Productive Replication of HIV-1 but Not SIVmac in Small Ruminant Cells
Abstract
:1. Introduction
2. Results
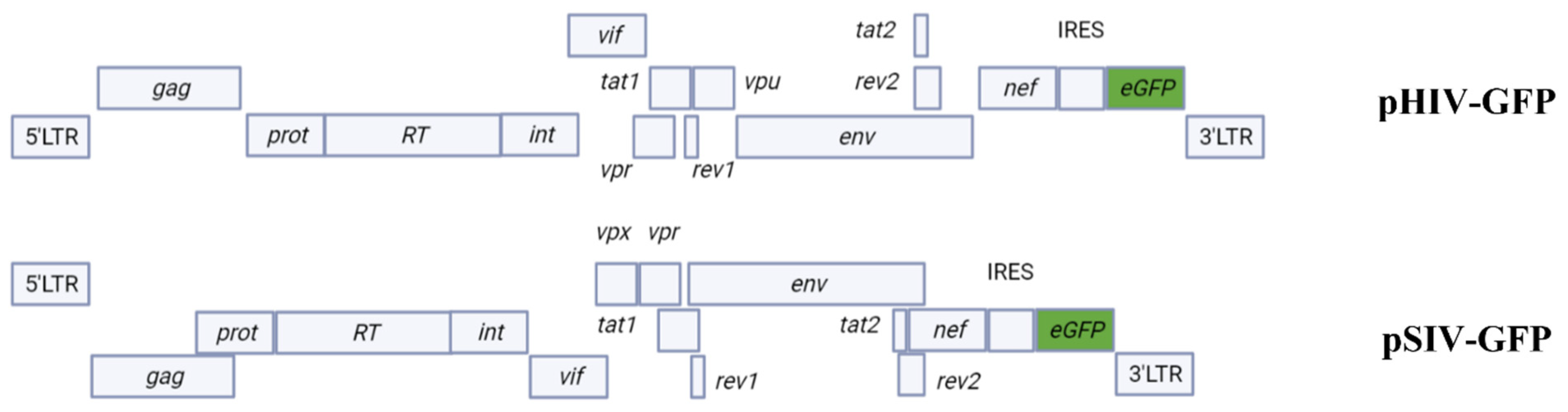
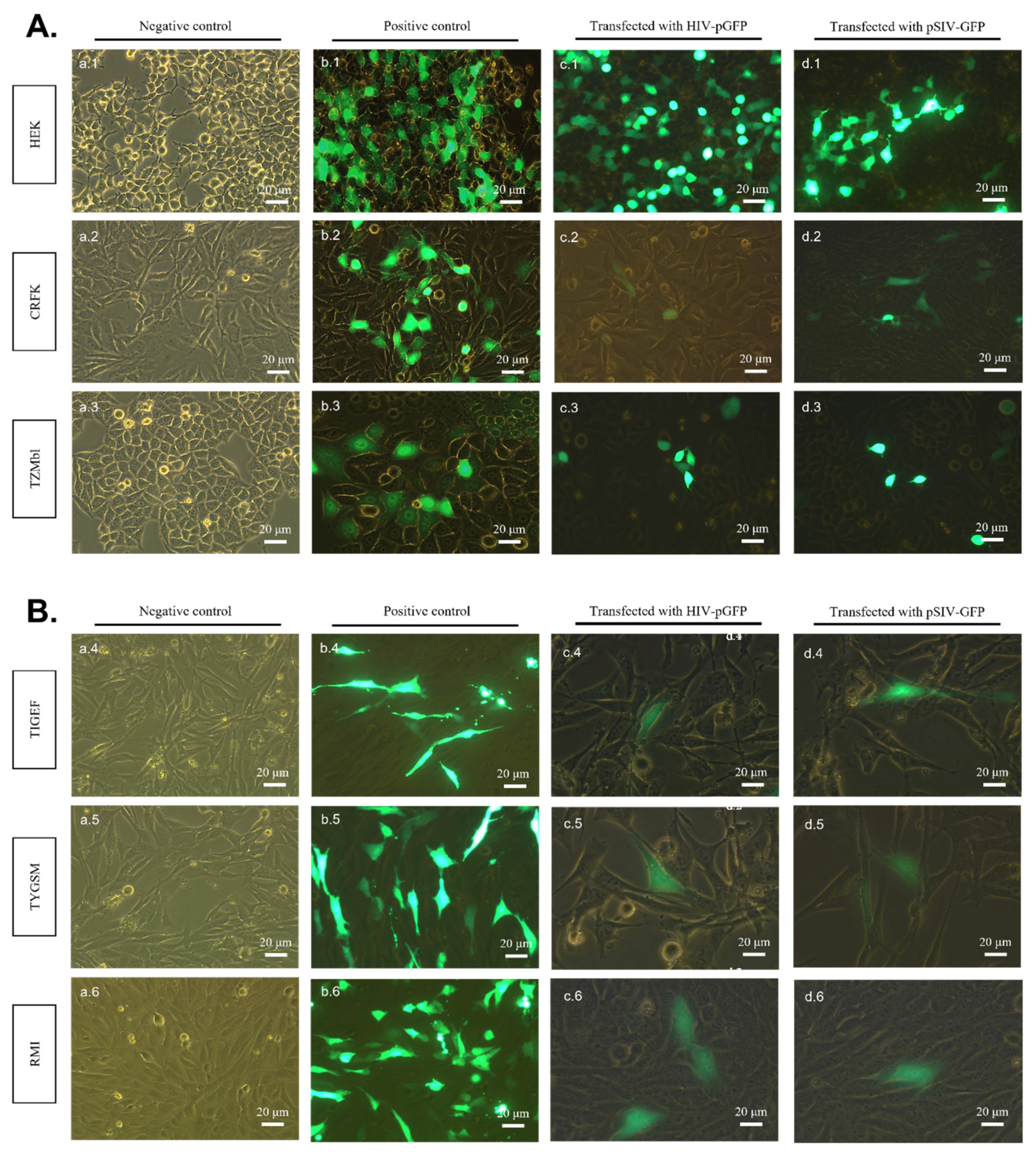
2.1. pSIV-GFP and pHIV-GFP DNA Plasmids Successfully Express Viral Genomes in All Transfected SR Cell Lines
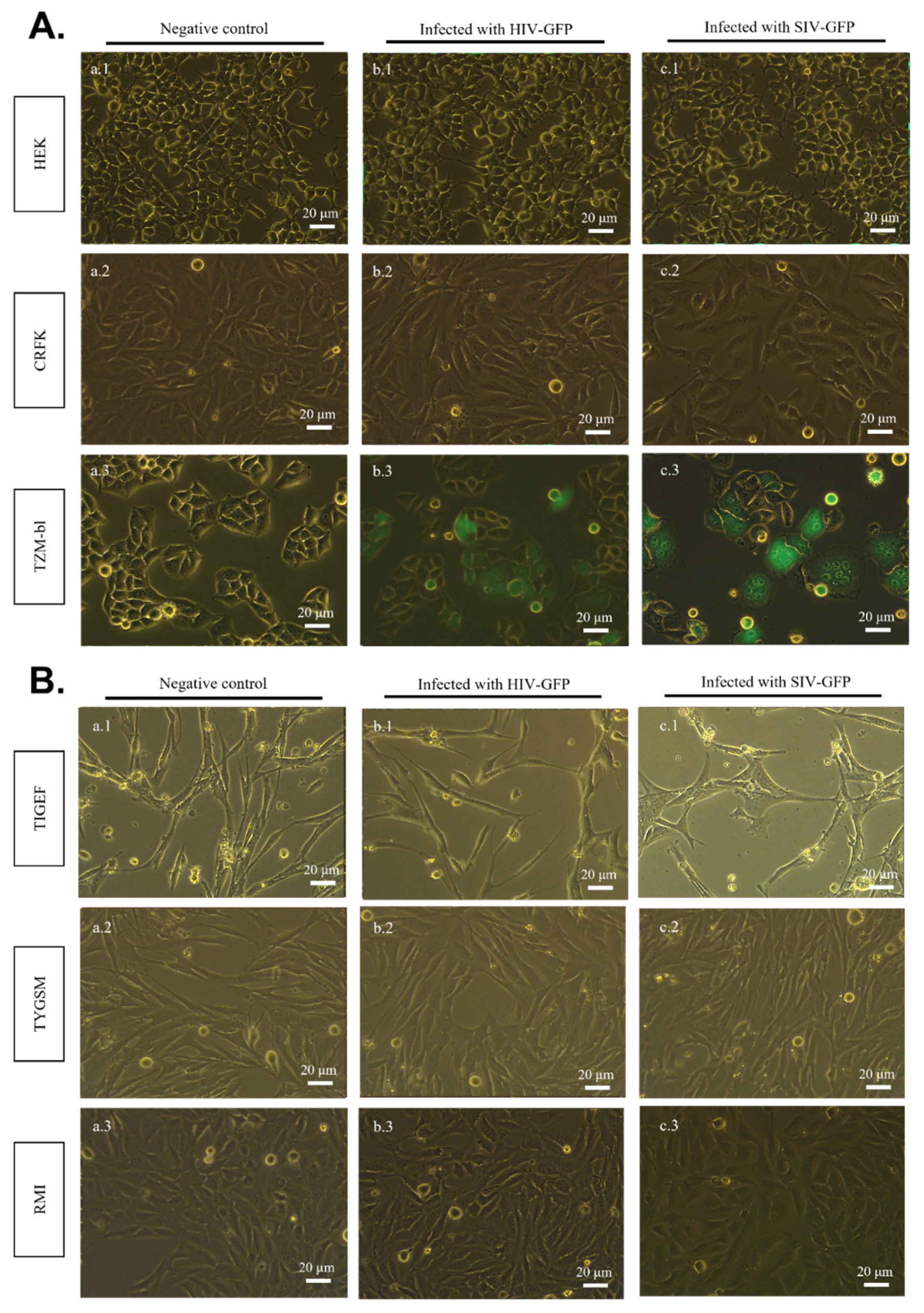
2.2. SIV-GFP and HIV-GFP Viruses Cannot Directly Infect SR Cell Lines
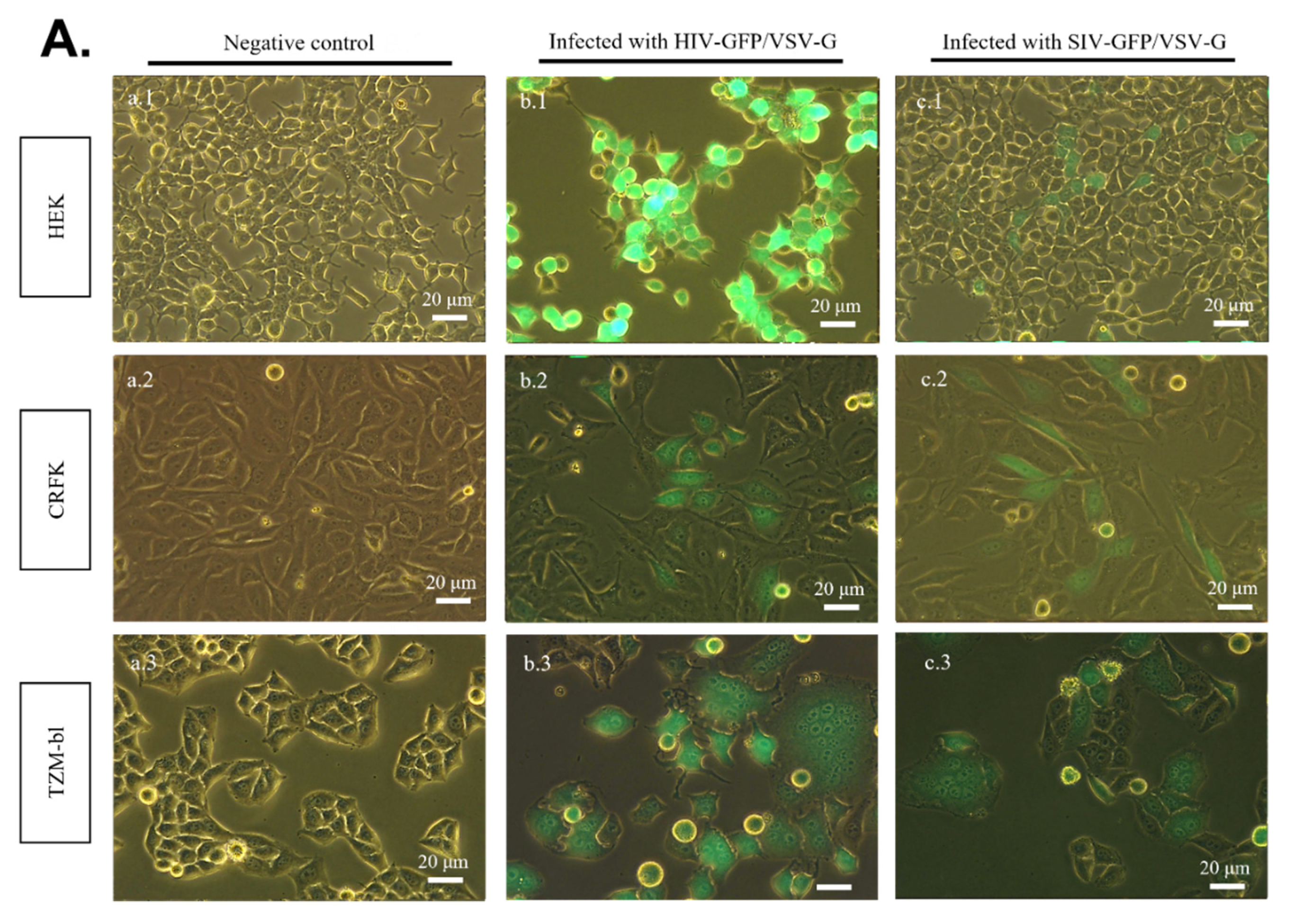
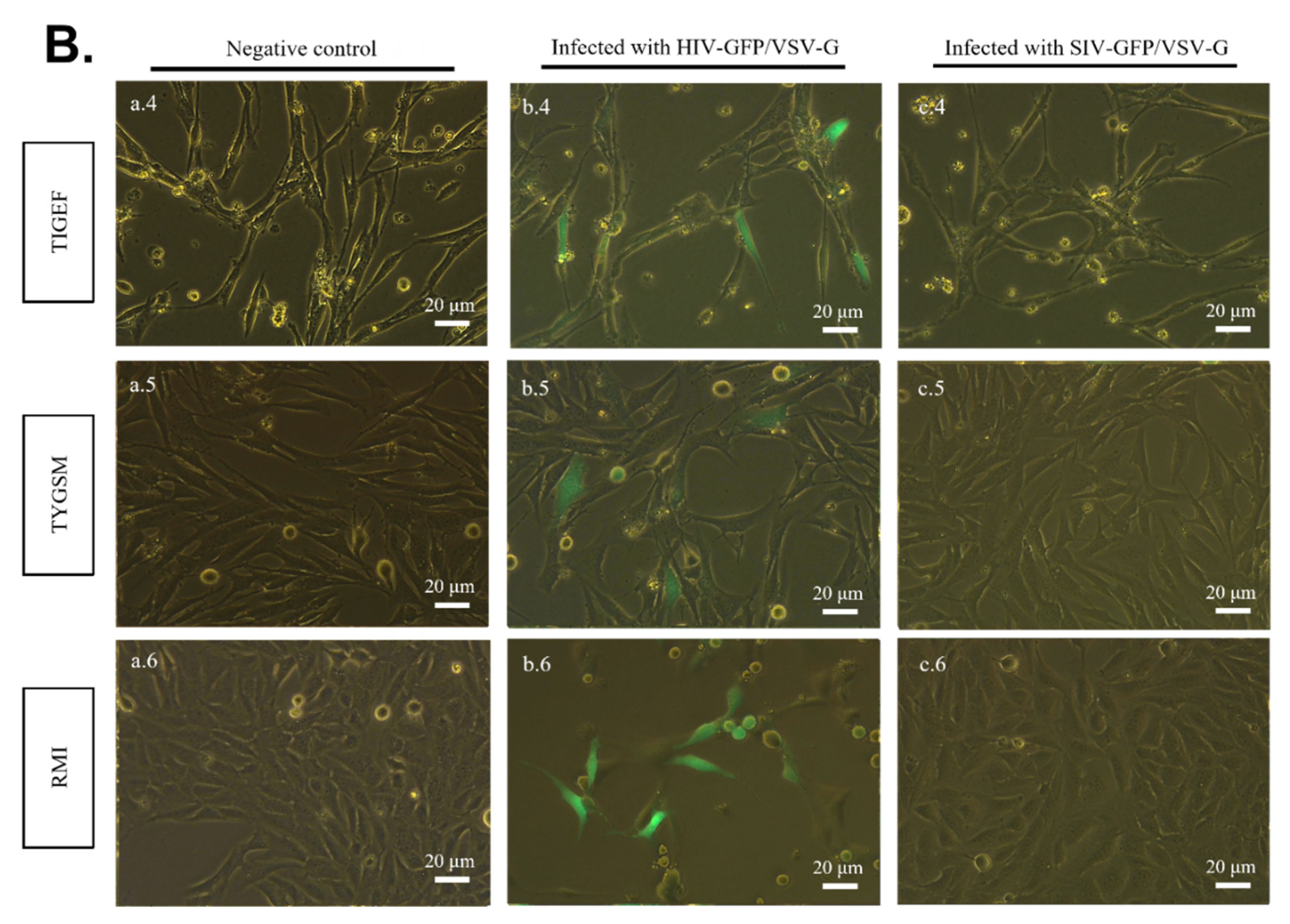
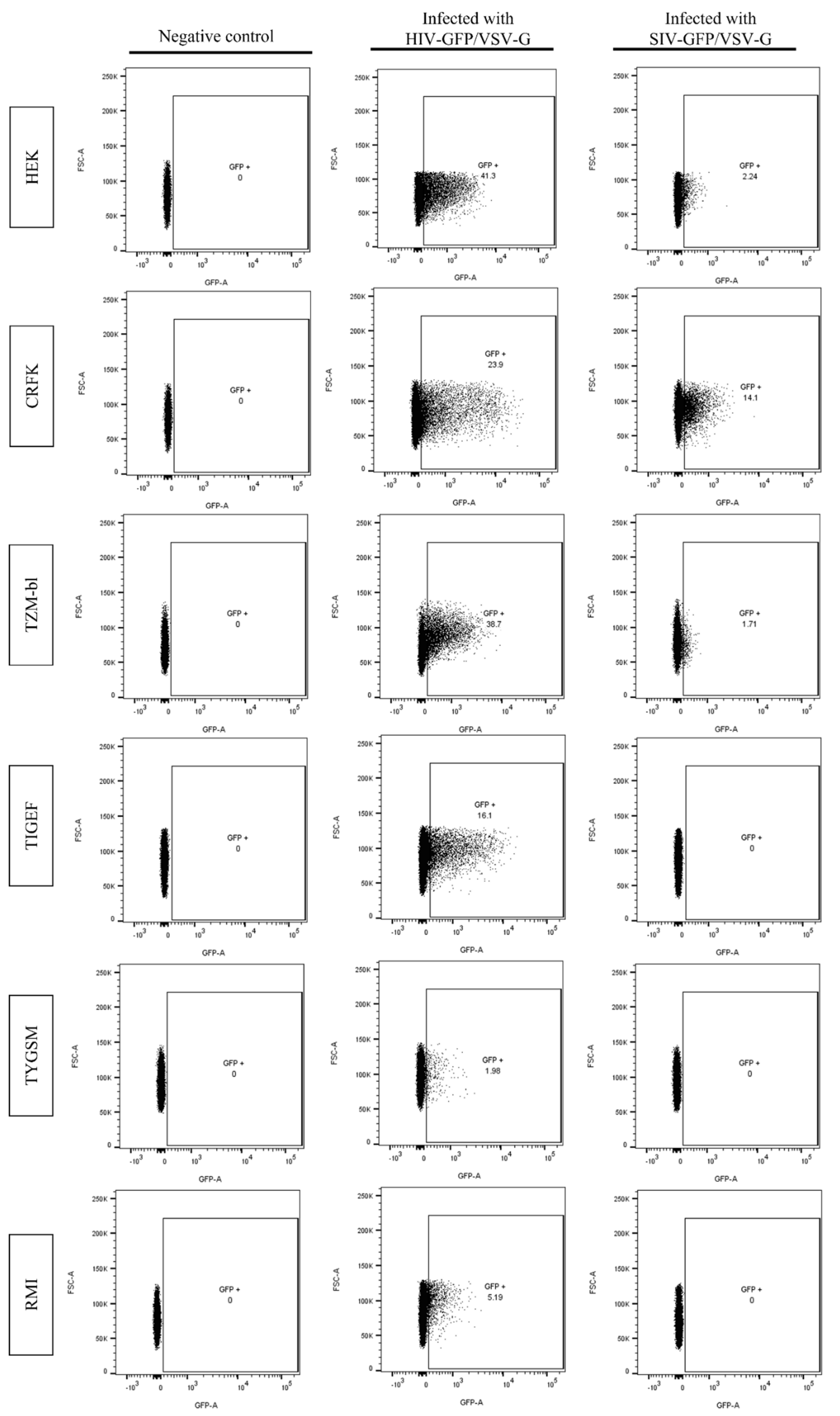
2.3. SR Cells Are Permissive to HIV-GFP, but Not SIV-GFP, Pseudotyped with VSV-G
3. Discussion
4. Material and Methods
4.1. Plasmids
4.2. Cell Culture
4.3. Cell Transfection
4.4. Production of Viral Stocks
4.5. Infection of Cell Lines
4.6. Flow Cytometry Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.K.; Hitchens, P.; Evans, T.S.; Goldstein, T.; Thomas, K.; Clements, A.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; Karesh, W.; et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 2015, 5, 14830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, P.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, B.H.; Shaw, G.M.; De Cock, K.M.; Sharp, P.M. AIDS as a Zoonosis: Scientific and Public Health Implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Bailes, E.; Robertson, D.L.; Chen, Y.; Rodenburg, C.M.; Michael, S.F.; Cummins, L.B.; Arthur, L.O.; Peeters, M.; Shaw, G.M.; et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 1999, 397, 436–441. [Google Scholar] [CrossRef]
