Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection
Abstract
:1. Introduction
2. Results
2.1. Prevalence of ILC2 in the Skin and Draining Lymph Nodes of Naïve Mice
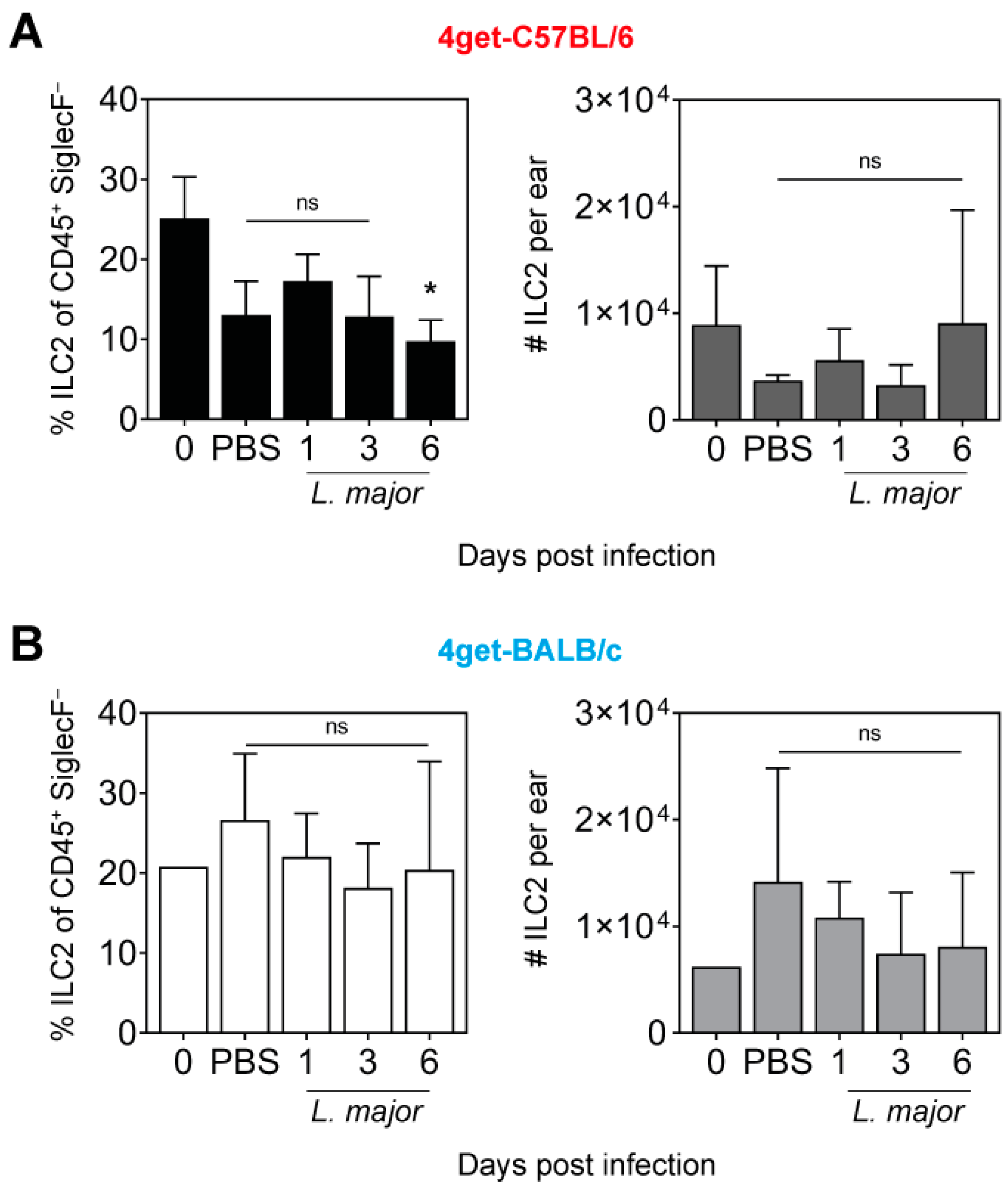
2.2. Prevalence of ILC2 in the Skin of L. major-Infected Mice
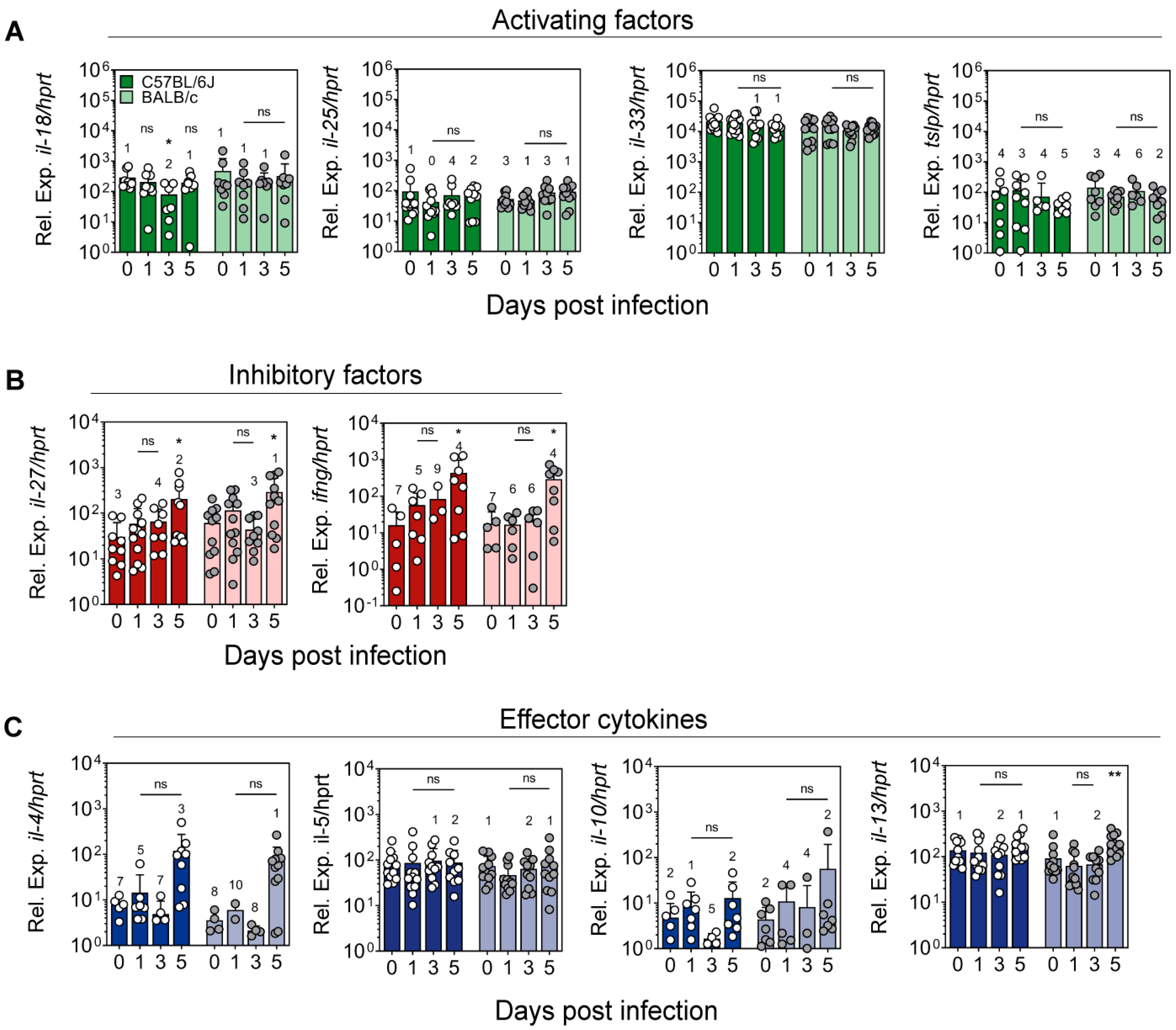
2.3. Expression of Cytokines Regulating ILC2 Activity in the Ear Skin of L. major-Infected Mice
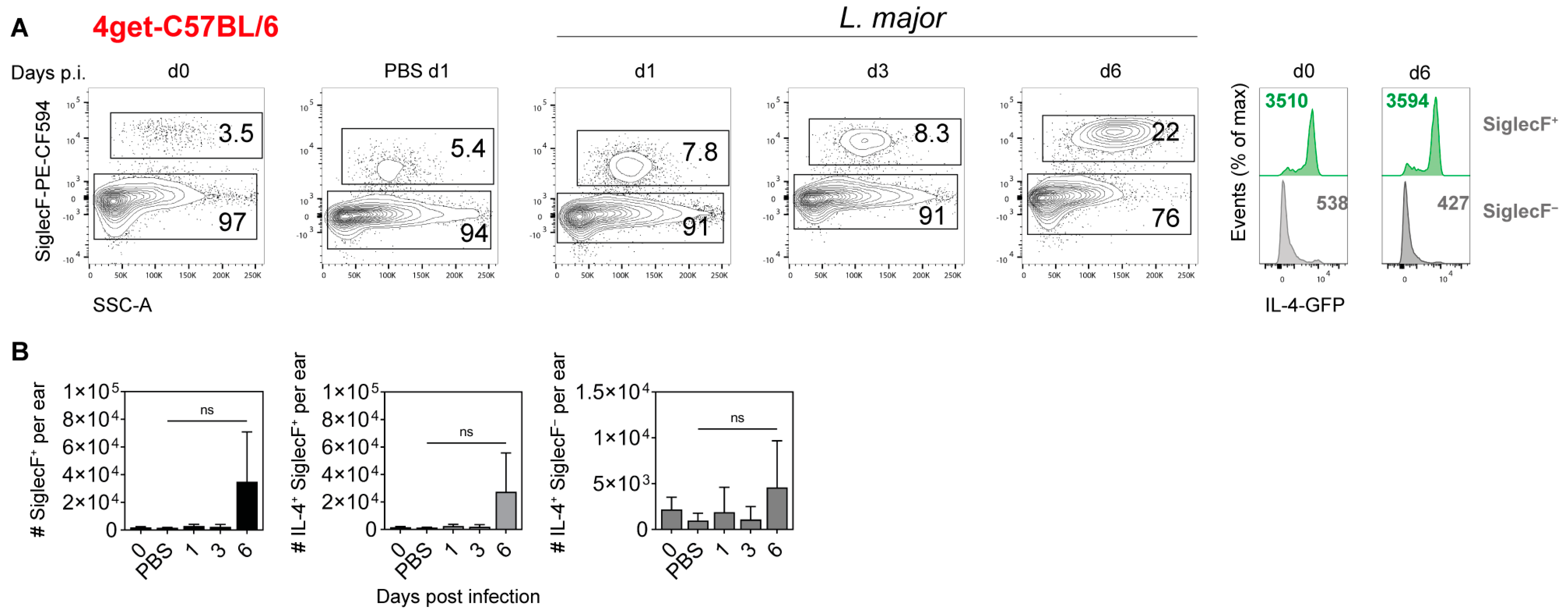
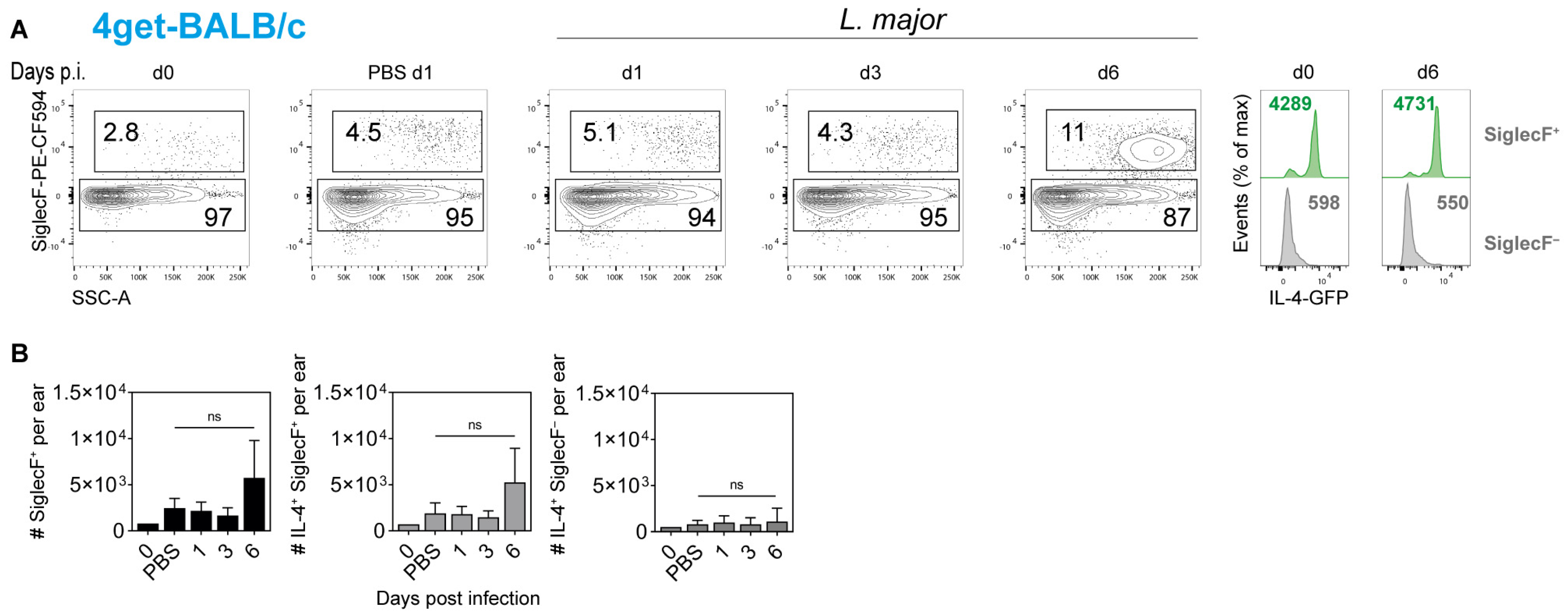
2.4. Cellular Source of IL-4 in the Ear Skin of L. major-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Parasites and Infection
4.3. Preparation of Single Cell Suspensions
4.4. Flow Cytometry
4.5. Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdan, C. Leishmaniasis in Rheumatology, Hematology, and Oncology: Epidemiological, immunological, and clinical aspects and caveats. Ann. Rheum. Dis. 2012, 71, i60–i66. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Sacks, D.L.; Noben-Trauth, N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002, 2, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Debus, A.; Sebald, H.; Rai, B.; Schafer, J.; Obermeyer, S.; Schleicher, U. Experimental Cutaneous Leishmaniasis: Mouse Models for Resolution of Inflammation Versus Chronicity of Disease. Methods Mol. Biol 2019, 1971, 315–349. [Google Scholar] [CrossRef] [PubMed]
- Brewig, N.; Kissenpfennig, A.; Malissen, B.; Veit, A.; Bickert, T.; Fleischer, B.; Mostbock, S.; Ritter, U. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J. Immunol. 2009, 182, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Von Stebut, E.; Tenzer, S. Cutaneous leishmaniasis: Distinct functions of dendritic cells and macrophages in the interaction of the host immune system with Leishmania major. Int. J. Med. Microbiol. 2018, 308, 206–214. [Google Scholar] [CrossRef]
