Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function
Abstract
:1. Introduction
2. Results
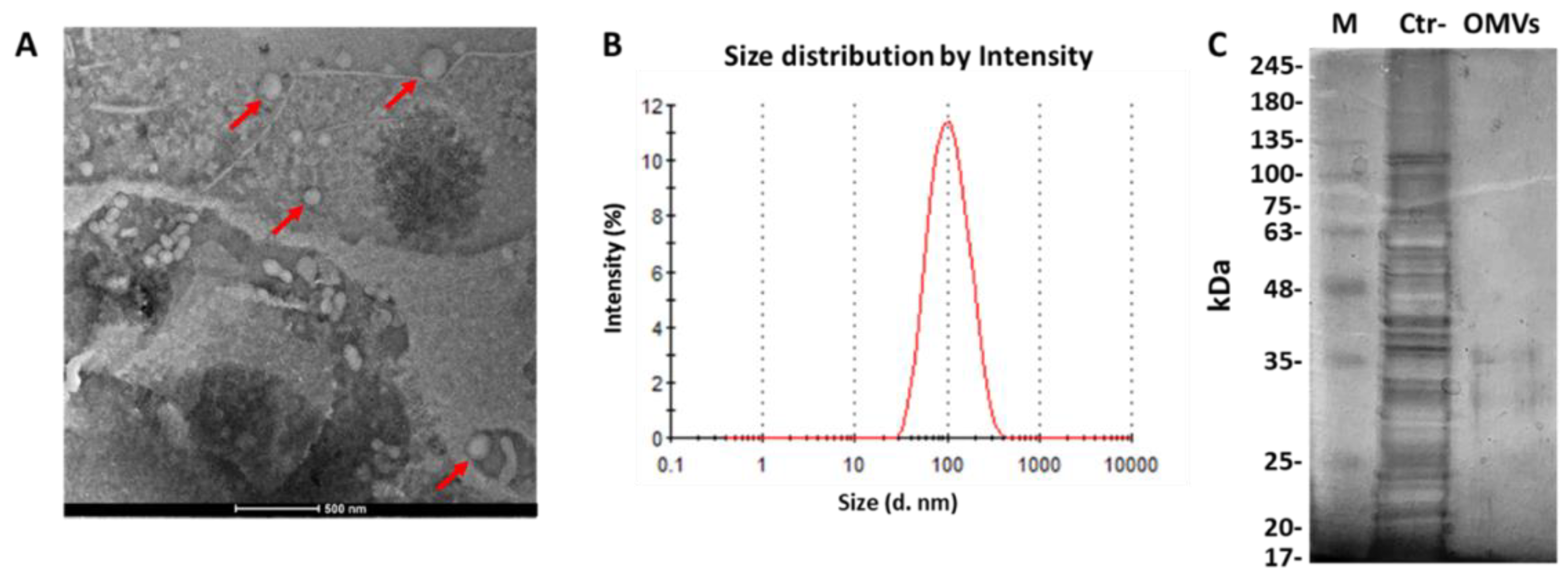
2.1. Identification of OMVs Derived from E. coli
2.2. Effect of E. coli OMVs on Sperm Quality
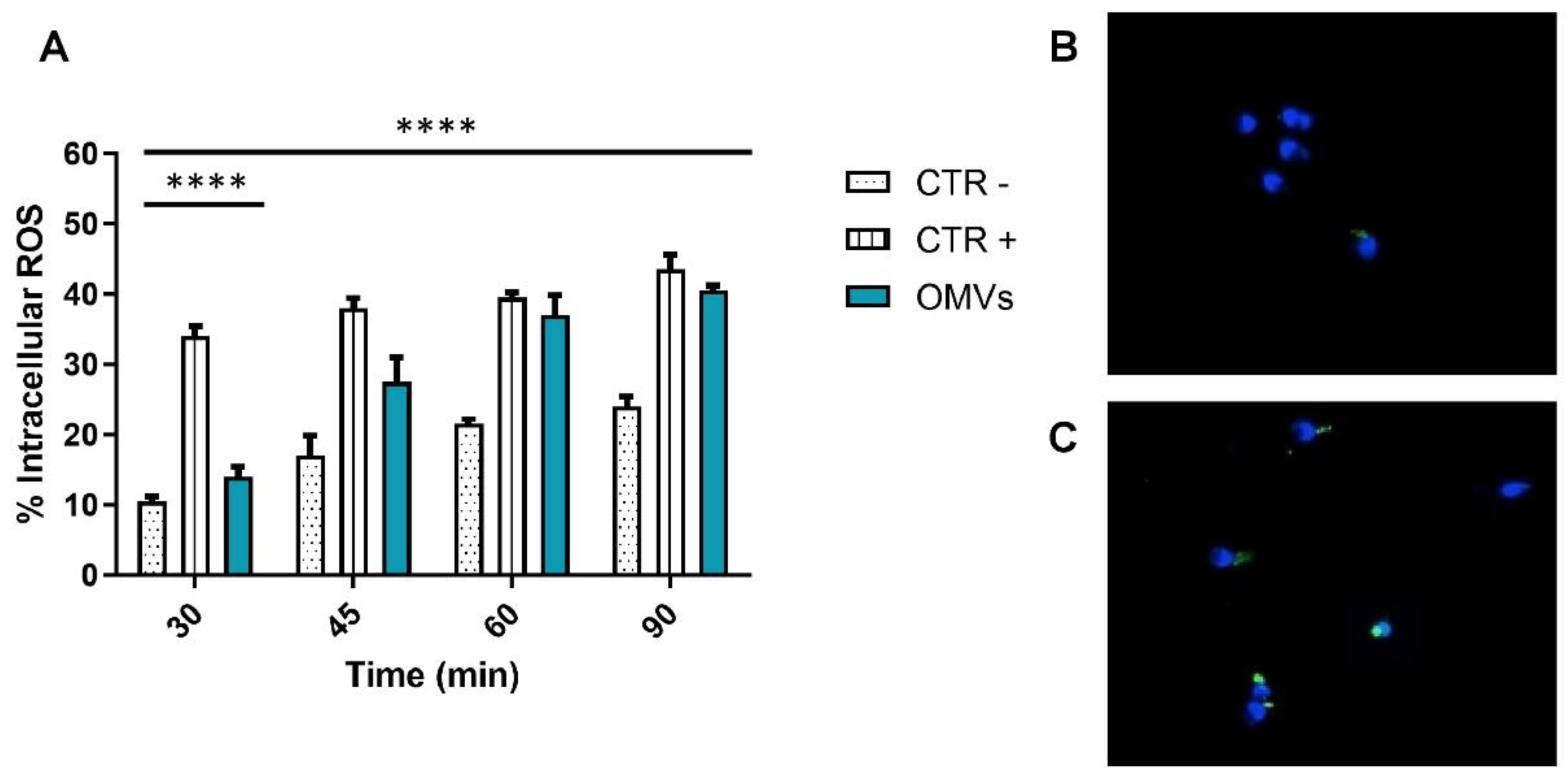
2.3. Assessment of Intracellular ROS Levels
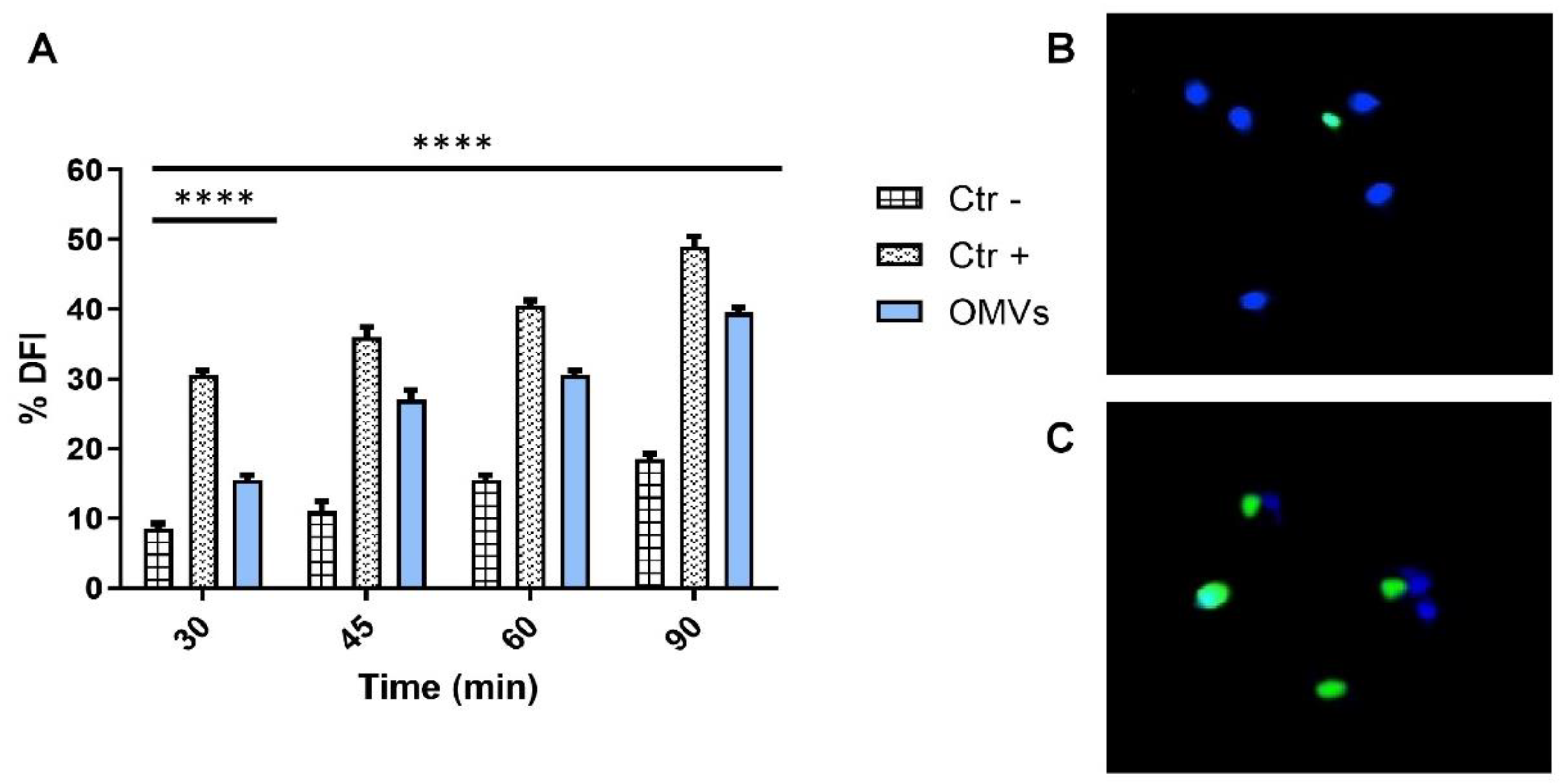
2.4. Evaluation of Sperm DNA Fragmentation
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Growth Conditions
4.2. OMV Purification
4.3. Dynamic Light Scattering (DLS) Analysis
4.4. Transmission Electron Microscopy (TEM)
4.5. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6. Human Semen Sample Collection and Purification
4.7. Exposure to OMVs and Semen Parameter Evaluation
4.8. Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) Test
4.9. Terminal Deoxynucleotidyl Transferase (TdT)dUTP Nick-End Labeling (TUNEL) Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.; Sophie, R.; Imam, A.M.; I Khan, F.; Ali, S.F.; Shaikh, A.; Farid-Ul-Hasnain, S. Knowledge, perceptions and myths regarding infertility among selected adult population in Pakistan: A cross-sectional study. BMC Public Health 2011, 11, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Sihag, P.; Tandon, A.; Pal, R.; Jain, B.K.; Bhatt, S.; Kaur, S.; Sinha, A. Sonography in male infertility: A look beyond the obvious. J. Ultrasound 2018, 21, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, H.-C.; Pilatz, A.; Hossain, H.; Diemer, T.; Wagenlehner, F.; Weidner, W. Urogenital Infection as a Risk Factor for Male Infertility. Dtsch. Ärzteblatt Int. 2017, 114, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Vilvanathan, S.; Kandasamy, B.; Jayachandran, A.L.; Sathiyanarayanan, S.; Singaravelu, V.T.; Krishnamurthy, V.; Elangovan, V. Bacteriospermia and Its Impact on Basic Semen Parameters among Infertile Men. