Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies
Abstract
:1. Introduction
2. Intracranial Hypertension in the Setting of CNS Cryptococcosis
3. Significance of Increased ICP in CM
4. Lessons from the Preclinical Studies
5. Pharmacological Considerations in the Treatment of CM
6. Clinical Studies on the Pharmacologic Management of Elevated ICP in CM and Non-CM Patients
7. Aquaporins (AQPs) as the Novel Therapeutic Targets for Treating Increased ICP
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, K.M.; Montrief, T.; Ramzy, M.; Koyfman, A.; Long, B. Cryptococcal meningitis: A review for emergency clinicians. Intern. Emerg. Med. 2021, 16, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life Cycle ofCryptococcus neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Brizendine, K.D.; Baddley, J.W.; Pappas, P.G. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS ONE 2013, 8, e60431. [Google Scholar] [CrossRef]
- Henao-Martinez, A.F.; Chastain, D.B.; Franco-Paredes, C. Treatment of cryptococcosis in non-HIV immunocompromised patients. Curr. Opin. Infect. Dis. 2018, 31, 278–285. [Google Scholar] [CrossRef]
- Alanio, A. Dormancy in Cryptococcus neoformans: 60 years of accumulating evidence. J. Clin. Investig. 2020, 130, 3353–3360. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.B.; Perlin, D.S.; Xue, C. Molecular mechanisms of cryptococcal meningitis. Virulence 2012, 3, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyaseela, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 2004, 72, 4985–4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio 2017, 8, e02183-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrell, T.C.; Juillard, P.G.; Djordjevic, J.T.; Kaufman-Francis, K.; Dietmann, A.; Milonig, A.; Combes, V.; Grau, G.E. Cryptococcal transmigration across a model brain blood-barrier: Evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes Infect. 2016, 18, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Wirth, F.; de Azevedo, M.I.; Pilla, C.; Aquino, V.R.; Neto, G.W.; Goldani, L.Z. Relationship between intracranial pressure and antifungal agents levels in the CSF of patients with cryptococcal meningitis. Med. Mycol. 2018, 56, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, J.N.; Harrison, T.S. HIV-associated cryptococcal meningitis. AIDS 2007, 21, 2119–2129. [Google Scholar] [CrossRef]
- Bicanic, T.; Brouwer, A.E.; Meintjes, G.; Rebe, K.; Limmathurotsakul, D.; Chierakul, W.; Teparrakkul, P.; Loyse, A.; White, N.J.; Wood, R.; et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009, 23, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Graybill, J.R.; Sobel, J.; Saag, M.; Van Der Horst, C.; Powderly, W.; Cloud, G.; Riser, L.; Hamill, R.; Dismukes, W.; NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Diagnosis and Management of Increased Intracranial Pressure in Patients with AIDS and Cryptococcal Meningitis. Clin. Infect. Dis. 2000, 30, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Y.; Hung, C.C.; Chang, S.C. Management of cryptococcal meningitis with extremely high intracranial pressure in HIV-infected patients. Clin. Infect. Dis. 2004, 38, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Womack, T.; Bohlmeyer, T.; Sellers, B.; Hays, A.; Patel, K.; Lizarazo, J.; Lockhart, S.R.; Siddiqui, W.; Marr, K.A. Management of Cryptococcus gattii meningoencephalitis. Lancet Infect. Dis. 2015, 15, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Warkentien, T.; Crum-Cianflone, N.F. An update on Cryptococcus among HIV-infected patients. Int. J. STD AIDS 2010, 21, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molloy, S.F.; Ross, B.; Kanyama, C.; Mfinanga, S.; Lesikari, S.; Heyderman, R.S.; Kalata, N.; Ellis, J.; Kouanfack, C.; Chanda, D.; et al. Fungal Burden and Raised Intracranial Pressure Are Independently Associated with Visual Loss in Human Immunodeficiency Virus-Associated Cryptococcal Meningitis. Open Forum Infect. Dis. 2021, 8, ofab066. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Franco-Paredes, C.; Chastain, D.B.; Rodriguez-Morales, A.J.; Marcos, L.A. Cryptococcal meningoencephalitis in HIV/AIDS: When to start antiretroviral therapy? Ann. Clin. Microbiol. Antimicrob. 2017, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, P.G.; Chetchotisakd, P.; Larsen, R.A.; Manosuthi, W.; Morris, M.I.; Anekthananon, T.; Sungkanuparph, S.; Supparatpinyo, K.; Nolen, T.L.; Zimmer, L.O.; et al. A Phase II Randomized Trial of Amphotericin B Alone or Combined with Fluconazole in the Treatment of HIV-Associated Cryptococcal Meningitis. Clin. Infect. Dis. 2009, 48, 1775–1783. [Google Scholar] [CrossRef] [Green Version]
- Loyse, A.; Wainwright, H.; Jarvis, J.N.; Bicanic, T.; Rebe, K.; Meintjes, G.; Harrison, T.S. Histopathology of the arachnoid granulations and brain in HIV-associated cryptococcal meningitis: Correlation with cerebrospinal fluid pressure. AIDS 2010, 24, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Robertson, E.J.; Najjuka, G.; Rolfes, M.A.; Akampurira, A.; Jain, N.; Anantharanjit, J.; Von Hohenberg, M.; Tassieri, M.; Carlsson, A.; Meya, D.B.; et al. Cryptococcus neoformans Ex Vivo Capsule Size is Associated with Intracranial Pressure and Host Immune Response in HIV-associated Cryptococcal Meningitis. J. Infect. Dis. 2014, 209, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.T.; Hong, J.; Lee, D.G.; Lee, M.; Cha, S.; Lim, Y.G.; Jung, K.W.; Hwangbo, A.; Lee, Y.; Yu, S.J.; et al. Fungal kinases and transcription factors regulating brain infection in Cryptococcus neoformans. Nat. Commun. 2020, 11, 1521. [Google Scholar] [CrossRef]
- Moodley, A.; Rae, W.; Bhigjee, A. Visual loss in HIV-associated cryptococcal meningitis: A case series and review of the mechanisms involved. S. Afr. J. HIV Med. 2015, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Moodley, A.; Rich, P.; Molloy, S.F.; Bicanic, T.; Bishop, L.; Rae, W.I.; Bhigjee, A.I.; Loubser, N.D.; Michowicz, A.J.; et al. Neurological, visual, and MRI brain scan findings in 87 South African patients with HIV-associated cryptococcal meningoencephalitis. J. Infect. 2015, 70, 668–675. [Google Scholar] [CrossRef]
- Johnston, S.R.; Corbett, E.L.; Foster, O.; Ash, S.; Cohen, J. Raised intracranial pressure and visual complications in AIDS patients with cryptococcal meningitis. J. Infect. 1992, 24, 185–189. [Google Scholar] [CrossRef]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care. Neurocritical Care 2014, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.N.; Ranger, A.; Singh, R.N.; Kornecki, A.; Seabrook, J.A.; Fraser, D.D. External ventricular drains in pediatric patients. Pediatr. Crit. Care Med. 2009, 10, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.B.; Dhillon, G.S. Ventriculostomy-related cerebral hemorrhages after endovascular aneurysm treatment. AJNR Am. J. Neuroradiol. 2003, 24, 1528–1531. [Google Scholar] [PubMed]
- Lenfeldt, N.; Koskinen, L.O.; Bergenheim, A.T.; Malm, J.; Eklund, A. CSF pressure assessed by lumbar puncture agrees with intracranial pressure. Neurology 2007, 68, 155–158. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children; World Health Organization: Geneva, Switzerland, 2018; p. 62. [Google Scholar]
