Cross-Sectional Study on the Prevalence of PCV Types 2 and 3 DNA in Suckling Piglets Compared to Grow–Finish Pigs in Downstream Production
Abstract
:1. Introduction
2. Results
2.1. Presence of PCV2 and PCV3 DNA in Suckling Piglets
2.2. Qualitative Evaluation
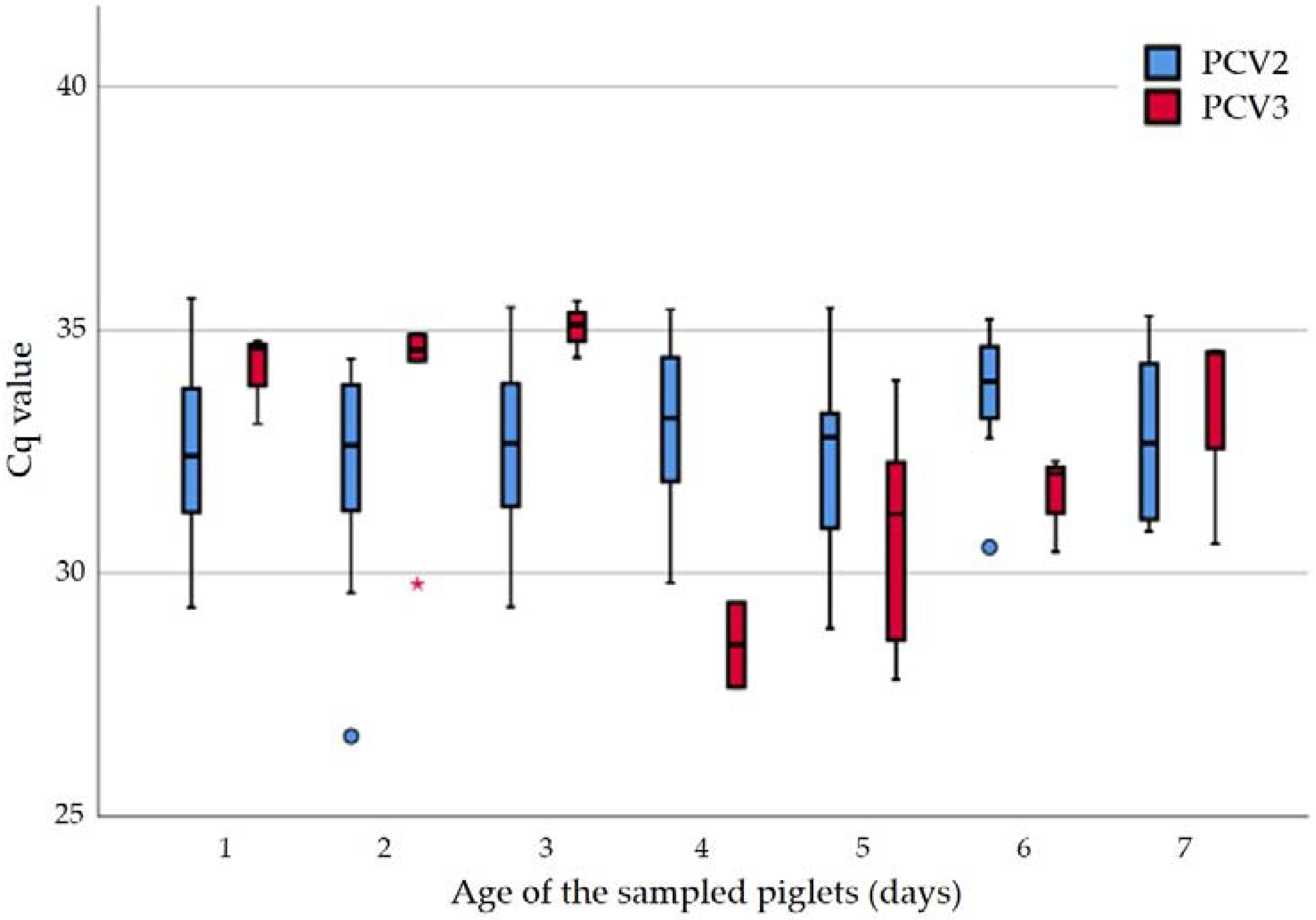
2.3. Quantitative Evaluation in Tissue
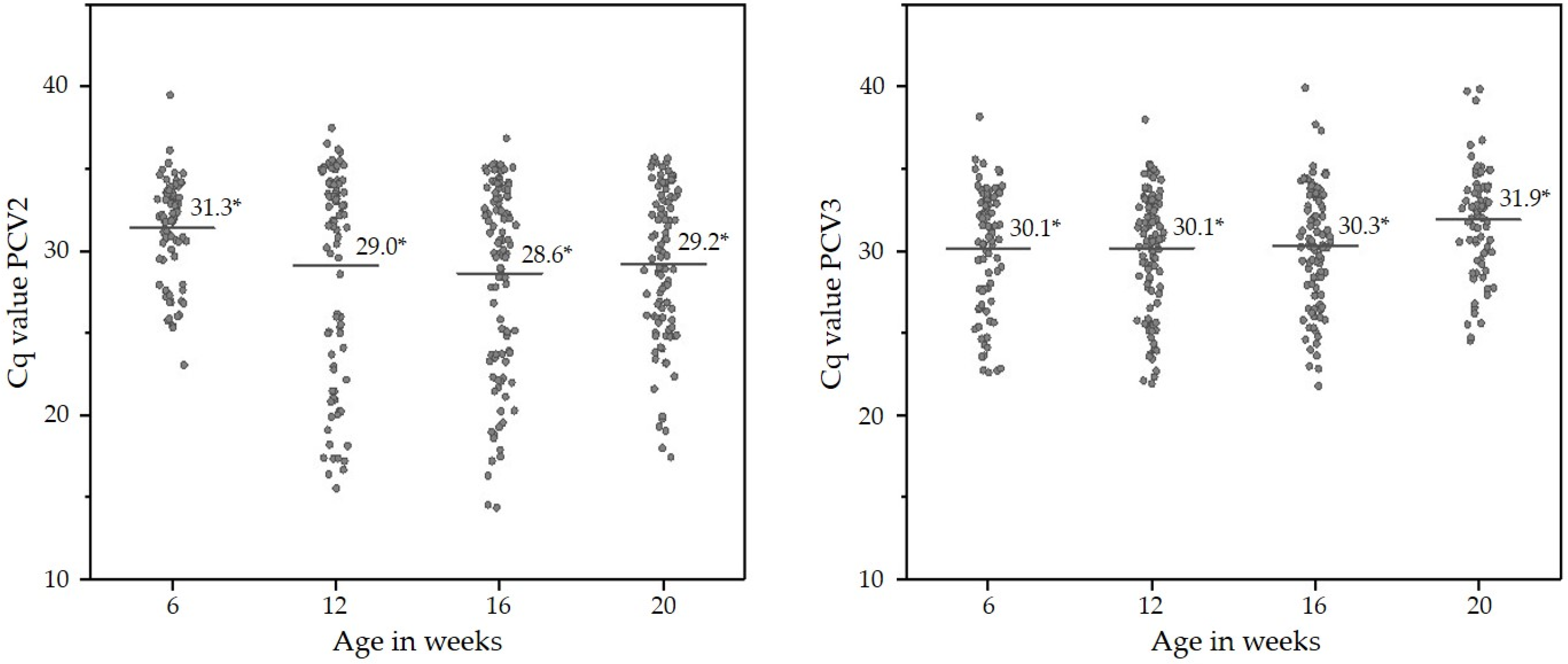
2.4. Presence of PCV2 and PCV3 DNA in OFS of Finisher Pigs
3. Discussion
4. Material and Methods
4.1. Farms and Animals
4.2. Collected Materials
4.2.1. Tissues
4.2.2. Oral Fluid Samples (OFS)
4.3. Molecular Biological Examinations
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segales, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Xiao, C.T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naive pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, R.E.; Nauwynck, H.J.; McNeilly, F.; Allan, G.M.; Pensaert, M.B. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Vet. Microbiol. 2001, 83, 169–176. [Google Scholar] [CrossRef]
- Rose, N.; Opriessnig, T.; Grasland, B.; Jestin, A. Epidemiology and transmission of porcine circovirus type 2 (PCV2). Virus Res. 2012, 164, 78–89. [Google Scholar] [CrossRef]
- Dvorak, C.M.; Lilla, M.P.; Baker, S.R.; Murtaugh, M.P. Multiple routes of porcine circovirus type 2 transmission to piglets in the presence of maternal immunity. Vet. Microbiol. 2013, 166, 365–374. [Google Scholar] [CrossRef]
- Gerber, P.F.; Garrocho, F.M.; Lana, A.M.; Lobato, Z.I. Fetal infections and antibody profiles in pigs naturally infected with porcine circovirus type 2 (PCV2). Can. J. Vet. Res. 2012, 76, 38–44. [Google Scholar]
- Shen, H.; Wang, C.; Madson, D.M.; Opriessnig, T. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Prev. Vet. Med. 2010, 97, 228–236. [Google Scholar] [CrossRef]
- Eddicks, M.; Koeppen, M.; Willi, S.; Fux, R.; Reese, S.; Sutter, G.; Stadler, J.; Ritzmann, M. Low prevalence of porcine circovirus type 2 infections in farrowing sows and corresponding pre-suckling piglets in southern German pig farms. Vet. Microbiol. 2016, 187, 70–74. [Google Scholar] [CrossRef]