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Folliero, V.; Caputo, P.; Della Rocca, M.T.; Chianese, A.; Galdiero, M.; Iovene, M.R.; Hay, C.; Franci, G.; Galdiero, M. Prevalence and Antimicrobial Susceptibility Patterns of Bacterial Pathogens in Urinary Tract Infections in University Hospital of Campania “Luigi Vanvitelli” between 2017 and 2018. Antibiotics 2020, 9, 215. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Sakka, V.; Gkoufa, A.; Sopilidis, O.; Chalikopoulos, D.; Alivizatos, G.; Giamarellou, E. Oral fosfomycin for the treatment of chronic bacterial prostatitis. J. Antimicrob. Chemother. 2019, 74, 1430–1437. [Google Scholar] [CrossRef] [Green Version]
- Monga, M.; Roberts, J.A. Spermagglutination by bacteria: Receptor-specific interactions. J. Androl. 1994, 15, 151–156. [Google Scholar]
- Diemer, T.; Huwe, P.; Ludwig, M.; Schroeder-Printzen, I.; Michelmann, H.W.; Schiefer, H.G.; Weidner, W. Influence of autogenous leucocytes and Escherichia coli on sperm motility parameters in vitro. Andrologia 2003, 35, 100–105. [Google Scholar] [CrossRef]
- Pant, N.C.; Singh, R.; Gupta, V.; Chauhan, A.; Mavuduru, R.; Prabha, V.; Sharma, P. Contraceptive efficacy of sperm agglutinating factor from Staphylococcus warneri, isolated from the cervix of a woman with inexplicable infertility. Reprod. Biol. Endocrinol. 2019, 17, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaraghavan, S.; Stephens, D.T.; Trautman, K.; Smith, G.D.; Khatra, B.; Silva, E.F.D.C.E.; Greengard, P. Sperm Motility Development in the Epididymis is Associated with Decreased Glycogen Synthase Kinase-3 and Protein Phosphatase 1 Activity1. Biol. Reprod. 