- Skipper, C.; Abassi, M.; Boulware, D.R. Diagnosis and Management of Central Nervous System Cryptococcal Infections in HIV-Infected Adults. J. Fungi 2019, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol. Life Sci. 2021, 78, 2429–2457. [Google Scholar] [CrossRef] [PubMed]
- Weiner, W.J.; Shulman, L.M. Emergent and Urgent Neurology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Li, L. Efficacy and Safety of Continuous Micro-Pump Infusion of 3% Hypertonic Saline combined with Furosemide to Control Elevated Intracranial Pressure. Med. Sci. Monit. 2015, 21, 1752–1758. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, S.; Westgate, C.S.J.; Uldall, M.S.; Jensen, R.H. Preclinical update on regulation of intracranial pressure in relation to idiopathic intracranial hypertension. Fluids Barriers CNS 2019, 16, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogh, B.P.; Godman, D.R. Timolol plus acetazolamide: Effect on formation of cerebrospinal fluid in cats and rats. Can. J. Physiol. Pharmacol. 1985, 63, 340–343. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.D.; Reed, D.J. The effect of acetazolamide and furosemide on cerebrospinal fluid production and choroid plexus carbonic anhydrase activity. J. Pharmacol. Exp. Ther. 1974, 189, 194–201. [Google Scholar] [PubMed]
- Uldall, M.; Botfield, H.; Jansen-Olesen, I.; Sinclair, A.; Jensen, R. Acetazolamide lowers intracranial pressure and modulates the cerebrospinal fluid secretion pathway in healthy rats. Neurosci. Lett. 2017, 645, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotton, W.J.; Botfield, H.F.; Westgate, C.S.; Mitchell, J.L.; Yiangou, A.; Uldall, M.S.; Jensen, R.H.; Sinclair, A.J. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 2019, 39, 209–218. [Google Scholar] [CrossRef]
- Thenuwara, K.; Todd, M.M.; Brian, J.E., Jr. Effect of mannitol and furosemide on plasma osmolality and brain water. Anesthesiology 2002, 96, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.B.; Wilkinson, H.A.; Rosenfeld, S.A.; Furuta, T. Intracranial hypertension and cerebrospinal fluid production in dogs: Effects of furosemide. Exp. Neurol. 1986, 94, 66–80. [Google Scholar] [CrossRef]
- Lorenzo, A.V.; Hornig, G.; Zavala, L.M.; Boss, V.; Welch, K. Furosemide lowers intracranial pressure by inhibiting CSF production. Z. Kinderchir. 1986, 41 (Suppl. 1), 10–12. [Google Scholar] [CrossRef]
- Pollay, M.; Fullenwider, C.; Roberts, P.A.; Stevens, F.A. Effect of mannitol and furosemide on blood-brain osmotic gradient and intracranial pressure. J. Neurosurg. 1983, 59, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plangger, C.; Völkl, H. Effect of amiloride and emopamil on intracranial pressure. Acta Neurol. Scand. 2009, 92, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.S.; Narayanan, S.P.; Somanath, P.R. Is amiloride a promising cardiovascular medication to persist in the COVID-19 crisis? Drug Discov. Ther. 2020, 14, 256–258. [Google Scholar] [CrossRef]
- Hakon, J.; Ruscher, K.; Romner, B.; Tomasevic, G. Preservation of the Blood Brain Barrier and Cortical Neuronal Tissue by Liraglutide, a Long Acting Glucagon-Like-1 Analogue, after Experimental Traumatic Brain Injury. PLoS ONE 2015, 10, e0120074. [Google Scholar] [CrossRef] [Green Version]
- Botfield, H.F.; Uldall, M.S.; Westgate, C.S.J.; Mitchell, J.L.; Hagen, S.M.; Gonzalez, A.M.; Hodson, D.J.; Jensen, R.H.; Sinclair, A.J. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci. Transl. Med. 2017, 9, eaan0972. [Google Scholar] [CrossRef] [Green Version]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [Green Version]
