRef] [PubMed]
- Linke-Serinsöz, E.; Fend, F.; Quintanilla-Martinez, L. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) related lymphomas, pathology view point. Semin. Diagn. Pathol. 2017, 34, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P. Epstein-Barr virus, malaria and endemic Burkitt lymphoma. EBioMedicine 2019, 39, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.; Fitzmaurice, C.; Naghavi, M.; Ahmed, L.A. Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990–2017. BMJ Open 2020, 10, e037505. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.L.; Suhrbier, A.; Miles, J.J.; Lawrence, G.; Pye, S.J.; Le, T.T.; Bharadwaj, M. Phase I Trial of a CD8 ϩ T-Cell Peptide Epitope-Based Vaccine for Infectious Mononucleosis. J. Virol. 2008, 82, 1448–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, L.; Tizard, E.J.; Morgan, A.J.; Cubitt, W.D.; Finerty, S.; Oyewole-Eletu, T.A.; Steven, N.M. A Phase I Trial of Epstein-Barr Virus Gp350 Vaccine for Children With Chronic Kidney Disease Awaiting Transplantation. Transplantation 2009, 88, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Bu, W.; Nguyen, L.A.; Batchelor, J.D.; Kim, J.; Pittaluga, S.; Fuller, J.R.; Nguyen, H.; Chou, T.