1996, 54, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. (IJRM) 2016, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kushawaha, B.; Yadav, R.S.; Swain, D.K.; Kumari, P.; Kumar, A.; Yadav, B.; Anand, M.; Yadav, S.; Singh, D.; Garg, S.K. Collapsed mitochondrial cristae in goat spermatozoa due to mercury result in lethality and compromised motility along with altered kinematic patterns. Sci. Rep. 2021, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Dell’Aversana, C.; Doti, N.; Donadio, G.; Piaz, F.D.; Izzo, V.; De Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef]
- Martora, F.; Pinto, F.; Folliero, V.; Cammarota, M.; Dell’Annunziata, F.; Squillaci, G.; Galdiero, M.; Morana, A.; Schiraldi, C.; Giovane, A.; et al. Isolation, characterization and analysis of pro-inflammatory potential of Klebsiella pneumoniae outer membrane vesicles. Microb. Pathog. 2019, 136, 103719. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Folliero, V.; Giugliano, R.; De Filippis, A.; Santarcangelo, C.; Izzo, V.; Daglia, M.; Galdiero, M.; Arciola, C.; Franci, G. Gene Transfer Potential of Outer Membrane Vesicles of Gram-Negative Bacteria. Int. J. Mol. Sci. 2021, 22, 5985. [Google Scholar] [CrossRef]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. Current Evidence on the Ocular Surface Microbiota and Related Diseases. Microorganisms 2020, 8, 1033. [Google Scholar] [CrossRef]
- Gao, H.; Gao, Y.; Yang, C.; Dong, D.; Yang, J.; Peng, G.; Peng, J.; Wang, Y.; Pan, C.; Dong, W. Influence of outer membrane vesicles of Proteus mirabilis isolated from boar semen on sperm function. Vet. Microbiol. 2018, 224, 34–42. [Google Scholar] [CrossRef]
- Villegas, J.; Schulz, M.; Soto, L.; Sanchez, R. Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis 2005, 10, 105–110. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Agarwal, A.; Barbăroșie, C.; Ambar, R.; Finelli, R. The Impact of Single- and Double-Strand DNA Breaks in Human Spermatozoa on Assisted Reproduction. Int. J. Mol. Sci. 2020, 21, 3882. [Google Scholar] [CrossRef]