- Cheong, J.W.; McCormack, J. Fluconazole resistance in cryptococcal disease: Emerging or intrinsic? Med. Mycol. 2013, 51, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bicanic, T.; Muzoora, C.; Brouwer, A.E.; Meintjes, G.; Longley, N.; Taseera, K.; Rebe, K.; Loyse, A.; Jarvis, J.; Bekker, L.G.; et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: Analysis of a combined cohort of 262 patients. Clin. Infect. Dis. 2009, 49, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Lawrence, D.S.; Meya, D.B.; Kagimu, E.; Kasibante, J.; Mpoza, E.; Rutakingirwa, M.K.; Ssebambulidde, K.; Tugume, L.; Rhein, J.; et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N. Engl. J. Med. 2022, 386, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Day, J.N.; Chau, T.T.H.; Wolbers, M.; Mai, P.P.; Dung, N.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Phong, N.D.; Thai, C.Q.; et al. Combination antifungal therapy for cryptococcal meningitis. N. Engl. J. Med. 2013, 368, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Bozzette, S.A.; Larsen, R.A.; Chiu, J.; Leal, M.A.; Jacobsen, J.; Rothman, P.; Robinson, P.; Gilbert, G.; McCutchan, J.A.; Tilles, J.; et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 1991, 324, 580–584. [Google Scholar] [CrossRef]
- Albright, A.L.; Latchaw, R.E.; Robinson, A.G. Intracranial and systemic effects of hetastarch in experimental cerebral edema. Crit. Care Med. 1984, 12, 496–500. [Google Scholar] [CrossRef]
- Kassamali, R.; Sica, D.A. Acetazolamide: A forgotten diuretic agent. Cardiol. Rev. 2011, 19, 276–278. [Google Scholar] [CrossRef]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef]
- Tanimura, Y.; Hiroaki, Y.; Fujiyoshi, Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J. Struct. Biol. 2009, 166, 16–21. [Google Scholar] [CrossRef]
- Pagan, F.L.; Restrepo, L.; Balish, M.; Patwa, H.S.; Houff, S. A new drug for an old condition? Headache 2002, 42, 695–696. [Google Scholar] [CrossRef]
- Palacio, E.; Rodero, L.; Pascual, J. Topiramate-responsive headache due to idiopathic intracranial hypertension in Behcet syndrome. Headache 2004, 44, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, W.E. Topiramate: A review of preclinical, pharmacokinetic, and clinical data. Clin. Ther. 1997, 19, 1294–1308. [Google Scholar] [CrossRef]
- Sills, G.J.; Leach, J.P.; Kilpatrick, W.S.; Fraser, C.M.; Thompson, G.G.; Brodie, M.J. Concentration-effect studies with topiramate on selected enzymes and intermediates of the GABA shunt. Epilepsia 2000, 41, 30–34. [Google Scholar] [CrossRef]
- Grande, P.O.; Romner, B. Osmotherapy in brain edema: A questionable therapy. J. Neurosurg. Anesth. 2012, 24, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, Y.; Cheng, J.; Cheng, C.; Chi, Y.; Wei, H. The use of mannitol in HIV-infected patients with symptomatic cryptococcal meningitis. Drug Discov. Ther. 2017, 10, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, P.; Tripathi, V.N.; Singh, R.P.; Sachan, D. Role of hypertonic saline and mannitol in the management of raised intracranial pressure in children: A randomized comparative study. J. Pediatr. Neurosci. 2010, 5, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Çelebisoy, N.; Gökçay, F.; Şirin, H.; Akyürekli, Ö. Treatment of idiopathic intracranial hypertension: Topiramate vs acetazolamide, an open-label study. Acta Neurol. Scand. 2007, 116, 322–327. [Google Scholar] [CrossRef]