- Hirsch, V.M.; Olmsted, R.A.; Murphey-Corb, M.; Purcell, R.H.; Johnson, P.R. An African primate lentivirus (SIVsmclosely related to HIV-2. Nature 1989, 339, 389–392. [Google Scholar] [CrossRef]
- Bibollet-Ruche, F.; Galat-Luong, A.; Cuny, G.; Sarni-Manchado, P.; Galat, G.; Durand, J.P.; Pourrut, X.; Veas, F. Simian Im-munodeficiency Virus Infection in a Patas Monkey (Erythrocebus Patas): Evidence for Cross-Species Transmission from African Green Monkeys (Cercopithecus Aethiops Sabaeus) in the Wild. J. Gen. Virol. 1996, 77, 773–781. [Google Scholar] [CrossRef]
- Bailes, E.; Gao, F.; Bibollet-Ruche, F.; Courgnaud, V.; Peeters, M.; Marx, P.A.; Hahn, B.H.; Sharp, P.M. Hybrid Origin of SIV in Chimpanzees. Science 2003, 300, 1713. [Google Scholar] [CrossRef] [Green Version]
- Caroline, L.; Minardi, C.J.; Jean-Francois, M. SRLVs: A Genetic Continuum of Lentiviral Species in Sheep and Goats with Cumulative Evidence of Cross Species Transmission. Curr. HIV Res. 2010, 8, 94–100. [Google Scholar] [CrossRef]
- Karr, B.M.; Chebloune, Y.; Leung, K.; Narayan, O. Genetic Characterization of Two Phenotypically Distinct North American Ovine Lentiviruses and Their Possible Origin from Caprine Arthritis-Encephalitis Virus. Virology 1996, 225, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rolland, M.; Mooney, J.; Valas, S.; Perrin, G.; Mamoun, R.Z. Characterisation of an Irish caprine lentivirus strain—SRLV phylogeny revisited. Virus Res. 2002, 85, 29–39. [Google Scholar] [CrossRef]
- Olech, M.; Osiński, Z.; Kuźmak, J. Seroprevalence of small ruminant lentivirus (SRLV) infection in wild cervids in Poland. Prev. Veter. Med. 2020, 176, 104905. [Google Scholar] [CrossRef] [PubMed]