- Chang, H.-W.; Li, R.-N.; Wang, H.-R.; Liu, J.-R.; Tang, J.-Y.; Huang, H.-W.; Chan, Y.-H.; Yen, C.-Y. Withaferin A Induces Oxidative Stress-Mediated Apoptosis and DNA Damage in Oral Cancer Cells. Front Physiol. 2017, 8, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pironti, C.; Dell’Annunziata, F.; Giugliano, R.; Folliero, V.; Galdiero, M.; Ricciardi, M.; Motta, O.; Proto, A.; Franci, G. Comparative analysis of peracetic acid (PAA) and permaleic acid (PMA) in disinfection processes. Sci. Total Environ. 2021, 797, 149206. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Ilisso, C.P.; Dell’Aversana, C.; Greco, G.; Coppola, A.; Martora, F.; Piaz, F.D.; Donadio, G.; Falanga, A.; Galdiero, M.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Influence the miRNA Expression Profile in Human Bronchial Epithelial BEAS-2B Cells. Microorganisms 2020, 8, 1985. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective Effects of Curcumin on the Outcome of Cryopreservation in Human Sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials 2020, 10, 1118. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Martora, F.; Della Pepa, M.E.; Folliero, V.; Luongo, L.; Bocelli, S.; Guida, F.; Mascolo, P.; Campobasso, C.P.; Maione, S.; et al. Postmortem interval assessment by MALDI-TOF mass spectrometry analysis in murine cadavers. J. Appl. Microbiol. 2021, 132, 707–714. [Google Scholar] [CrossRef] [PubMed]
| OMV–Solvent Concentration | Time of Treatment (min) | Vitality (%) | Motility (P + NP) (%) | Immobile (%) | Normal Morphology (%) |
|---|---|---|---|---|---|
| 8 µg/mL | 30 | 70.5 ± 0.71 ● | 54.5 ± 1.4 ** | 45.5 ± 3.5 □□ | 19.5 ± 2.1 ● |
| 45 | 72 ± 1.41 ● | 51 ± 2.8 *** | 49 ± 0.71 ** | 19 ± 0.70 ● | |
| 60 | 66.5 ± 1.32 ● | 42.5 ± 0.71 ▪▪▪ | 57.5 ± 6.1 *** | 18.5 ± 4.9 ● | |
| 90 | 64 ± 0.89 ● | 39 ± 0.71 ◦◦◦ | 61 ± 2.8 ◊◊◊ | 17 ± 2.8 ● | |
| CTR− | 30 | 71.5 ± 0.78 | 65.5 ± 6.36 | 34.5 ± 2.8 | 21 ± 1.9 |
| 45 | 71.3 ± 0.35 | 64 ± 1.4 | 36 ± 2.1 | 20.5 ± 1.4 | |
| 60 | 66 ± 1.39 | 63 ± 2.1 | 37 ± 1.4 | 19.5 ± 1.23 | |
