The Impact of RNA Interference in Tick Research
Abstract
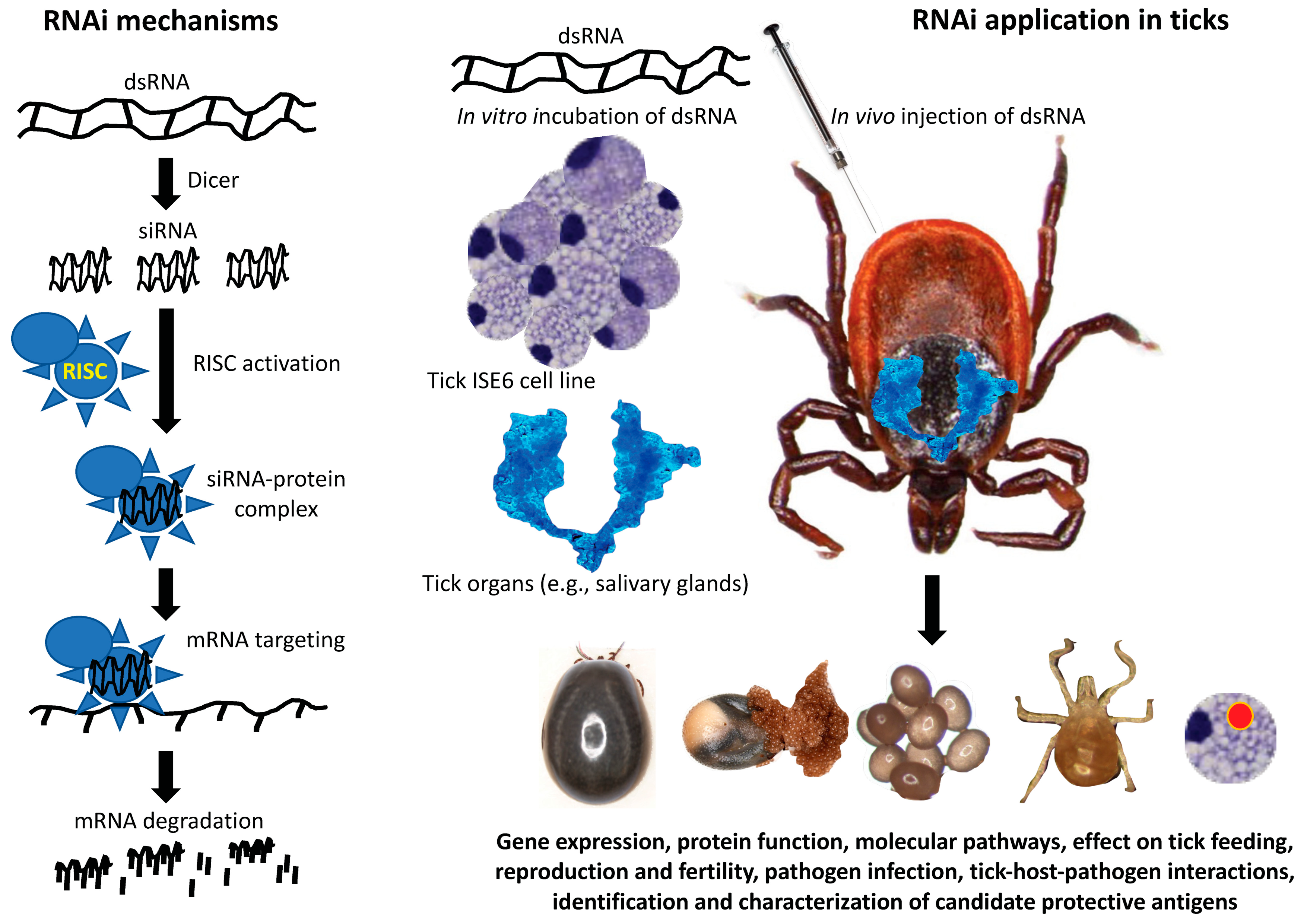
:1. Introduction
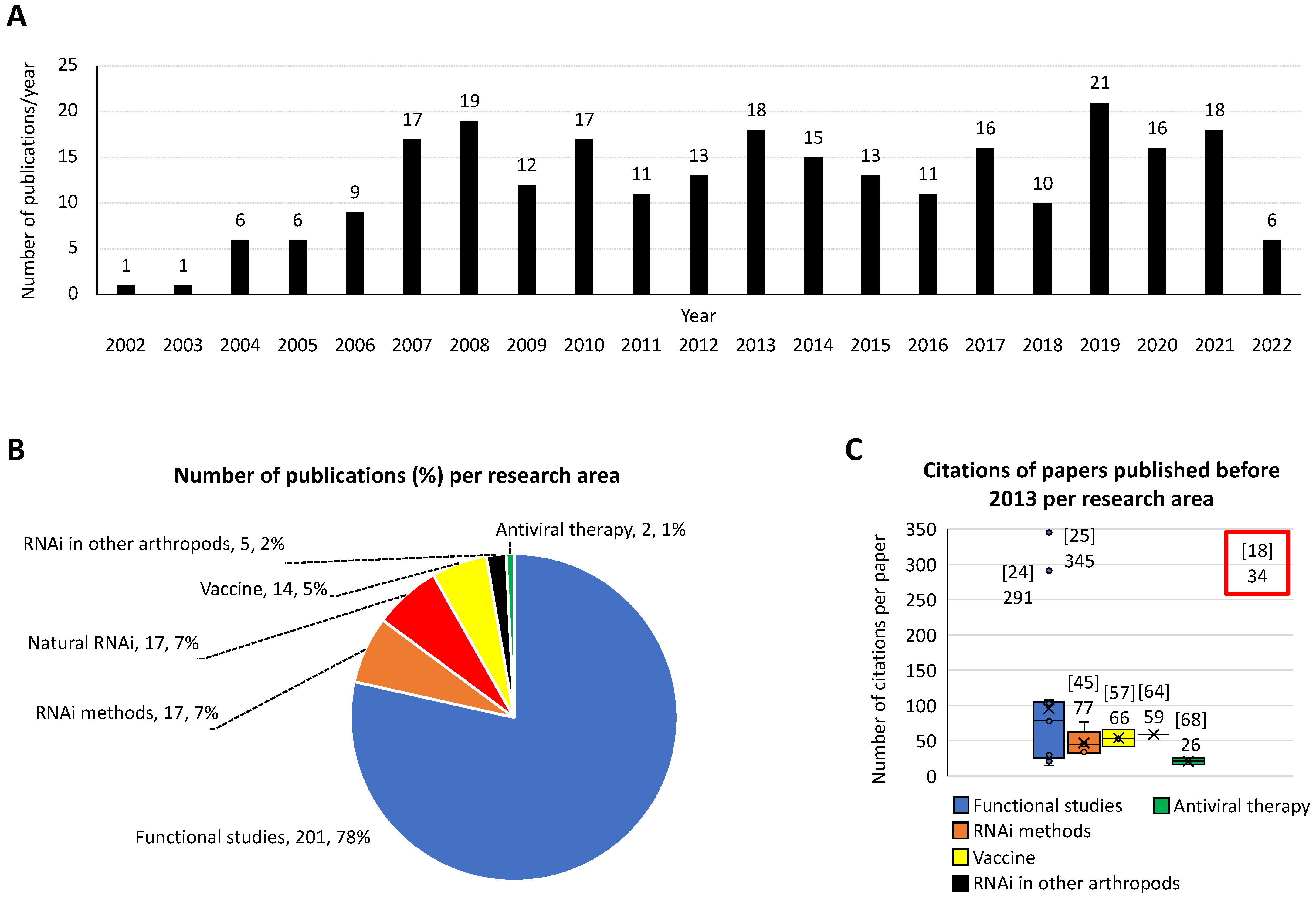
2. Discovery/Methodology
3. Impact
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. FASEB J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. Overview ofRNAInterference and Related Processes. Curr. Protoc. Mol. Biol. 2003, 62, 26.1.1–26.1.6. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K. RNA interference: Historical overview and significance. Methods Mol. Biol. 2004, 265, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Romano, N.; Macino, G. Quelling: Transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Guo, S.; Kemphues, K.J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Grishok, A.; Tabara, H.; Mello, C.C. Genetic Requirements for Inheritance of RNAi in C. elegans. Science 2000, 287, 2494–2497. [Google Scholar] [CrossRef] [Green Version]
- Voinnet, O.; Baulcombe, D. Systemic signalling in gene silencing. Nature 1997, 389, 553. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Baulcombe, D.C. A Species of Small Antisense RNA in Posttranscriptional Gene Silencing in Plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [Green Version]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-Stranded RNA Directs the ATP-Dependent Cleavage of mRNA at 21 to 23 Nucleotide Intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Martinez, J.; Patkaniowska, A.; Urlaub, H.; Lührmann, R.; Tuschl, T. Single-Stranded Antisense siRNAs Guide Target RNA Cleavage in RNAi. Cell 2002, 110, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Hutvágner, G.; Zamore, P.D. RNAi: Nature abhors a double-strand. Curr. Opin. Genet. Dev. 2002, 12, 225–232. [Google Scholar] [CrossRef]
- Aljamali, M.N.; Sauer, J.R.; Essenberg, R.C. RNA Interference: Applicability in Tick Research. Exp. Appl. Acarol. 2002, 28, 89–96. [Google Scholar] [CrossRef]
- de la Fuente, J.; Kocan, K.M.; Almazán, C.; Blouin, E.F. RNA interference for the study and genetic manipulation of ticks. Trends Parasitol. 2007, 23, 427–433. [Google Scholar] [CrossRef]
- Kocan, K.M.; Blouin, E.; De La Fuente, J. RNA Interference in Ticks. J. Vis. Exp. 2011, e2474. [Google Scholar] [CrossRef] [Green Version]
- Aljamali, M.N.; Bior, A.D.; Sauer, J.R.; Essenberg, R.C. RNA interference in ticks: A study using histamine binding protein dsRNA in the female tick Amblyomma americanum. Insect Mol. Biol. 2003, 12, 299–305. [Google Scholar] [CrossRef]
- Narasimhan, S.; Montgomery, R.R.; DePonte, K.; Tschudi, C.; Marcantonio, N.; Anderson, J.F.; Sauer, J.R.; Cappello, M.; Kantor, F.S.; Fikrig, E. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc. Natl. Acad. Sci. USA 2004, 101, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, J.; Almazán, C.; Naranjo, V.; Blouin, E.F.; Meyer, J.M.; Kocan, K.M. Autocidal control of ticks by silencing of a single gene by RNA interference. Biochem. Biophys. Res. Commun. 2006, 344, 332–338. [Google Scholar] [CrossRef]