- Morin, T.; Guiguen, F.; Bouzar, B.A.; Villet, S.; Greenland, T.; Grezel, D.; Gounel, F.; Gallay, K.; Garnier, C.; Durand, J.; et al. Clearance of a Productive Lentivirus Infection in Calves Experimentally Inoculated with Caprine Arthritis-Encephalitis Virus. J. Virol. 2003, 77, 6430–6437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mselli-Lakhal, L.; Guiguen, F.; Greenland, T.; Mornex, J.-F.; Chebloune, Y. In vitro cross-species infections using a caprine arthritis encephalitis lentivirus carrying the GFP marker gene. J. Virol. Methods 2007, 143, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, F.; Mselli-Lakhal, L.; Durand, J.; Du, J.; Favier, C.; Fornazero, C.; Grezel, D.; Balleydier, S.; Hausmann, E.; Chebloune, Y. Experimental infection of Mouflon-domestic sheep hybrids with caprine arthritis-encephalitis virus. Am. J. Veter. Res. 2000, 61, 456–461. [Google Scholar] [CrossRef]
- Shaw, G.M.; Hunter, E. HIV Transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef]
- Lakhal, L.M.; Guiguen, F.; Fornazero, C.; Du, J.; Favier, C.; Durand, J.; Grézel, D.; Balleydier, S.; Mornex, J.F.; Chebloune, Y. Goat Milk Epithelial Cells Are Highly Permissive to CAEV Infection in Vitro. Virology 1999, 259, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Reeves, J.D.; Hibbitts, S.; Simmons, G.; McKnight, A.; Azevedo-Pereira, J.M.; Moniz-Pereira, J.; Clapham, P.R. Primary Human Immunodeficiency Virus Type 2 (HIV-2) Isolates Infect CD4-Negative Cells via CCR5 and CXCR4: Comparison with HIV-1 and Simian Immunodeficiency Virus and Relevance to Cell Tropism In Vivo. J. Virol. 1999, 73, 7795–7804. [Google Scholar] [CrossRef] [Green Version]