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Haeberlein, S.; Sebald, H.; Schleicher, U.; Bogdan, C. Leishmania-infected macrophages are targets of NK cell-derived cytokines, but not of NK cell cytotoxicity. Infect. Immun. 2011, 79, 2699–2708. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, C. Natural killer cells in experimental and human leishmaniasis. Front. Cell. Infect. Microbiol. 2012, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biedermann, T.; Zimmermann, S.; Himmelrich, H.; Gumy, A.; Egeter, O.; Sakrauski, A.K.; Seegmuller, I.; Voigt, H.; Launois, P.; Levine, A.D.; et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2001, 2, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Roebrock, K.; Foell, D.; Nippe, N.; von Stebut, E.; Weiss, J.M.; Munck, N.A.; Viemann, D.; Varga, G.; Muller-Tidow, C.; et al. Keratinocytes determine Th1 immunity during early experimental leishmaniasis. PLoS Pathog. 2010, 6, e1000871. [Google Scholar] [CrossRef] [PubMed]
- Stenger, S.; Solbach, W.; Röllinghoff, M.; Bogdan, C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL-4. Eur. J. Immunol. 1991, 21, 1669–1675. [Google Scholar] [CrossRef]
- Hurdayal, R.; Nieuwenhuizen, N.E.; Revaz-Breton, M.; Smith, L.; Hoving, J.C.; Parihar, S.P.; Reizis, B.; Brombacher, F. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS Pathog. 2013, 9, e1003699. [Google Scholar] [CrossRef] [Green Version]
- Hurdayal, R.; Nieuwenhuizen, N.E.; Khutlang, R.; Brombacher, F. Inflammatory dendritic cells, regulated by il-4 receptor alpha signaling, control replication, and dissemination of leishmania major in mice. Front. Cell. Infect. Microbiol. 2019, 9, 479. [Google Scholar] [CrossRef]
- Launois, P.; Maillard, I.; Pingel, S.; Swihart, K.G.; Xenarios, I.; Acha-Orbea, H.; Diggelmann, H.; Locksley, R.M.; MacDonald, H.R.; Louis, J.A. IL-4 rapidly produced by Vβ4Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 1997, 6, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Himmelrich, H.; Launois, P.; Maillard, I.; Biedermann, T.; Tacchini-Cottier, F.; Locksley, R.M.; Rocken, M.; Louis, J.A. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 2000, 164, 4819–4825. [Google Scholar] [CrossRef] [Green Version]