- Dieste-Perez, L.; van Nes, A.; van Maanen, K.; Duinhof, T.; Tobias, T. The prevalence of PCV2 viremia in newborn piglets on four endemically infected Dutch sow farms is very low. Prev. Vet. Med. 2018, 153, 42–46. [Google Scholar] [CrossRef]
- Sydler, T.; Bragger, S.; Handke, M.; Hartnack, S.; Lewis, F.I.; Sidler, X.; Brugnera, E. Latent porcine circovirus type 2-infected domestic pigs: A potential infection model for the effective development of vaccines against latent or chronic virus induced diseases. Vaccine 2016, 34, 1047–1053. [Google Scholar] [CrossRef]
- Eddicks, M.; Beuter, B.; Stuhldreier, R.; Nolte, T.; Reese, S.; Sutter, G.; Ritzmann, M.; Fux, R. Cross-sectional study on viraemia and shedding of porcine circovirus type 2 in a subclinically infected multiplier sow herd. Vet. Rec 2019, 184, 189. [Google Scholar] [CrossRef] [PubMed]
- Mateusen, B.; Sanchez, R.E.; Van Soom, A.; Meerts, P.; Maes, D.G.; Nauwynck, H.J. Susceptibility of pig embryos to porcine circovirus type 2 infection. Theriogenology 2004, 61, 91–101. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segales, J.; Lopez-Soria, S.; Callen, A.; Merdy, O.; Joisel, F.; Sibila, M. Exploratory field study on the effect of Porcine circovirus 2 (PCV2) sow vaccination on serological, virological and reproductive parameters in a PCV2 subclinically infected sow herd. BMC Vet. Res. 2018, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Pineyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol 2017, 91, e01879-16. [Google Scholar] [CrossRef] [Green Version]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Lumyai, M.; Kesdangsakonwut, S.; Teankum, K.; Jittimanee, S.; Thanawongnuwech, R. Porcine circovirus type 3 (PCV3) infection in grower pigs from a Thai farm suffering from porcine respiratory disease complex (PRDC). Vet. Microbiol. 2018, 215, 71–76. [Google Scholar] [CrossRef]
- Chen, G.H.; Mai, K.J.; Zhou, L.; Wu, R.T.; Tang, X.Y.; Wu, J.L.; He, L.L.; Lan, T.; Xie, Q.M.; Sun, Y.; et al. Detection and genome sequencing of porcine circovirus 3 in neonatal pigs with congenital tremors in South China. Transbound Emerg. Dis. 2017, 64, 1650–1654. [Google Scholar] [CrossRef]