- Etienne, L.; Bibollet-Ruche, F.; Sudmant, P.; Wu, L.I.; Hahn, B.; Emerman, M. The Role of the Antiviral APOBEC3 Gene Family in Protecting Chimpanzees against Lentiviruses from Monkeys. PLoS Pathog. 2015, 11, e1005149. [Google Scholar] [CrossRef]
- Nakano, Y.; Aso, H.; Soper, A.; Yamada, E.; Moriwaki, M.; Juarez-Fernandez, G.; Koyanagi, Y.; Sato, K. A conflict of interest: The evolutionary arms race between mammalian APOBEC3 and lentiviral Vif. Retrovirology 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Mariani, R.; Chen, D.; Schröfelbauer, B.; Navarro, F.; König, R.; Bollman, B.; Münk, C.; Nymark-McMahon, H.; Landau, N.R. Species-Specific Exclusion of APOBEC3G from HIV-1 Virions by Vif. Cell 2003, 114, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Bogerd, H.P.; Doehle, B.P.; Wiegand, H.L.; Cullen, B.R. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. USA 2004, 101, 3770–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, S.M.; Emerman, M. Controlling lentiviruses: Single amino acid changes can determine specificity. Proc. Natl. Acad. Sci. USA 2004, 101, 3725–3726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Rapid evolution of primate antiviral enzyme APOBEC3G. Hum. Mol. Genet. 2004, 13, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Hatziioannou, T.; Perez-Caballero, D.; Yang, A.; Cowan, S.; Bieniasz, P.D. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc. Natl. Acad. Sci. USA 2004, 101, 10774–10779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willey, S.J.; Reeves, J.D.; Hudson, R.; Miyake, K.; Dejucq, N.; Schols, D.; De Clercq, E.; Bell, J.; McKnight, A.; Clapham, P.R. Identification of a Subset of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus Strains Able To Exploit an Alternative Coreceptor on Untransformed Human Brain and Lymphoid Cells. J. Virol. 