- Kropf, P.; Herath, S.; Weber, V.; Modolell, M.; Muller, I. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 2003, 25, 439–447. [Google Scholar] [CrossRef]
- Gurung, P.; Karki, R.; Vogel, P.; Watanabe, M.; Bix, M.; Lamkanfi, M.; Kanneganti, T.D. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J. Clin. Investig. 2015, 125, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Iniesta, V.; Carcelen, J.; Molano, I.; Peixoto, P.M.V.; Redondo, E.; Parra, P.; Mangas, M.; Monroy, I.; Campo, M.L.; Nieto, C.G.; et al. Arginase I induction during Leishmania major infection mediates the development of disease. Infect. Immun. 2005, 73, 6085–6090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleicher, U.; Paduch, K.; Debus, A.; Obermeyer, S.; Konig, T.; Kling, J.C.; Ribechini, E.; Dudziak, D.; Mougiakakos, D.; Murray, P.J.; et al. TNF-Mediated restriction of arginase 1 expression in myeloid cells triggers type 2 no synthase activity at the site of infection. Cell. Rep. 2016, 15, 1062–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacchini-Cottier, F.; Zweifel, C.; Belkaid, Y.; Mukankundiye, C.; Vasei, M.; Launois, P.; Milon, G.; Louis, J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000, 165, 2628–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Chaves, M.M.; Kamenyeva, O.; Gazzinelli-Guimaraes, P.H.; Kang, B.; Pessenda, G.; Passelli, K.; Tacchini-Cottier, F.; Kabat, J.; Jacobsen, E.A.; et al. M2-like, dermal macrophages are maintained via IL-4/CCL24-mediated cooperative interaction with eosinophils in cutaneous leishmaniasis. Sci. Immunol. 2020, 5, eaaz4415. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Germain, R.N.; Huang, Y. ILC2s—resident lymphocytes pre-adapted to a specific tissue or migratory effectors that adapt to where they move? Curr. Opin. Immunol. 2019, 56, 76–81. [Google Scholar] [CrossRef]