- Bruce, B.B.; Digre, K.B.; McDermott, M.P.; Schron, E.B.; Wall, M. Quality of life at 6 months in the Idiopathic Intracranial Hypertension Treatment Trial. Neurology 2016, 87, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Wall, M.; McDermott, M.P.; Kieburtz, K.D.; Corbett, J.J.; Feldon, S.E.; Friedman, D.I.; Katz, D.M.; Keltner, J.L.; Schron, E.B.; Kupersmith, M.J. Effect of Acetazolamide on Visual Function in Patients with Idiopathic Intracranial Hypertension and Mild Visual Loss. JAMA 2014, 311, 1641. [Google Scholar] [CrossRef]
- Newton, P.; Thai, L.H.; Tip, N.Q.; Short, J.M.; Chierakul, W.; Rajanuwong, A.; Pitisuttithum, P.; Chasombat, S.; Phonrat, B.; Maek-A-Nantawat, W.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Acetazolamide for the Treatment of Elevated Intracranial Pressure in Cryptococcal Meningitis. Clin. Infect. Dis. 2002, 35, 769–772. [Google Scholar] [CrossRef] [Green Version]
- Wongkhumjan, T.; Srisulait, K.; Kalayanalarp, S. Effects of acetazolamide in children with meningitis with increased intracranial pressure: A retrospective cohort study. Clin. Acad. 2018, 42, 170–180. [Google Scholar]
- Ware, M.L.; Nemani, V.M.; Meeker, M.; Lee, C.; Morabito, D.J.; Manley, G.T. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: A preliminary study. Neurosurgery 2005, 57, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Wolbers, M.; Kibengo, F.M.; Ggayi, A.B.; Kamali, A.; Cuc, N.T.; Binh, T.Q.; Chau, N.V.; Farrar, J.; Merson, L.; et al. Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N. Engl. J. Med. 2016, 374, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.K.; Howman, A.; Wheatley, K.; Burdon, M.A.; Matthews, T.; Jacks, A.S.; Lawden, M.; Sivaguru, A.; Furmston, A.; Howell, S.; et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. J. Neurol. 2011, 258, 874–881. [Google Scholar] [CrossRef]
- Gamba, G.; Friedman, P.A. Thick ascending limb: The Na(+):K (+):2Cl (-) co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflug. Arch. 2009, 458, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Rogawski, M.A.; Loscher, W.; Rho, J.M. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016, 6, a022780. [Google Scholar] [CrossRef]
- Bragadottir, G.; Redfors, B.; Ricksten, S.E. Mannitol increases renal blood flow and maintains filtration fraction and oxygenation in postoperative acute kidney injury: A prospective interventional study. Crit. Care 2012, 16, R159. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Castilla, L.; Gopinath, S.; Robertson, C.S. Management of intracranial hypertension. Neurol. Clin. 2008, 26, 521–541. [Google Scholar] [CrossRef]
- Tenny, S.; Patel, R.; Thorell, W. Mannitol; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kerwin, A.J.; Schinco, M.A.; Tepas, J.J., 3rd; Renfro, W.H.; Vitarbo, E.A.; Muehlberger, M. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: A pilot study. J. Trauma. 2009, 67, 277–282. [Google Scholar] [CrossRef]
- Mason, A.; Malik, A.; Ginglen, J.G. Hypertonic Fluids; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Battison, C.; Andrews, P.J.; Graham, C.; Petty, T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit. Care Med. 2005, 33, 196–202. [Google Scholar] [CrossRef]
- da Silva, J.C.; de Lima, M.F.; Valenca, M.M.; de Azevedo, H.R.F. Hypertonic saline more efficacious than mannitol in lethal intracranial hypertension model. Neurol. Res. 2010, 32, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Marko, N.F. Hypertonic saline, not mannitol, should be considered gold-standard medical therapy for intracranial hypertension. Crit. Care 2012, 16, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, M.C.; McIntyre, P.; Prasad, K.; van de Beek, D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 2015, 2015, CD004405. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.K.; Huang, T.Y.; Wu, A.Y.; Chen, H.H.; Liu, C.P.; Jong, A. How Cryptococcus interacts with the blood-brain barrier. Future Microbiol. 2015, 10, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Zhu, H.M.; Wu, J.H.; Wen, H.; Liu, C.J. Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J. Int. Med. Res. 2014, 42, 85–92. [Google Scholar] [CrossRef]
