First Human Case of Tick-Borne Encephalitis in Non-Endemic Region in Italy: A Case Report
Abstract
:1. Introduction
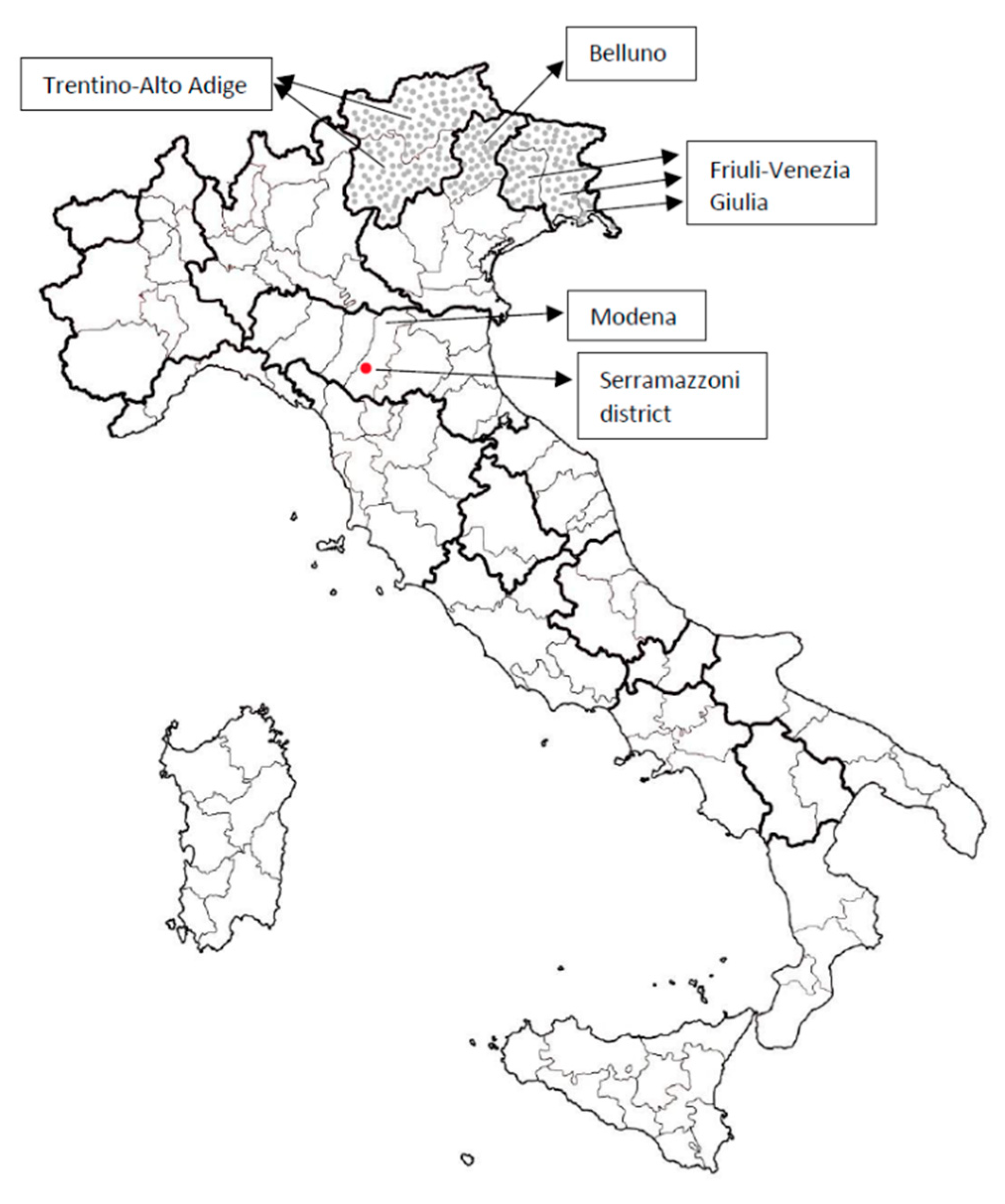
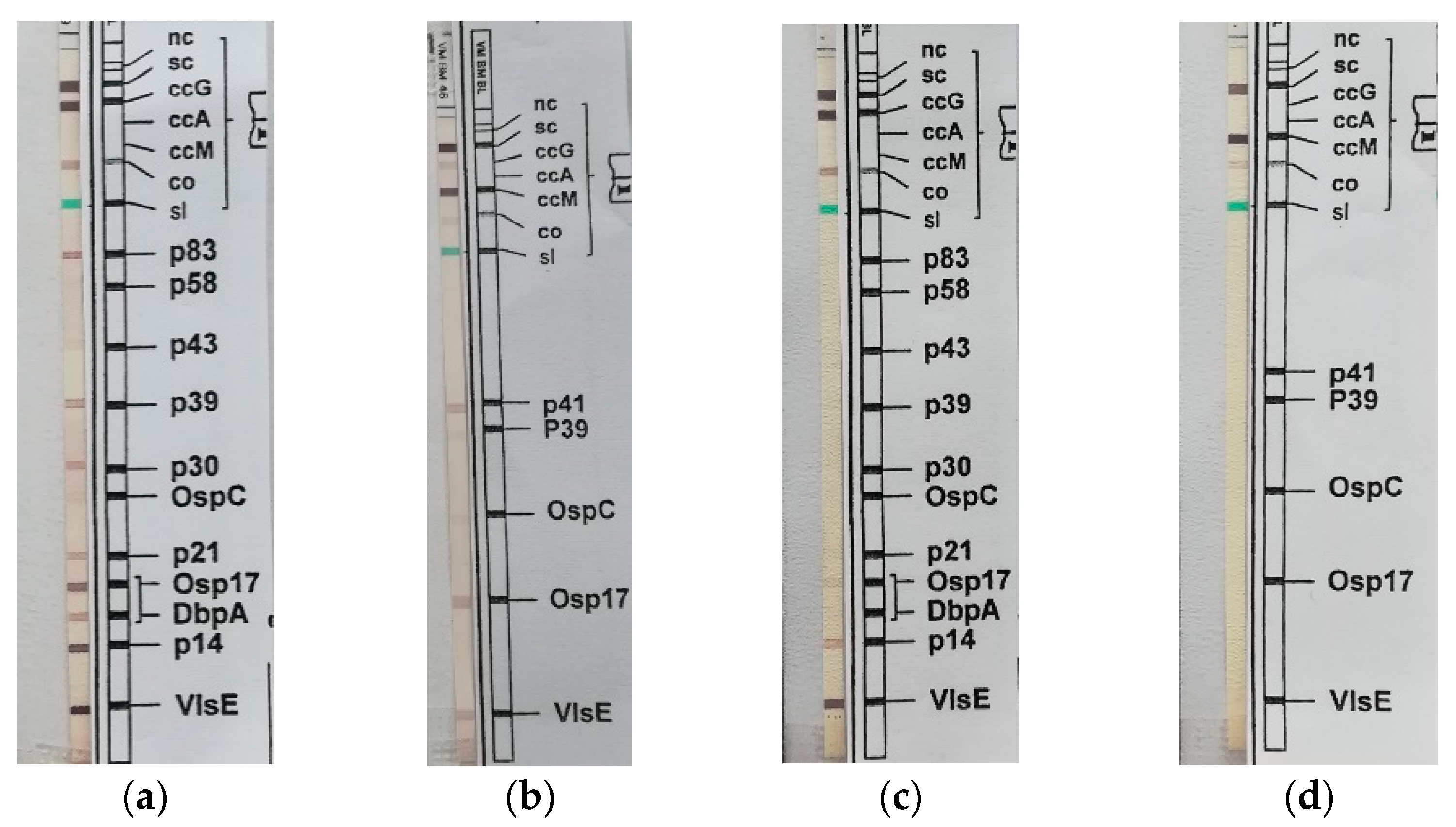
2. The Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruzek, D.; Županc, T.A.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Barp, N.; Trentini, A.; Di Nuzzo, M.; Mondardini, V.; Francavilla, E.; Contini, C. Clinical and laboratory findings in tick-borne en-cephalitis virus infection. Parasite Epidemiol. Control 2020, 19, e00160. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Lotric-Furlan, S.; Strle, F. What tick-borne encephalitis may look like: Clinical signs and symptoms. Travel Med. Infect. Dis. 2010, 8, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Holzmann, H. Laboratory findings in tick-borne encephalitis–correlation with clinical outcome. Infection 2000, 28, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1800201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piano Nazionale di Prevenzione, Sorveglianza e Risposta alle Arbovirosi 2020–2025. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 10 June 2022).
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gmao.gsfc.nasa.gov/reanalysis/MERRA-2/ (accessed on 1 April 2020).
- Available online: https://www2.jpl.nasa.gov/srtm/ (accessed on 1 April 2020).