- Horiuchi, K.; Higashiyama, M.; Kurihara, C.; Matsumura, K.; Tanemoto, R.; Ito, S.; Mizoguchi, A.; Nishii, S.; Wada, A.; Inaba, K.; et al. Intestinal inflammations increase efflux of innate lymphoid cells from the intestinal mucosa to the mesenteric lymph nodes through lymph-collecting ducts. Microcirculation 2021, 28, e12694. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J.; et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018, 19, 1093–1099. [Google Scholar] [CrossRef]
- Seehus, C.R.; Kadavallore, A.; Torre, B.; Yeckes, A.R.; Wang, Y.; Tang, J.; Kaye, J. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat. Commun. 2017, 8, 1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bando, J.K.; Gilfillan, S.; Di Luccia, B.; Fachi, J.L.; Secca, C.; Cella, M.; Colonna, M. ILC2s are the predominant source of intestinal ILC-derived IL-10. J. Exp. Med. 2020, 217, e20191520. [Google Scholar] [CrossRef] [PubMed]
- Duerr, C.U.; McCarthy, C.D.; Mindt, B.C.; Rubio, M.; Meli, A.P.; Pothlichet, J.; Eva, M.M.; Gauchat, J.F.; Qureshi, S.T.; Mazer, B.D.; et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat. Immunol. 2016, 17, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Kabata, H.; Tanabe, M.; Koga, S.; Takeno, N.; Mochizuki, M.; Fukunaga, K.; Asano, K.; Betsuyaku, T.; Koyasu, S. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. 2016, 17, 76–86. [Google Scholar] [CrossRef]
- Bonne-Annee, S.; Nutman, T.B. Human innate lymphoid cells (ILCs) in filarial infections. Parasite Immunol. 2018, 40, e12442. [Google Scholar] [CrossRef]
- Filbey, K.; Bouchery, T.; Le Gros, G. The role of ILC2 in hookworm infection. Parasite Immunol. 2018, 40, e12429. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.R.; Douglas, B.; Zullo, K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef] [Green Version]