- Faccini, S.; Barbieri, I.; Gilioli, A.; Sala, G.; Gibelli, L.R.; Moreno, A.; Sacchi, C.; Rosignoli, C.; Franzini, G.; Nigrelli, A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound Emerg. Dis. 2017, 64, 1661–1664. [Google Scholar] [CrossRef]
- Tochetto, C.; Lima, D.A.; Varela, A.P.M.; Loiko, M.R.; Paim, W.P.; Scheffer, C.M.; Herpich, J.I.; Cerva, C.; Schmitd, C.; Cibulski, S.P.; et al. Full-Genome Sequence of Porcine Circovirus type 3 recovered from serum of sows with stillbirths in Brazil. Transbound Emerg. Dis. 2018, 65, 5–9. [Google Scholar] [CrossRef]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Kesdangsakonwut, S.; Tummaruk, P.; Teankum, K.; Assavacheep, P.; Jittimanee, S.; et al. Porcine circovirus type 3 (PCV3) shedding in sow colostrum. Vet. Microbiol. 2018, 220, 12–17. [Google Scholar] [CrossRef]
- Gerber, P.F.; Garrocho, F.M.; Lana, A.M.Q.; Lobato, Z.I.P. Serum antibodies and shedding of infectious porcine circovirus 2 into colostrum and milk of vaccinated and unvaccinated naturally infected sows. Vet. J. 2011, 188, 240–242. [Google Scholar] [CrossRef]
- Shibata, I.; Okuda, Y.; Kitajima, K.; Asai, T. Shedding of porcine circovirus into colostrum of sows. J. Vet. Med. B 2006, 53, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Mora-Diaz, J.; Pineyro, P.; Shen, H.; Schwartz, K.; Vannucci, F.; Li, G.; Arruda, B.; Gimenez-Lirola, L. Isolation of PCV3 from Perinatal and Reproductive Cases of PCV3-Associated Disease and In Vivo Characterization of PCV3 Replication in CD/CD Growing Pigs. Viruses 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fux, R.; Soeckler, C.; Link, E.K.; Renken, C.; Krejci, R.; Sutter, G.; Ritzmann, M.; Eddicks, M. Full genome characterization of porcine circovirus type 3 isolates reveals the existence of two distinct groups of virus strains. Virol. J. 2018, 15, 25. [Google Scholar] [CrossRef] [Green Version]