- Maioli, G.; Pistone, D.; Bonilauri, P.; Pajoro, M.; Barbieri, I.; Mulatto, P.; Vicari, N.; Dottori, M. Etiological agents of rick-ettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp. Appl. Acarol. 2012, 57, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Süss, J.; Schrader, C.; Abel, U.; Voigt, W.P.; Schosser, R. Annual and seasonal variation of tick-borne encephalitis virus (TBEV) prevalence in ticks in selected hot spot areas in Germany using a nRT-PCR: Results from 1997 and 1998. Zent. Bakteriol. 1999, 289, 564–578. [Google Scholar] [CrossRef]
- Orlinger, K.K.; Hofmeister, Y.; Fritz, R.; Holzer, G.W.; Falkner, F.G.; Unger, B.; Loew-Baselli, A.; Poellabauer, E.-M.; Ehrlich, H.J.; Barrett, P.N.; et al. A Tick-Borne Encephalitis Virus Vaccine Based on the European Prototype Strain Induces Broadly Reactive Cross-Neutralizing Antibodies in Humans. J. Infect. Dis. 2011, 203, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Czupryna, P.; Moniuszko, A.; Pancewicz, S.A.; Grygorczuk, S.; Kondrusik, M.; Zajkowska, J. Tick-borne encephalitis in Poland in years 1993-2008--epidemiology and clinical presentation. A retrospective study of 687 patients. Eur. J. Neurol. 2011, 18, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Goettner, G.; Schulte-Spechtel, U.; Hillermann, R.; Liegl, G.; Wilske, B.; Fingerle, V. Improvement of Lyme Borreliosis Serodiagnosis by a Newly Developed Recombinant Immunoglobulin G (IgG) and IgM Line Immunoblot Assay and Addition of VlsE and DbpA Homologues. J. Clin. Microbiol. 2005, 43, 3602–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological Diagnosis of Human Borrelioses: Current Knowledge and Perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Wilske, B.; Fingerle, V.; Herzer, P.; Hofmann, A.; Lehnert, G.; Peters, H.; Pfister, H.W.; Preac-Mursic, V.; Soutschek, E.; Weber, K. Re-combinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and en-zyme-linked immunosorbent assay. Med. Microbiol. Immunol. 1993, 182, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.T.; Aberer, E.; Cinco, M.; Gern, L.; Hu, C.M.; Lobet, Y.N.; Ruscio, M.; Voet, P.E., Jr.; Weynants, V.E.; Philipp, M.T. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 2000, 182, 1455–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, U.; Lehnert, G.; Wilske, B. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiag-nosis of Lyme borreliosis based on sera collected throughout Europe. J. Clin. Microbiol. 1999, 37, 2241–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Date | Event |
|---|---|
| 29 March 2022 | Fever, asthenia (First phase) |
| 3 April 2022–11 April 2022 | Asymptomatic interval |
| 11 April 2022 | Fever, headache, ideomotor slowing, ataxia, constipation, dyspnea, high blood pressure values (Second phase) |
| 14 April 2022 | Tremors, facial palsy |
| 15 April 2022 | Stop fever and dyspnea |
| 21 April 2022 | Discharged |
| 6 May 2022 | Tremors and asthenia persistence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barp, N.; Cappi, C.; Meschiari, M.; Battistel, M.; Libbra, M.V.; Ferri, M.A.; Ballestri, S.; Gallerani, A.; Ferrari, F.; Meacci, M.; et al. First Human Case of Tick-Borne Encephalitis in Non-Endemic Region in Italy: A Case Report. Pathogens 2022, 11, 854. https://doi.org/10.3390/pathogens11080854
Barp N, Cappi C, Meschiari M, Battistel M, Libbra MV, Ferri MA, Ballestri S, Gallerani A, Ferrari F, Meacci M, et al. First Human Case of Tick-Borne Encephalitis in Non-Endemic Region in Italy: A Case Report. Pathogens. 2022; 11(8):854. https://doi.org/10.3390/pathogens11080854
Chicago/Turabian StyleBarp, Nicole, Cinzia Cappi, Marianna Meschiari, Marzia Battistel, Maria Vittoria Libbra, Maria Alice Ferri, Stefano Ballestri, Altea Gallerani, Filippo Ferrari, Marisa Meacci, and et al. 2022. "First Human Case of Tick-Borne Encephalitis in Non-Endemic Region in Italy: A Case Report" Pathogens 11, no. 8: 854. https://doi.org/10.3390/pathogens11080854
APA StyleBarp, N., Cappi, C., Meschiari, M., Battistel, M., Libbra, M. V., Ferri, M. A., Ballestri, S., Gallerani, A., Ferrari, F., Meacci, M., Sarti, M., Capitelli, M., Mussini, C., & Franceschini, E. (2022). First Human Case of Tick-Borne Encephalitis in Non-Endemic Region in Italy: A Case Report. Pathogens, 11(8), 854. https://doi.org/10.3390/pathogens11080854