2003, 77, 6138–6152. [Google Scholar] [CrossRef] [Green Version]
- Thippeshappa, R.; Ruan, H.; Kimata, J.T. Breaking Barriers to an AIDS Model with Macaque-Tropic HIV-1 Derivatives. Biology 2012, 1, 134–164. [Google Scholar] [CrossRef]
- Van Damme, N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.C.; Stephens, E.B.; Guatelli, J. The Interferon-Induced Protein BST-2 Restricts HIV-1 Release and Is Downregulated from the Cell Surface by the Viral Vpu Protein. Cell Host Microbe 2008, 3, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Serra-Moreno, R.; Neidermyer, M., Jr.; Rahmberg, A.; Mackey, J.; Ben Fofana, I.; Johnson, W.E.; Westmoreland, S.; Evans, D.T. Species-Specific Activity of SIV Nef and HIV-1 Vpu in Overcoming Restriction by Tetherin/BST2. PLoS Pathog. 2009, 5, e1000429. [Google Scholar] [CrossRef] [Green Version]
- le Tortorec, A.; Neil, S.J.D. Antagonism to and Intracellular Sequestration of Human Tetherin by the Human Immunodefi-ciency Virus Type 2 Envelope Glycoprotein. J. Virol. 2009, 83, 11966–11978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Pan, Q.; Rong, L.; Liu, S.-L.; Liang, C. The IFITM Proteins Inhibit HIV-1 Infection. J. Virol. 2011, 85, 2126–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Pan, Q.; Liu, S.-L.; Liang, C. HIV-1 mutates to evade IFITM1 restriction. Virology 2014, 454–455, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.-Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-Inducible Cholesterol-25-Hydroxylase Broadly Inhibits Viral Entry by Production of 25-Hydroxycholesterol. Immunity 2012, 38, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Izumida, M.; Yashima, Y.; Yoshii-Kamiyama, H.; Tanaka, Y.; Yasui, K.; Hayashi, H.; Matsuyama, T. Gamma-interferon-inducible, lysosome/endosome-localized thiolreductase, GILT, has anti-retroviral activity and its expression is counteracted by HIV-1. Oncotarget 2016, 7, 71255–71273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usami, Y.; Wu, Y.; Göttlinger, H.G. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature 2015, 526, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, D.C.; Ennis-Adeniran, V.; Hedden, J.J.; Groom, H.C.T.; Rice, G.I.; Christodoulou, E.; Walker, P.A.; Kelly, G.; Haire, L.F.; Yap, M.W.; et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011, 480, 379–382. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Ségéral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]
- Baldauf, H.-M.; Pan, X.; Erikson, E.; Schmidt, S.; Daddacha, W.; Burggraf, M.; Schenkova, K.; Ambiel, I.; Wabnitz, G.H.; Gramberg, T.; et al. SAMHD1 restricts HIV-1 infection in resting CD4+ T cells. Nat. Med. 2012, 18, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Mselli-Lakhal, L.; Favier, C.; Leung, K.; Guiguen, F.; Grezel, D.; Miossec, P.; Mornex, J.-F.O.; Narayan, O.; Querat, G.; Chebloune, Y. Lack of Functional Receptors Is the Only Barrier That Prevents Caprine Arthritis-Encephalitis Virus from In-fecting Human Cells. J. Virol. 2000, 74, 8343–8348. [Google Scholar] [CrossRef] [Green Version]