-H.; Cohen, J.I.; et al. A bivalent Epstein-Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl. Med. 2022, 14, eabf3685. [Google Scholar] [CrossRef]
- Murata, T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Cintolo, J.A.; Datta, J.; Mathew, S.J.; Czerniecki, B.J. Dendritic-cell based vaccines: Barriers and opportunities. Future Oncol. 2012, 8, 1273–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, G.S.; Haigh, T.A.; Gudgeon, N.H.; Phelps, R.; Lee, S.P.; Steven, N.M.; Rickinson, A.B. Dual Stimulation of Epstein-Barr Virus (EBV) -Specific CD4 ϩ- and CD8 ϩ -T-Cell Responses by a Chimeric Antigen Construct: Potential Therapeutic Vaccine for EBV-Positive Nasopharyngeal Carcinoma. J. Virol. 2004, 78, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altenburg, A.F.; Kreijtz, J.H.; De Vries, R.D.; Song, F.; Fux, R.; Rimmelzwaan, G.F.; Volz, A. Modified vaccinia virus ankara (MVA) as production platform for vaccines against influenza and other viral respiratory Diseases. Viruses 2014, 6, 2735–2761. [Google Scholar] [CrossRef] [PubMed]
- Rühl, J.; Leung, C.S.; Münz, C. Vaccination against the Epstein–Barr virus. Cell Mol. Life Sci. 2020, 77, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Herbert, A.P.; Barlow, P.N.; Holers, V.M.; Hannan, J.P. Molecular Basis of the Interaction between Complement Receptor Type 2 (CR2/CD21) and Epstein-Barr Virus Glycoprotein gp350. J. Virol. 2008, 82, 11217–11227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
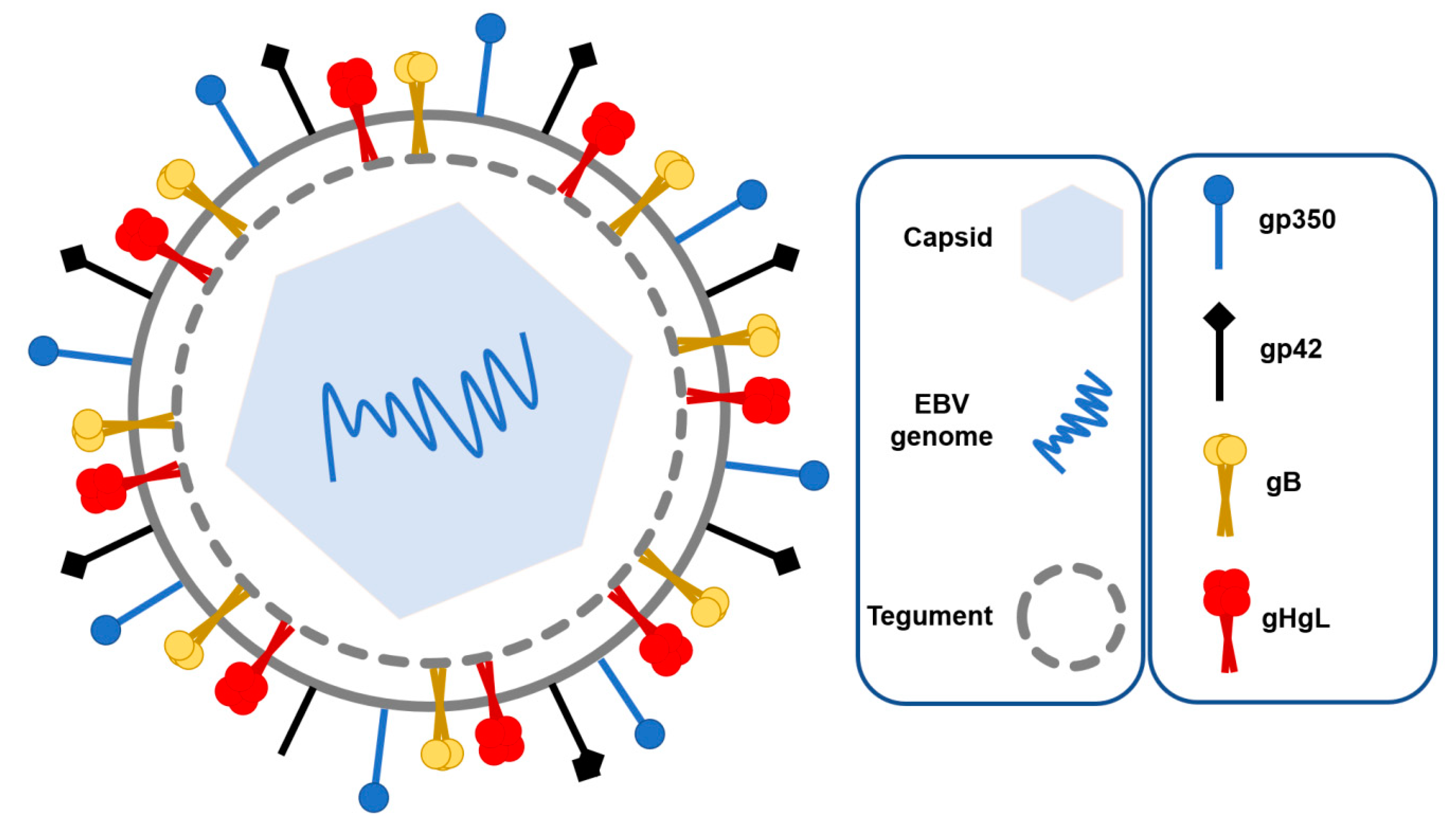
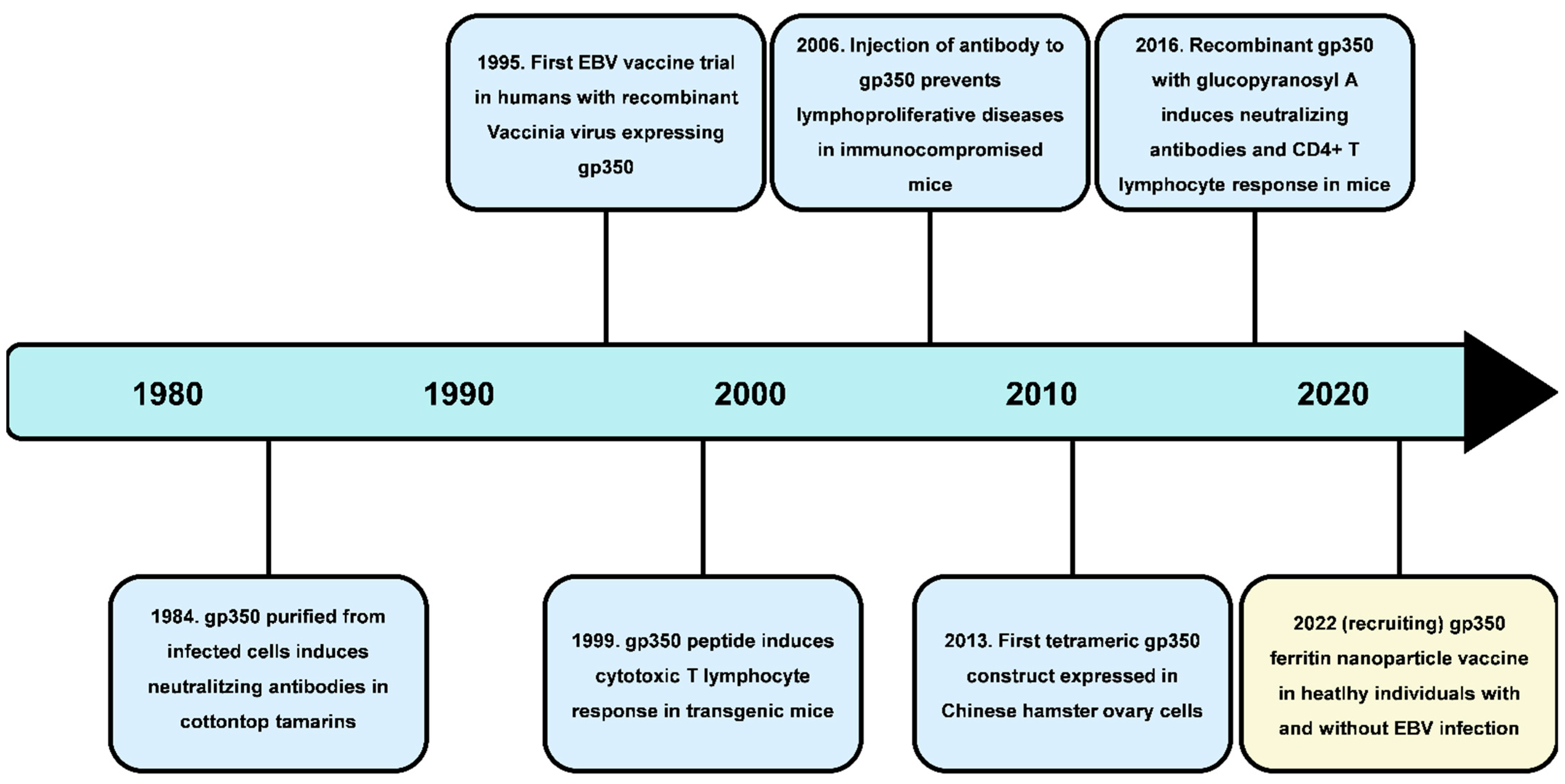
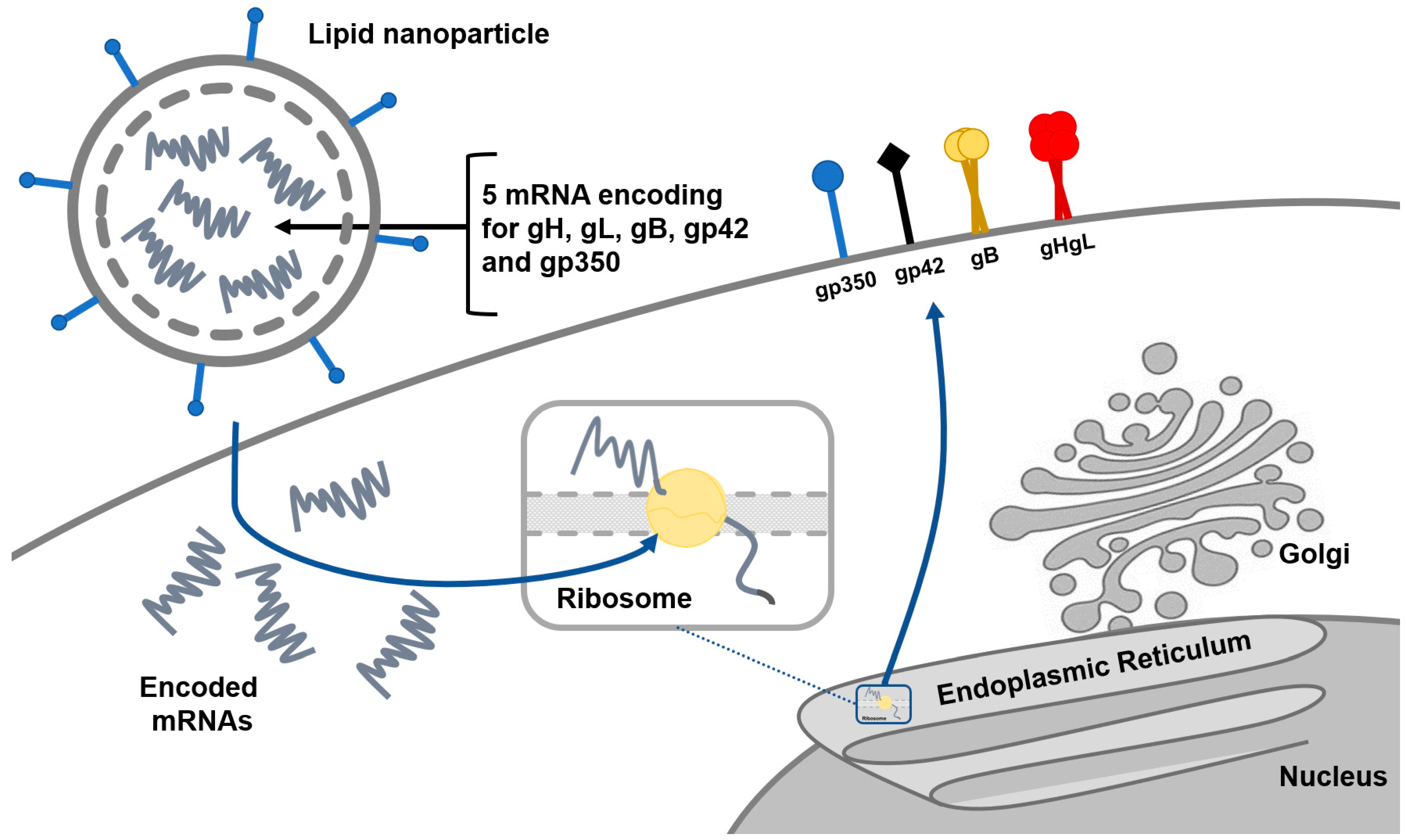
| Latency Type | Expressed Genes | Cells |
|---|---|---|
| 0 | EBERs (noncoding RNAs) | Memory B lymphocytes |
| I | EBERs, EBNA1 | Memory B lymphocytes |
| II | EBERs, EBNA1, LMP1, LMP2A, LMP2B | Proliferating and maturing EBV-infected lymphocytes |