- Rafei-Shamsabadi, D.A.; van de Poel, S.; Dorn, B.; Kunz, S.; Martin, S.F.; Klose, C.S.N.; Arnold, S.J.; Tanriver, Y.; Ebert, K.; Diefenbach, A.; et al. Lack of Type 2 Innate Lymphoid Cells Promotes a Type I-Driven Enhanced Immune Response in Contact Hypersensitivity. J. Investig. Dermatol. 2018, 138, 1962–1972. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, S.; Schulz-Kuhnt, A.; Neurath, M.F.; Atreya, I. Functional Contribution and Targeted Migration of Group-2 Innate Lymphoid Cells in Inflammatory Lung Diseases: Being at the Right Place at the Right Time. Front. Immunol. 2021, 12, 688879. [Google Scholar] [CrossRef]
- Mohrs, M.; Shinkai, K.; Mohrs, K.; Locksley, R.M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 2001, 15, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Tsang, J.C.; Wang, C.; Clare, S.; Wang, J.; Chen, X.; Brandt, C.; Kane, L.; Campos, L.S.; Lu, L.; et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature 2016, 539, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Moro, K. Tissue-Specific Diversity of Group 2 Innate Lymphoid Cells in the Skin. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Rodriguez, O.L.; Lugo, D.A.; Cabrera, M.; Sanchez, M.A.; Zerpa, O.; Tapia, F.J. Innate lymphoid cells in peripheral blood of patients with American Cutaneous Leishmaniasis. Exp. Dermatol. 2021, 30, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Carvalho, A.M.; Sacramento, L.A.; Grice, E.A.; Scott, P. Microbiota instruct IL-17A-producing innate lymphoid cells to promote skin inflammation in cutaneous leishmaniasis. PLoS Pathog. 2021, 17, e1009693. [Google Scholar] [CrossRef]
- Launois, P.; Himmelreich, H.; Tacchini-Cottier, F.; Milon, G.; Louis, J.A. New insight into the mechanisms underlying Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Microbes Infect. 1999, 1, 59–64. [Google Scholar] [CrossRef]