- Chebloune, Y.; Sheffer, D.; Karr, B.M.; Stephens, E.; Narayan, O. Restrictive Type of Replication of Ovine/Caprine Lentiviruses in Ovine Fibroblast Cell Cultures. Virology 1996, 222, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnier, C.; Takeuchi, Y.; Towers, G. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 2002, 99, 11920–11925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, D.B.; Huebner, K.; Williams, W.V.; Greene, M.I. Human Genes Other than CD4 Facilitate HIV-1 Infection of Murine Cells. Pathobiology 1991, 59, 361–371. [Google Scholar] [CrossRef]
- Browning, J.; Horner, J.W.; Pettoello-Mantovani, M.; Raker, C.; Yurasov, S.; DePinho, R.A.; Goldstein, H. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 1997, 94, 14637–14641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, M.E.; Wei, P.; KewalRamani, V.N.; Mayall, T.P.; Herrmann, C.H.; Rice, A.P.; Littman, D.R.; Jones, K.A. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998, 12, 3512–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krisko, J.F.; Martinez-Torres, F.; Foster, J.L.; Garcia, J.V. HIV Restriction by APOBEC3 in Humanized Mice. PLoS Pathog. 2013, 9, e1003242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munch, J.; Rajan, D.; Schindler, M.; Specht, A.; Rucker, E.; Novembre, F.J.; Nerrienet, E.; Muller-Trutwin, M.C.; Peeters, M.; Hahn, B.H.; et al. Nef-Mediated Enhancement of Virion Infectivity and Stimulation of Viral Replication Are Fundamental Properties of Primate Lentiviruses. J. Virol. 2007, 81, 13852–13864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 Cell Surface Concentrations on Infections by Macrophagetropic Isolates of Human Immunodeficiency Virus Type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [Green Version]