| 90 | 64.5 ± 2.12 | 60 ± 2.8 | 42 ± 1.3 | 18 ± 2.6 | |
| CTR+ | 30 | 65.5 ±2.2 * | 48 ± 5.40 *** | 52 ± 1.2 *** | 18 ± 1.3 ● |
| 45 | 59 ± 8.6 ** | 42 ± 6.80 ✩✩✩ | 58 ± 3.4 △△△ | 16 ± 2.2 ** | |
| 60 | 48 ± 4.5 *** | 35 ± 4.40 **** | 65 ± 1.5 ▲▲▲ | 14 ± 3.5 ⬢⬢ | |
| 90 | 40 ± 2.4 §§§ | 27 ± 1.50 ▽▽▽ | 71 ± 3.2 **** | 13 ± 0.60 ## |
| Sperm Parameters | Mean ± SD |
|---|---|
| Semen volume (mL) | 3.1 ± 0.42 |
| pH | 7.5 ± 0.21 |
| Sperm concentration (106 sperm/mL) | 60 ± 83.6 |
| Vitality (%) | 76 ± 2.83 |
| Progressive motility (%) | 71 ± 2.75 |
| Non-progressive motility (%) | 15 ± 1.89 |
| Immobile (%) | 19 ± 3.32 |
| Normal morphology (%) | 21 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folliero, V.; Santonastaso, M.; Dell’Annunziata, F.; De Franciscis, P.; Boccia, G.; Colacurci, N.; De Filippis, A.; Galdiero, M.; Franci, G. Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function. Pathogens 2022, 11, 782. https://doi.org/10.3390/pathogens11070782
Folliero V, Santonastaso M, Dell’Annunziata F, De Franciscis P, Boccia G, Colacurci N, De Filippis A, Galdiero M, Franci G. Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function. Pathogens. 2022; 11(7):782. https://doi.org/10.3390/pathogens11070782
Chicago/Turabian StyleFolliero, Veronica, Marianna Santonastaso, Federica Dell’Annunziata, Pasquale De Franciscis, Giovanni Boccia, Nicola Colacurci, Anna De Filippis, Massimiliano Galdiero, and Gianluigi Franci. 2022. "Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function" Pathogens 11, no. 7: 782. https://doi.org/10.3390/pathogens11070782
APA StyleFolliero, V., Santonastaso, M., Dell’Annunziata, F., De Franciscis, P., Boccia, G., Colacurci, N., De Filippis, A., Galdiero, M., & Franci, G. (2022). Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function. Pathogens, 11(7), 782. https://doi.org/10.3390/pathogens11070782