- Karim, S.; Ramakrishnan, V.G.; Tucker, J.S.; Essenberg, R.C.; Sauer, J.R. Amblyomma americanum salivary glands: Double-stranded RNA-mediated gene silencing of synaptobrevin homologue and inhibition of PGE2 stimulated protein secretion. Insect Biochem. Mol. Biol. 2004, 34, 407–413. [Google Scholar] [CrossRef]
- Pal, U.; Li, X.; Wang, T.; Montgomery, R.R.; Ramamoorthi, N.; Desilva, A.M.; Bao, F.; Yang, X.; Pypaert, M.; Pradhan, D.; et al. TROSPA, an Ixodes scapularis Receptor for Borrelia burgdorferi. Cell 2004, 119, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthi, N.; Narasimhan, S.; Pal, U.; Bao, F.; Yang, X.F.; Fish, D.; Anguita, J.; Norgard, M.V.; Kantor, F.S.; Anderson, J.F.; et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 2005, 436, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Karim, S.; Kenny, B.; Troiano, E.; Mather, T.N. RNAi-mediated gene silencing in tick synganglia: A proof of concept study. BMC Biotechnol. 2008, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Hajdusek, O.; Sojka, D.; Kopacek, P.; Buresova, V.; Franta, Z.; Sauman, I.; Winzerling, J.; Grubhoffer, L. Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proc. Natl. Acad. Sci. USA 2009, 106, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Booth, C.J.; Paley, M.A.; Wang, X.; DePonte, K.; Fikrig, E.; Narasimhan, S.; Montgomery, R.R. Inhibition of Neutrophil Function by Two Tick Salivary Proteins. Infect. Immun. 2009, 77, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
- Mulenga, A.; Khumthong, R. Silencing of three Amblyomma americanum (L.) insulin-like growth factor binding protein-related proteins prevents ticks from feeding to repletion. J. Exp. Biol. 2010, 213, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Narasimhan, S.; Zhang, L.; Liu, L.; Wang, P.; Fikrig, E. Tick Histamine Release Factor Is Critical for Ixodes scapularis Engorgement and Transmission of the Lyme Disease Agent. PLoS Pathog. 2010, 6, e1001205. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.M.; Boldbaatar, D.; Umemiya-Shirafuji, R.; Liao, M.; Xuenan, X.; Suzuki, H.; Galay, R.L.; Tanaka, T.; Fujisaki, K. Scavenger Receptor Mediates Systemic RNA Interference in Ticks. PLoS ONE 2011, 6, e28407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, F.A.; Pohl, P.C.; Gandara, A.C.; Ferreira, J.D.S.; Nascimento-Silva, M.C.; Bechara, G.H.; Sorgine, M.H.F.; Almeida, I.C.; Vaz, I.D., Jr.; Oliveira, P.L. ATP Binding Cassette Transporter Mediates Both Heme and Pesticide Detoxification in Tick Midgut