| III | EBERs, EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C LMP1, LMP2A, LMP2B | Naive B lymphocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman, M.; Korać, P.; Jambrosic, K.; Židovec Lepej, S. Progress in Prophylactic and Therapeutic EBV Vaccine Development Based on Molecular Characteristics of EBV Target Antigens. Pathogens 2022, 11, 864. https://doi.org/10.3390/pathogens11080864
Rozman M, Korać P, Jambrosic K, Židovec Lepej S. Progress in Prophylactic and Therapeutic EBV Vaccine Development Based on Molecular Characteristics of EBV Target Antigens. Pathogens. 2022; 11(8):864. https://doi.org/10.3390/pathogens11080864
Chicago/Turabian StyleRozman, Marija, Petra Korać, Karlo Jambrosic, and Snjezana Židovec Lepej. 2022. "Progress in Prophylactic and Therapeutic EBV Vaccine Development Based on Molecular Characteristics of EBV Target Antigens" Pathogens 11, no. 8: 864. https://doi.org/10.3390/pathogens11080864
APA StyleRozman, M., Korać, P., Jambrosic, K., & Židovec Lepej, S. (2022). Progress in Prophylactic and Therapeutic EBV Vaccine Development Based on Molecular Characteristics of EBV Target Antigens. Pathogens, 11(8), 864. https://doi.org/10.3390/pathogens11080864