- Aaron, P.A.; Jamklang, M.; Uhrig, J.P.; Gelli, A. The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor. Cell. Microbiol. 2018, 20, e12811. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, M.A.; Noverr, M.C.; Chen, G.H.; Toews, G.B.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004, 164, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Vu, K.; Garcia, J.A.; Gelli, A. Cryptococcal Meningitis and Anti-virulence Therapeutic Strategies. Front. Microbiol. 2019, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [Green Version]
- Verkman, A.S.; Smith, A.J.; Phuan, P.W.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Assentoft, M.; Larsen, B.R.; MacAulay, N. Regulation and Function of AQP4 in the Central Nervous System. Neurochem. Res. 2015, 40, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Speake, T.; Freeman, L.J.; Brown, P.D. Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim. Biophys. Acta 2003, 1609, 80–86. [Google Scholar] [CrossRef]
- Yao, X.; Derugin, N.; Manley, G.T.; Verkman, A.S. Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci. Lett. 2015, 584, 368–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.N.; Xie, L.L.; Liang, R.; Sun, X.L.; Fan, Y.; Hu, G. AQP4 Knockout Aggravates Ischemia/Reperfusion Injury in Mice. CNS Neurosci. Ther. 2012, 18, 388–394. [Google Scholar] [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005, 280, 13906–13912. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zador, Z.; Verkman, A.S. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J. Biol. Chem. 2008, 283, 15280–15286. [Google Scholar] [CrossRef] [Green Version]
- Bloch, O.; Papadopoulos, M.C.; Manley, G.T.; Verkman, A.S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005, 95, 254–262. [Google Scholar] [CrossRef] [PubMed]
| Drug | Structure (PubChem) | Mechanism of Action | Ref. |
|---|---|---|---|
| Furosemide CAS: CAS 54-31-9 MF: C12H11ClN2O5S MW: 222.3 g/mol |  |
| [39,43,44,45,58] |
| Amiloride CAS: 2609-46-3 MF: C6H8ClN7O MW: 330.74 g/mL | 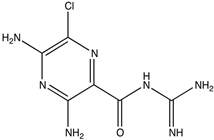 |
| [47] |
| Acetazolamide (CAS: 59-66-5) MF: C4H6N4O3S2 MW: 229.63 g/mol | 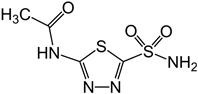 |
| [40,41,59,60,61] |
| Topiramate CAS: 97240-79-4 MF: C12H21NO8S MW: 339.36 g/mol | 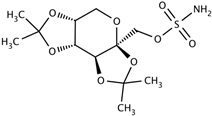 |
| [38,62,63,64,65] |
| Exendin-4 CAS: 141758-74-9 MF: C184H282N50O60S MW: 4187 g/mol |  |
| [50] |
| Liraglutide CAS: 204656-20-2 MF: C172H265N43O51 MW: 3751 g/mol | 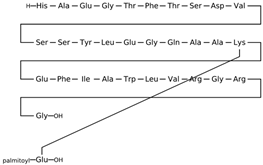 |
| [49] |
| Mannitol CAS: 69-65-8 MF: C6H14O6 MW: 182.17 g/mol |  |
| [43,46,66,67,68] |
| Drug | Animal | Effect | ||
|---|---|---|---|---|
| Healthy Animals | Disease Model | Ref. | ||
| Furosemide | Rats | No effect on intracranial pressure (ICP) with a clinically relevant dose but a high dose reduces brain volume. | [43] | |
| Dogs | Robust reduction in ICP on a high dose. | A slight reduction in ICP. | [44] | |
| Rabbits | Prominent reduction in ICP. | [40] | ||
| Amiloride | Rats | No change in ICP. | Lowers elevated ICP. | [42] |
| Acetazolamide | Rats | Mixed response: While one study revealed a reduction in ICP at a 200 mg dose, another study reported no change despite administering high doses (oral or subcutaneous). | 55% reduction in cerebrospinal fluid production. | [41,42,60] |