Cells. PLoS ONE 2015, 10, e0134779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzugaray, M.F.; Parizi, L.F.; Seixas, A.; Benavides, U.; Vaz, I.D.S. Molecular and functional characterization of Bm05br antigen from Rhipicephalus microplus. Ticks Tick-Borne Dis. 2017, 8, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, J.M.; Gulia-Nuss, M.; Kuhn, R.J.; Hill, C.A. RNAi reveals proteins for metabolism and protein processing associated with Langat virus infection in Ixodes scapularis (black-legged tick) ISE6 cells. Parasites Vectors 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, S.; Schuijt, T.J.; Abraham, N.M.; Rajeevan, N.; Coumou, J.; Graham, M.; Robson, A.; Wu, M.-J.; Daffre, S.; Hovius, J.W.; et al. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun. 2017, 8, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, J.M.; Tsetsarkin, K.A.; Long, D.; Scott, D.P.; Rosenke, R.; Schwan, T.G.; Mlera, L.; Offerdahl, D.K.; Pletnev, A.G.; Bloom, M.E. Flavivirus Infection of Ixodes scapularis (Black-Legged Tick) Ex Vivo Organotypic Cultures and Applications for Disease Control. mBio 2017, 8, e01255-17. [Google Scholar] [CrossRef] [Green Version]
- Carroll, E.E.M.; Wang, X.; Shaw, D.K.; O’Neal, A.J.; Chávez, A.S.O.; Brown, L.J.; Boradia, V.M.; Hammond, H.L.; Pedra, J.H.F. p47 licenses activation of the immune deficiency pathway in the tick Ixodes scapularis. Proc. Natl. Acad. Sci. USA 2019, 116, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.E.; Johnson, W.C.; Taus, N.S.; Suarez, C.E.; Scoles, G.A.; Ueti, M.W. Silencing expression of the Rhipicephalus microplus vitellogenin receptor gene blocks Babesia bovis transmission and interferes with oocyte maturation. Parasites Vectors 2019, 12, 7. [Google Scholar] [CrossRef]
- Wu, F.; Luo, J.; Chen, Z.; Ren, Q.; Xiao, R.; Liu, W.; Hao, J.; Liu, X.; Guo, J.; Qu, Z.; et al. MicroRNA let-7 regulates the expression of ecdysteroid receptor (ECR) in Hyalomma asiaticum (Acari: Ixodidae) ticks. Parasites Vectors 2019, 12, 235. [Google Scholar] [CrossRef]
- Agwunobi, D.O.; Wang, N.; Huang, L.; Zhang, Y.; Chang, G.; Wang, K.; Li, M.; Wang, H.; Liu, J. Phosphoproteomic Analysis of Haemaphysalis longicornis Saliva Reveals the Influential Contributions of Phosphoproteins to Blood-Feeding Success. Front. Cell. Infect. Microbiol. 2022, 11, 769026. [Google Scholar] [CrossRef]
- Andzhaparidze, O.G.; Bogomolova, N.N.; Boriskin, I.; Desiatskova, R.G.; Bektemirova, M.S. Sravnitel’noe izuchenie ersistentsii trekh RNK-soderzhashchikh virusov v perevivaemoĭ kul’ture kletok chelovecheskogo proiskhozhdeniia [Comparative study of the persistence of 3 RNA-containing viruses in a continuous cell culture of human origin]. Vopr. Virusol. 1980, 3, 323–327. [Google Scholar]