- Stadejek, T.; Wozniak, A.; Milek, D.; Biernacka, K. First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transbound Emerg. Dis. 2017, 64, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Yoo, S.J.; Park, C.K.; Lyoo, Y.S. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet. Microbiol. 2017, 207, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, G.L.; Vidigal, P.M.P.; Fietto, J.L.R.; Bressan, G.C.; Silva Junior, A.; de Almeida, M.R. Evolutionary analysis of Porcine circovirus 3 (PCV3) indicates an ancient origin for its current strains and a worldwide dispersion. Virus Genes 2018, 54, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Correa-Fiz, F.; Franzo, G.; Sibila, M.; Nunez, J.I.; Segales, J. Current Knowledge on Porcine circovirus 3 (PCV-3): A Novel Virus With a Yet Unknown Impact on the Swine Industry. Front. Vet. Sci 2018, 5, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipos, W.; Duvigneau, C.; Pietschmann, P.; Holler, K.; Hartl, R.; Wahl, K.; Steinborn, R.; Gemeiner, M.; Willheim, M.; Schmoll, F. Parameters of humoral and cellular immunity following vaccination of pigs with a European modified-live strain of porcine reproductive and respiratory syndrome virus (PRRSV). Viral Immunol. 2003, 16, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.; Simer, R.; Christopher-Hennings, J.; Yoon, K.J.; Evans, R.B.; Zimmerman, J.J. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: A longitudinal study under experimental conditions. J. Vet. Diagn. Investig. 2008, 20, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Suckling Piglets (Up to 1 Week of Age) | Grow–Finish Pigs (6–20 Weeks of Age) | |||||
|---|---|---|---|---|---|---|
| Farm | Tissue Pools by Litters | Tissue Pools for Each Piglet | Oral Fluids | |||
| PCV2 % (n) | PCV3 % (n) | PCV2 % (n) | PCV3 % (n) | PCV2 %(n) | PCV3 % (n) | |
| 1 | 41.7% (5/12) | 8.3% (1/12) | 38.5% (5/13) | 7.7% (1/13) | 100.0% (32/32) | 65.6% (21/32) |
| 2 | 68.8% (11/16) | 6.3% (1/16) | 66.7% (12/18) | 5.6% (1/18) | 60.0% (21/35) | 65.7% (23/35) |
| 3 | 75.0% (6/8) | 37.5% (3/8) | 77.8% (7/9) | 33.3% (3/9) | 63.9% (23/36) | 30.6% (11/36) |
| 4 | 87.5% (14/16) | 0.0% (0/16) | 78.6% (22/28) | 0.0% (0/28) | 63.9% (23/36) | 22.2% (8/36) |
| 5 | 42.9% (3/7) | 28.6% (2/7) | 44.4% (4/9) | 22.2% (2/9) | 62.2% (23/37) | 100.0% (37/37) |