- Seridi, N.; Hamidouche, M.; Belmessabih, N.; El Kennani, S.; Gagnon, J.; Martinez, G.; Coutton, C.; Marchal, T.; Chebloune, Y. Immortalization of primary sheep embryo kidney cells. Cell Dev. Biol. Anim. 2021, 57, 76–85. [Google Scholar] [CrossRef]
- Teixeira, M.F.D.S.; Lambert, V.; Mselli-Lakahl, L.; Chettab, A.; Chebloune, Y.; Mornex, J.F. Immortalization of caprine fibroblasts permissive for replication of small ruminant lentiviruses. Am. J. Vet. Res. 1997, 58, 579–584. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chergui, H.E.; Idres, T.; Chaudesaigues, C.; Noueihed, D.; Gagnon, J.; Chebloune, Y. Productive Replication of HIV-1 but Not SIVmac in Small Ruminant Cells. Pathogens 2022, 11, 799. https://doi.org/10.3390/pathogens11070799
Chergui HE, Idres T, Chaudesaigues C, Noueihed D, Gagnon J, Chebloune Y. Productive Replication of HIV-1 but Not SIVmac in Small Ruminant Cells. Pathogens. 2022; 11(7):799. https://doi.org/10.3390/pathogens11070799
Chicago/Turabian StyleChergui, Hibet Errahmane, Takfarinas Idres, Chloé Chaudesaigues, Diana Noueihed, Jean Gagnon, and Yahia Chebloune. 2022. "Productive Replication of HIV-1 but Not SIVmac in Small Ruminant Cells" Pathogens 11, no. 7: 799. https://doi.org/10.3390/pathogens11070799
APA StyleChergui, H. E., Idres, T., Chaudesaigues, C., Noueihed, D., Gagnon, J., & Chebloune, Y. (2022). Productive Replication of HIV-1 but Not SIVmac in Small Ruminant Cells. Pathogens, 11(7), 799. https://doi.org/10.3390/pathogens11070799