- Schnettler, E.; Tykalová, H.; Watson, M.; Sharma, M.; Sterken, M.; Obbard, D.; Lewis, S.H.; McFarlane, M.; Bell-Sakyi, L.; Barry, G.; et al. Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res. 2014, 42, 9436–9446. [Google Scholar] [CrossRef]
- Hart, C.E.; Thangamani, S. Tick-virus interactions: Current understanding and future perspectives. Parasite Immunol. 2021, 43, e12815. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, Z.; Ren, Y.; Ma, L.; Ye, Z.; Gao, C.; Wang, J.; Li, Y. Antiviral RNA interference in disease vector (Asian longhorned) ticks. PLoS Pathog. 2021, 17, e1010119. [Google Scholar] [CrossRef]
- de la Fuente, J.; Blouin, E.F.; Manzano-Roman, R.; Naranjo, V.; Almazán, C.; de la Lastra, J.M.P.; Zivkovic, Z.; Jongejan, F.; Kocan, K.M. Functional genomic studies of tick cells in response to infection with the cattle pathogen, Anaplasma marginale. Genomics 2007, 90, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Kocan, K.M.; Manzano-Roman, R.; De La Fuente, J. Transovarial silencing of the subolesin gene in three-host ixodid tick species after injection of replete females with subolesin dsRNA. Parasitol. Res. 2007, 100, 1411–1415. [Google Scholar] [CrossRef]
- Kröber, T.; Guerin, P.M. In vitro feeding assays for hard ticks. Trends Parasitol. 2007, 23, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Karim, S.; Troiano, E.; Mather, T.N. Functional genomics tool: Gene silencing in Ixodes scapularis eggs and nymphs by electroporated dsRNA. BMC Biotechnol. 2010, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Usme-Ciro, J.A.; Campillo-Pedroza, N.; Almazán, F.; Gallego-Gomez, J.C. Cytoplasmic RNA viruses as potential vehicles for the delivery of therapeutic small RNAs. Virol. J. 2013, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Tuckow, A.P.; Temeyer, K.B. Discovery, adaptation and transcriptional activity of two tick promoters: Construction of a dual luciferase reporter system for optimization of RNA interference in Rhipicephalus (Boophilus) microplus cell lines. Insect Mol. Biol. 2015, 24, 454–466. [Google Scholar] [CrossRef]
- Ruiz, N.; De Abreu, L.A.; Parizi, L.F.; Kim, T.K.; Mulenga, A.; Braz, G.R.C.; Vaz, I.D.S.; Logullo, C. Non-Invasive Delivery of dsRNA into De-Waxed Tick Eggs by Electroporation. PLoS ONE 2015, 10, e0130008. [Google Scholar] [CrossRef]
- Galay, R.L.; Hernandez, E.P.; Talactac, M.R.; Maeda, H.; Kusakisako, K.; Umemiya-Shirafuji, R.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. Induction of gene silencing in Haemaphysalis longicornis ticks through immersion in double-stranded RNA. Ticks Tick-Borne Dis. 2016, 7, 813–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Zhou, Y.; Cao, J.; Gong, H.; Zhang, H.; Zhou, J. Liposome mediated double-stranded RNA delivery to silence ribosomal protein P0 in the tick Rhipicephalus haemaphysaloides. Ticks Tick-Borne Dis. 2018, 9, 638–644. [Google Scholar] [CrossRef]