- Anderson, C.F.; Mendez, S.; Sacks, D.L. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 2005, 174, 2934–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beil, W.J.; Meinardus-Hager, G.; Neugebauer, D.C.; Sorg, C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J. Leukoc. Biol. 1992, 52, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Soares, M.J.; Moriearty, P.L. Tissue eosinophilia and Leishmania mexicana mexicana eosinophil interactions in murine cutaneous leishmaniosis. Parasite Immunol. 1984, 6, 735–739. [Google Scholar] [CrossRef]
- McElrath, M.J.; Kaplan, G.; Nusrat, A.; Cohn, Z.A. Cutaneous leishmaniasis: The defect in T cell influx in BALB/c mice. J. Exp. Med. 1987, 165, 546–559. [Google Scholar] [CrossRef] [Green Version]
- Pompeu, M.L.; Freitas, L.A.; Santos, M.L.; Khouri, M.; Barral-Netto, M. Granulocytes in the inflammatory process of BALB/c mice infected by Leishmania amazonensis. A quantitative approach. Acta Trop. 1991, 48, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Pompeu, M.L.; Freitas, L.A.; Santos, M.L.; Barral, A.; Barral-Netto, M. Leishmania amazonensis infection: A comparison of in vivo leishmanicidal mechanisms between immunized and naive infected BALB/c mice. Exp. Parasitol. 1992, 74, 169–176. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Cardoso, F.; de Souza Cda, S.; Mendes, V.G.; Abreu-Silva, A.L.; Goncalves da Costa, S.C.; Calabrese, K.S. Immunopathological studies of Leishmania amazonensis infection in resistant and in susceptible mice. J. Infect. Dis. 2010, 201, 1933–1940. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, P.F.; Dos Santos, M.A.; De Souza, W. Fine structure and cytochemistry of the interaction between Leishmania mexicana amazonensis and rat neutrophils and eosinophils. J. Submicrosc. Cytol. 1987, 19, 387–395. [Google Scholar]
- Oliveira, S.H.P.; Fonseca, S.G.; Romao, P.R.T.; Figueiredo, F.; Ferreira, S.H.; Cunha, F.Q. Microbicidal activity of eosinophils is associated with activation of the arginine-NO pathway. Parasite Immunol. 1998, 20, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Stenger, S.; Röllinghoff, M.; Solbach, W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-γ to activate murine macrophages for killing of Leishmania major amastigotes. Eur. J. Immunol. 