| 6 | 75.0% (3/4) | 0.0% (0/4) | 75.0% (6/8) | 0.0% (0/8) | 53.1% (17/32) | 87.5% (28/32) |
| 7 | 100.0% (9/9) | 0.0% (0/9) | 92.3% (12/13) | 0.0% (0/13) | 42.9% (15/35) | 62.9% (22/35) |
| 8 | 100.0% (8/8) | 12.5% (1/8) | 75.0% (12/16) | 6.3% (1/16) | 96.9% (31/32) | 81.3% (26/32) |
| 9 | 89.5% (17/19) | 5.3% (1/19) | 85.7% (18/21) | 4.8% (1/21) | 29.4% (10/34) | 73.5% (25/34) |
| 10 | 80.0% (4/5) | 100.0% (5/5) | 72.7% (8/11) | 100.0% (11/11) | 57.1% (16/28) | 92.9% (26/28) |
| 11 | 50.0% (1/2) | 0.0% (0/2) | 50.0% (1/2) | 0.0% (0/2) | 100.0% (36/36) | 61.1% (22/36) |
| 12 | 50.0% (3/6) | 50.0% (3/6) | 36.4% (4/11) | 27.3% (3/11) | 100.0% (38/38) | 94.7% (36/38) |
| 13 | 11.1% (1/9) | 11.1% (1/9) | 8.3% (1/12) | 8.3% (1/12) | 100.0% (24/24) | 95.8% (23/24) |
| 14 | 33.3% (1/3) | 0.0% (0/3) | 33.3% (1/3) | 0.0% (0/3) | 40.0% (8/20) | 0.0% (0/20) |
| 15 | 0.0% (0/7) | 0.0% (0/7) | 0.0% (0/7) | 0.0% (0/7) | 87.0% (20/23) | 39.1% (9/23) |
| 16 | 33.3% (1/3) | 0.0% (0/3) | 25.0% (1/4) | 0.0% (0/4) | 100.0% (37/37) | 78.4% (29/37) |
| Total | 64.9% (87/134) | 13.4% (18/134) | 61.6% (114/185) | 13.0% (24/185) | 72.6% (374/515) | 67.2% (346/515) |
| Independent Variable | Dependent Variable | p-Value Chi2 Test | p-Value Binary Logistic Regression | OR | Upper CI | Lower CI |
|---|---|---|---|---|---|---|
| PCV2 sow vaccination | PCV2 DNA- positive piglet | <0.001 | 0.001 | 0.279 | 0.134 | 0.578 |
| Mycoplasma hyopneumoniae sow vaccination | 0.004 | 0.464 | - | - | - | |
| Own replacement gilts | 0.007 | 0.056 | - | - | - |
| Independent Variable | Dependent Variable | p Value Chi2 Test | p-Value Binary Logistic Regression | OR | Upper CI | Lower CI |
|---|---|---|---|---|---|---|
| Porcine reproductive and respiratory syndrome virus sow vaccination | PCV3 DNA- positive piglet | 0.001 | 0.002 | 0.252 | 0.104 | 0.610 |
| Actinobacillus pleuropneumoniae sow vaccination | 0.048 | 0.184 | - | - | - |
| PCV2 Sow Vaccination | PCV2 DNA-Positive Piglets % (n) | PRRSV Sow Vaccination | PCV3 DNA-Positive Piglets % (n) |
|---|---|---|---|
| Yes | 37.5% (CI: 22.2–53.7%) (15/40) | Yes | 8.1% (CI: 3.7–13.2%) (11/135) |
| No | 68.3% (CI: 60.8–75.7%) (99/145) | No | 26.0% (CI: 14.9–38.5%) (13/50) |