- Koosha, M.; Vatandoost, H.; Karimian, F.; Choubdar, N.; Oshaghi, M.A. Delivery of a Genetically Marked Serratia AS1 to Medically Important Arthropods for Use in RNAi and Paratransgenic Control Strategies. Microb. Ecol. 2019, 78, 185–194. [Google Scholar] [CrossRef]
- Grabowski, J.M.; Kissinger, R. Ixodid Tick Dissection and Tick Ex Vivo Organ Cultures for Tick-Borne Virus Research. Curr. Protoc. Microbiol. 2020, 59, e118. [Google Scholar] [CrossRef]
- Stockmal, K.A.; Downs, L.P.; Davis, A.N.; Kemp, L.K.; Karim, S.; Morgan, S.E. Cationic Glycopolyelectrolytes for RNA Interference in Tick Cells. Biomacromolecules 2022, 23, 34–46. [Google Scholar] [CrossRef]
- De La Fuente, J.; Almazán, C.; Blouin, E.F.; Naranjo, V.; Kocan, K.M. RNA interference screening in ticks for identification of protective antigens. Parasitol. Res. 2005, 96, 137–141. [Google Scholar] [CrossRef]
- De La Fuente, J.; Almazán, C.; Naranjo, V.; Blouin, E.F.; Kocan, K.M. Synergistic effect of silencing the expression of tick protective antigens 4D8 and Rs86 in Rhipicephalus sanguineus by RNA interference. Parasitol. Res. 2006, 99, 108–113. [Google Scholar] [CrossRef]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Peña, A.E.; Kocan, K.M.; De La Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef]
- Mulenga, A.; Kim, T.K.; Ibelli, A.M.G. Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int. J. Parasitol. 2013, 43, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjunathachar, H.V.; Azhahianambi, P.; Kumar, B.; Ghosh, S. Screening for the “Achilles Heel” of Hyalomma anatolicum Ticks by RNA Interference Technology and an Update on Anti-Tick Vaccine Design. Methods Mol. Biol. 2022, 2411, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Drolia, R.; Von Ohlen, T.; Chapes, S.K. Ehrlichia chaffeensis replication sites in adult Drosophila melanogaster. Int. J. Med Microbiol. 2013, 303, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichner, C.; Øvergård, A.-C.; Nilsen, F.; Dalvin, S. Molecular characterization and knock-down of salmon louse (Lepeophtheirus salmonis) prostaglandin E synthase. Exp. Parasitol. 2015, 159, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Budge, G.E.; Bowman, A.S. Gene-knockdown in the honey bee mite Varroa destructor by a non-invasive approach: Studies on a glutathione S-transferase. Parasites Vectors 2010, 3, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.Y.; Bian, G.; Xi, Z.; Xie, X. Genes important for survival or reproduction inVarroa destructoridentified by RNAi. Insect Sci. 2019, 26, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Letinić, B.D.; Dahan-Moss, Y.; Koekemoer, L.L. Characterising the effect of Akirin knockdown on Anopheles arabiensis (Diptera: Culicidae) reproduction and survival, using RNA-mediated interference. PLoS ONE 2020, 15, e0228576. [Google Scholar] [CrossRef] [Green Version]