1991, 21, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hamaguchi-Tsuru, E.; Morimoto, N.; Nishio, Y.; Yagyu, K.; Konishi, Y.; Tominaga, M.; Miyazaki, J.; Furuya, M.; Tominaga, A. IL-5-Induced Eosinophils Suppress the Growth of Leishmania amazonensis In Vivo and Kill Promastigotes In Vitro in Response to Either IL-4 or IFN-gamma. DNA Cell Biol. 2004, 23, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Stenger, S.; Donhauser, N.; Thüring, H.; Röllinghoff, M.; Bogdan, C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 1996, 183, 1501–1514. [Google Scholar] [CrossRef] [Green Version]
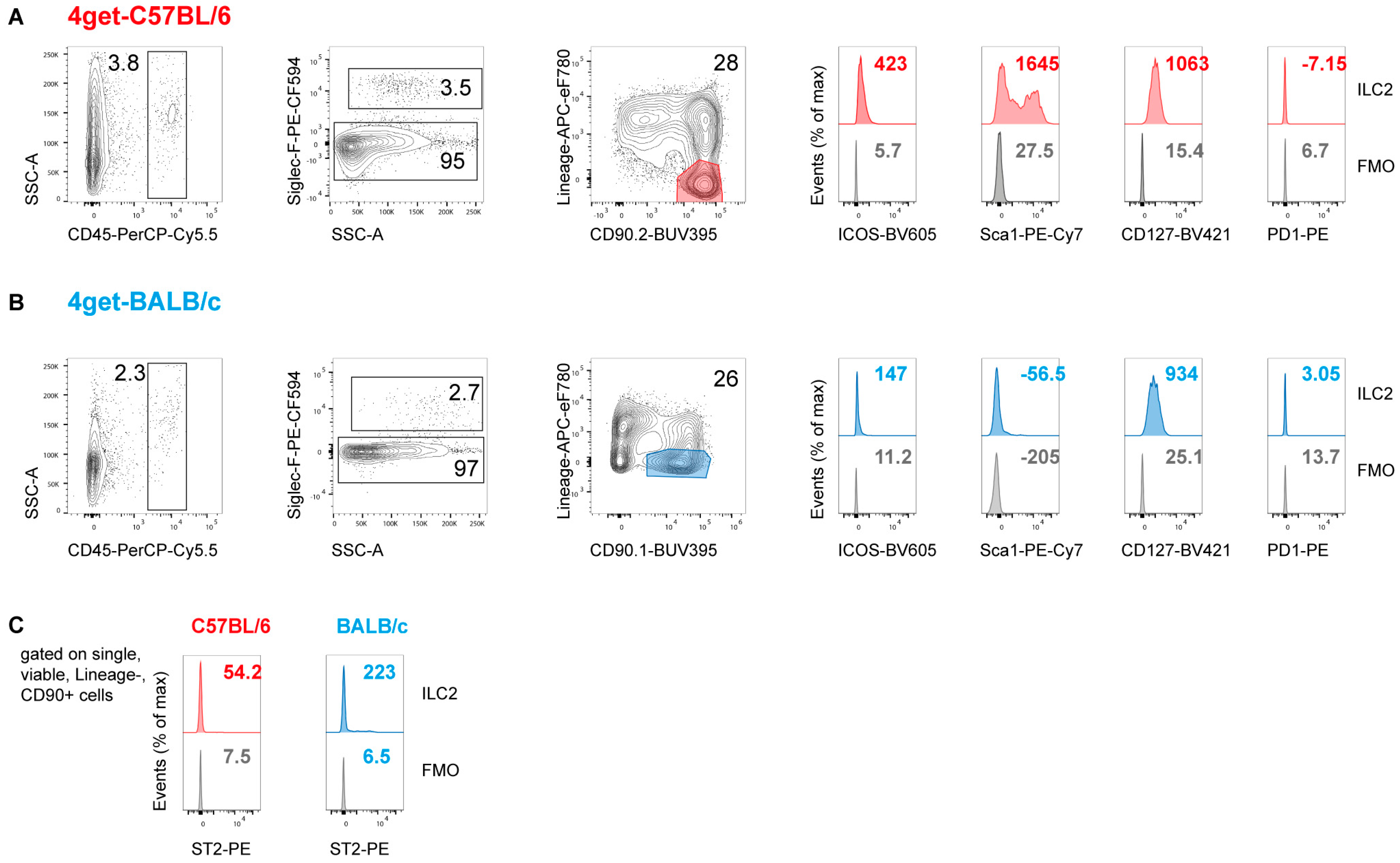

| Antibody | Source | Order No. | Batch, Clone | Fluorochrome Conjugation | Dilution |
|---|---|---|---|---|---|
| Rat anti-mouse CD2 | BioLegend | 100104 | RM2-5 | Biotin | 1:100 |
| Hamster anti-mouse CD3 | BioLegend | 100304 | 145-2C11 | Biotin | 1:100 |
| Rat anti-mouse CD5 | BioLegend | 100604 | 53–7.3 | Biotin | 1:100 |
| Rat anti-mouse CD11b | BioLegend | 101204 | M1/70 | Biotin | 1:100 |
| Hamster anti-mouse CD11c | BD | 553800 | HL3 | Biotin | 1:100 |
| Rat anti-mouse CD19 | BioLegend | 115504 | 6D5 | Biotin | 1:100 |
| Rat anti-mouse CD45 | BioLegend | 103132 | 30-F11 | PerCP-Cy5.5 | 1:100 |
| Hamster ant-mouse CD49b | BD | 740250 | HMα2 | BUV395 | 1:100 |
| Mouse anti-mouse CD90.1 | BD | 740261 | OX-7 | BUV395 | 1:300 |
| Rat anti-mouse CD90.2 | BD | 565257 | 53–2.1 | BUV395 | 1:300 |
| Rat anti-mouse CD117 (ckit) | BioLegend | 105812 | 2B8 | APC | 1:100 |
| Rat anti-mouse CD127 | BioLegend | 135027 | A7R34 | BV421 | 1:100 |
| Rat anti-mouse CD170 (SiglecF) | BioLegend | 61-1702-82 | 1RNM44N | PE-Dazzle594 | 1:200 |
| Rat anti-mouse CD200R3 | BioLegend | 142206 | Ba13 | PE | 1:100 |
| Hamster anti-mouse CD278 (ICOS) | BioLegend | 313538 | C398.4A | BV605 | 1:100 |