| Week of Life | PCV2 DNA- Positive OFS % (n) | 95% Confidence Interval % | PCV3 DNA- Positive OFS % (n) | 95% Confidence Interval % | PCV2 + PCV3 DNA-Positive OFS % (n) | 95% Confidence Interval % |
|---|---|---|---|---|---|---|
| 6 | 65.6% (82/125) | 56.8–73.6 | 65.6% (82/125) | 56.8–73.6 | 44.8% (56/125) | 36.0–53.6 |
| 12 | 67.2% (90/134) | 59.0–74.6 | 75.4% (101/134) | 68.7–82.8 | 56.0% (75/134) | 47.8–63.4 |
| 16 | 79.2% (103/130) | 71.7–86.2 | 70.8% (92/130) | 63.1–78.5 | 56.9% (74/130) | 48.5–65.4 |
| 20 | 78.6% (99/126) | 71.4–85.7 | 56.3% (71/126) | 47.6–64.3 | 46.0% (58/126) | 37.3–54.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddicks, M.; Maurer, R.; Deffner, P.; Eddicks, L.; Sipos, W.; Reese, S.; Cvjetković, V.; Krejci, R.; Opriessnig, T.; Ritzmann, M.; et al. Cross-Sectional Study on the Prevalence of PCV Types 2 and 3 DNA in Suckling Piglets Compared to Grow–Finish Pigs in Downstream Production. Pathogens 2022, 11, 671. https://doi.org/10.3390/pathogens11060671
Eddicks M, Maurer R, Deffner P, Eddicks L, Sipos W, Reese S, Cvjetković V, Krejci R, Opriessnig T, Ritzmann M, et al. Cross-Sectional Study on the Prevalence of PCV Types 2 and 3 DNA in Suckling Piglets Compared to Grow–Finish Pigs in Downstream Production. Pathogens. 2022; 11(6):671. https://doi.org/10.3390/pathogens11060671
Chicago/Turabian StyleEddicks, Matthias, Roland Maurer, Pauline Deffner, Lina Eddicks, Wolfgang Sipos, Sven Reese, Vojislav Cvjetković, Roman Krejci, Tanja Opriessnig, Mathias Ritzmann, and et al. 2022. "Cross-Sectional Study on the Prevalence of PCV Types 2 and 3 DNA in Suckling Piglets Compared to Grow–Finish Pigs in Downstream Production" Pathogens 11, no. 6: 671. https://doi.org/10.3390/pathogens11060671
APA StyleEddicks, M., Maurer, R., Deffner, P., Eddicks, L., Sipos, W., Reese, S., Cvjetković, V., Krejci, R., Opriessnig, T., Ritzmann, M., & Fux, R. (2022). Cross-Sectional Study on the Prevalence of PCV Types 2 and 3 DNA in Suckling Piglets Compared to Grow–Finish Pigs in Downstream Production. Pathogens, 11(6), 671. https://doi.org/10.3390/pathogens11060671