- Achazi, K.; Patel, P.; Paliwal, R.; Radonić, A.; Niedrig, M.; Donoso-Mantke, O. RNA interference inhibits replication of tick-borne encephalitis virus in vitro. Antivir. Res. 2012, 93, 94–100. [Google Scholar] [CrossRef] [Green Version]
- David, A.S.; Stein, D.A.; Shi, P.-Y. Nucleic acid-based inhibition of flavivirus infections. Front. Biosci. 2008, 13, 1385–1395. [Google Scholar] [CrossRef]
- Sharma, S.R.; Crispell, G.; Mohamed, A.; Cox, C.; Lange, J.; Choudhary, S.; Commins, S.P.; Karim, S. Alpha-Gal Syndrome: Involvement of Amblyomma americanum α-D-Galactosidase and β-1,4 Galactosyltransferase Enzymes in α-Gal Metabolism. Front. Cell. Infect. Microbiol. 2021, 11, 775371. [Google Scholar] [CrossRef]
- Nganso, B.T.; Pines, G.; Soroker, V. Insights into gene manipulation techniques for Acari functional genomics. Insect Biochem. Mol. Biol. 2022, 143, 103705. [Google Scholar] [CrossRef]
- Aguilar-Díaz, H.; Quiroz-Castañeda, R.E.; Cobaxin-Cárdenas, M.; Salinas-Estrella, E.; Amaro-Estrada, I. Advances in the Study of the Tick Cattle Microbiota and the Influence on Vectorial Capacity. Front. Vet. Sci. 2021, 8, 710352. [Google Scholar] [CrossRef]
- Choubdar, N.; Karimian, F.; Koosha, M.; Oshaghi, M.A. An integrated overview of the bacterial flora composition of Hyalomma anatolicum, the main vector of CCHF. PLoS Negl. Trop. Dis. 2021, 15, e0009480. [Google Scholar] [CrossRef]
- de la Fuente, J. Translational biotechnology for the control of ticks and tick-borne diseases. Ticks Tick-borne Dis. 2021, 12, 101738. [Google Scholar] [CrossRef]
- Sharma, A.; Pham, M.N.; Reyes, J.B.; Chana, R.; Yim, W.C.; Heu, C.C.; Kim, D.; Chaverra-Rodriguez, D.; Rasgon, J.L.; Harrell, R.A.; et al. Cas9-mediated gene editing in the black-legged tick, Ixodes scapularis, by embryo injection and ReMOT Control. iScience 2022, 25, 103781. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente, J.; Kocan, K.M. The Impact of RNA Interference in Tick Research. Pathogens 2022, 11, 827. https://doi.org/10.3390/pathogens11080827
de la Fuente J, Kocan KM. The Impact of RNA Interference in Tick Research. Pathogens. 2022; 11(8):827. https://doi.org/10.3390/pathogens11080827
Chicago/Turabian Stylede la Fuente, José, and Katherine M. Kocan. 2022. "The Impact of RNA Interference in Tick Research" Pathogens 11, no. 8: 827. https://doi.org/10.3390/pathogens11080827
APA Stylede la Fuente, J., & Kocan, K. M. (2022). The Impact of RNA Interference in Tick Research. Pathogens, 11(8), 827. https://doi.org/10.3390/pathogens11080827