| Rat anti-mouse CD279 (PD-1) | BioLegend | 135205 | 29F.1A12 | PE | 1:100 |
| Rat anti-mouse CD335 (Nkp46) | BioLegend | 137616 | 29A1.4 | Biotin | 1:100 |
| Rat anti-mouse F4/80 | BioLegend | 123114 | BM8 | PE-Cy7 | 1:100 |
| Hamster anti-mouse FcεR1α | BioLegend | 134304 | MAR-1 | Biotin | 1:100 |
| Rat anti-mouse/human GATA3 | ebiosciences | 12-9966-42 | TWAJ | PE | 5 µL/test |
| Rat anti-mouse Ly6A/E (Sca-1) | BD | 561021 | D7 | PE-Cy7 | 1:100 |
| Rat anti-mouse Ly6G | BioLegend | 127604 | 1A8 | Biotin | 1:100 |
| Rat anti-mouse ST2 (IL-33R) | MD Bioproducts | 101001PE | DJ8 | PE | 1:100 |
| Rat anti-mouse Ter-119 | BD | 553672 | Ly-76 | Biotin | 1:100 |
| Streptavidin | ebiosciences | 47-4317 | N/A | APC-eFluor™ 780 | 1:100 |
| Gene | Assay ID |
|---|---|
| Hprt | Mm00446968_m1 |
| Ifng | Mm00801778_m1 |
| Il4 | Mm00445259_m1 |
| Il5 | Mm00439646_m1 |
| Il10 | Mm01288386_m1 |
| Il13 | Mm00434204_m1 |
| Il18 | Mm00434225_m1 |
| Il25 | Mm00499822_m1 |
| Il27 | Mm00461164_m1 |
| Il33 | Mm00505403_m1 |
| TSLP | Mm01157588_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasse, C.; Barinberg, D.; Obermeyer, S.; Debus, A.; Schleicher, U.; Bogdan, C. Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection. Pathogens 2022, 11, 828. https://doi.org/10.3390/pathogens11080828
Sasse C, Barinberg D, Obermeyer S, Debus A, Schleicher U, Bogdan C. Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection. Pathogens. 2022; 11(8):828. https://doi.org/10.3390/pathogens11080828
Chicago/Turabian StyleSasse, Carolin, David Barinberg, Stephanie Obermeyer, Andrea Debus, Ulrike Schleicher, and Christian Bogdan. 2022. "Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection" Pathogens 11, no. 8: 828. https://doi.org/10.3390/pathogens11080828
APA StyleSasse, C., Barinberg, D., Obermeyer, S., Debus, A., Schleicher, U., & Bogdan, C. (2022). Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection. Pathogens, 11(8), 828. https://doi.org/10.3390/pathogens11080828