| Topiramate | Rats | Significant decrease in ICP at low and high clinically related doses by 32% and 21%, respectively. | [42,65] | |
| Exendin-4 | Rats | Reduced ICP. | Reduced ICP in hydrocephalus | [50] |
| Liraglutide | Rats | Reduced cerebral edema in peri-contusional regions in the traumatic brain injury (TBI) at a dose of 200 μg/kg. | [49] | |
| Drug | Study | Population | Outcome | Adverse Effects | Ref. |
|---|---|---|---|---|---|
| Acetazolamide | Multicenter, randomized, double-masked, placebo-controlled trial. | Subjects undergoing Idiopathic intracranial hypertension (IIH) and mild visual loss. | In combination with a low-sodium weight-reduction diet, it moderately improved visual field function. It also improved the quality-of-life outcomes at six months. | Changed taste, nausea, fatigue, and tingling of the hands and feet. | [38] |
| A randomized, double-blinded, placebo-controlled trial. | Adults with HIV + Cryptococcus Meningoencephalitis (CM) + headache + >20 cm H2O CSF opening pressure. | Discontinued for safety reasons. | Electrolyte imbalance, particularly in bicarbonate and chloride levels, often hyperchloremic acidosis. | [72] | |
| A randomized controlled trial. | IIH but not CM. | Failed due to insufficient sample size. | 48% of the subjects discontinued the drug for its side effects. | [61] | |
| Retrospective cohort study. | 3–15-year-old children with non-Cryptococcus-related meningitis. | AZA as adjunctive therapy to the standard therapeutics showed no added advantage of AZA in reducing elevated CSF. | [73] | ||
| Furosemide | Single-center retrospective study. | Non-CM neurological subjects with >25 cm H2O. | Continuous infusion of 3% HS with Furosemide was safe and effective in controlling ICP. | [37] | |
| Topiramate | Uncontrolled open-label study. | Non-CM patients with IIH. | It was effective and well-tolerated for the management of IIH. | Distal paresthesia and concentration difficulties, in addition to weight loss. | [69] |
| Hypertonic saline | Non-CM patients with traumatic brain injury (TBI). | Effective at lowering ICP in concentrations ranging from 3% to 23.4%. | Acute heart or kidney failure, severe pulmonary edema, and myelinolysis. | [74] | |
| Dexamethasone | A randomized controlled trial. | CM patients. | The study was stopped prematurely for no disparity in mortality rate or immune reconstitution inflammatory syndrome (IRIS) among two groups at 10 weeks. | Risks of disability and clinical adverse events were higher. | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.H.; Adil, M.S.; Lin, X.; Chastain, D.B.; Henao-Martínez, A.F.; Franco-Paredes, C.; Somanath, P.R. Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies. Pathogens 2022, 11, 783. https://doi.org/10.3390/pathogens11070783
Alanazi AH, Adil MS, Lin X, Chastain DB, Henao-Martínez AF, Franco-Paredes C, Somanath PR. Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies. Pathogens. 2022; 11(7):783. https://doi.org/10.3390/pathogens11070783
Chicago/Turabian StyleAlanazi, Abdulaziz H., Mir S. Adil, Xiaorong Lin, Daniel B. Chastain, Andrés F. Henao-Martínez, Carlos Franco-Paredes, and Payaningal R. Somanath. 2022. "Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies" Pathogens 11, no. 7: 783. https://doi.org/10.3390/pathogens11070783
APA StyleAlanazi, A. H., Adil, M. S., Lin, X., Chastain, D. B., Henao-Martínez, A. F., Franco-Paredes, C., & Somanath, P. R. (2022). Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies. Pathogens, 11(7), 783. https://doi.org/10.3390/pathogens11070783








